Abstract
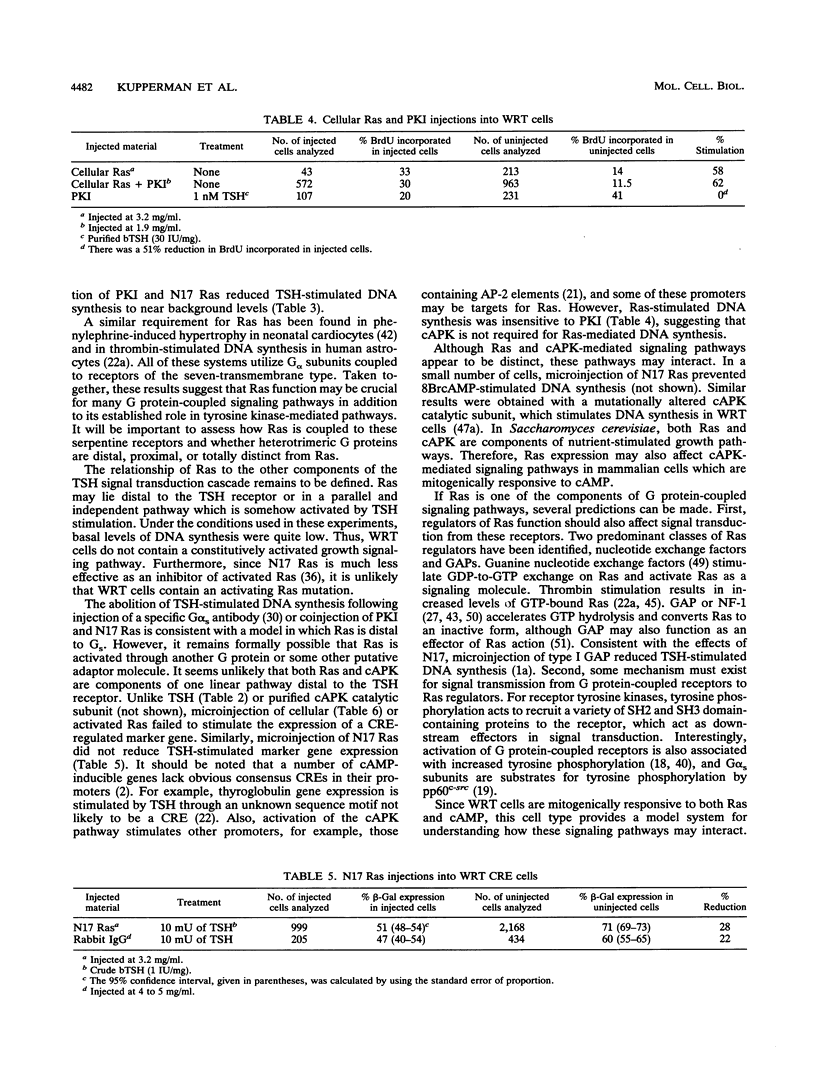
Microinjection of a dominant interfering mutant of Ras (N17 Ras) caused a significant reduction in thyrotropin (thyroid-stimulating hormone [TSH])-stimulated DNA synthesis in rat thyroid cells. A similar reduction was observed following injection of the heat-stable protein kinase inhibitor of the cyclic AMP-dependent protein kinase. Coinjection of both inhibitors almost completely abolished TSH-induced DNA synthesis. In contrast to TSH, overexpression of cellular Ras protein did not stimulate the expression of a cyclic AMP response element-regulated reporter gene. Similarly, injection of N17 Ras had no effect on TSH-stimulated reporter gene expression. Moreover, overexpression of cellular Ras protein stimulated similar levels of DNA synthesis in the presence or absence of the heat-stable protein kinase inhibitor. Together, these results suggest that in Wistar rat thyroid cells, a full mitogenic response to TSH requires both Ras and cyclic APK-dependent protein kinase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney T. O., Carswell L. S. Gonadotropin regulation of Leydig cell DNA synthesis. Mol Cell Endocrinol. 1986 May;45(2-3):157–165. doi: 10.1016/0303-7207(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Albanese C., Kay T. W., Troccoli N. M., Jameson J. L. Novel cyclic adenosine 3',5'-monophosphate response element in the human chorionic gonadotropin beta-subunit gene. Mol Endocrinol. 1991 May;5(5):693–702. doi: 10.1210/mend-5-5-693. [DOI] [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Medema R. H., Maassen J. A., van de Wetering M. L., van der Eb A. J., McCormick F., Bos J. L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991 May;10(5):1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Szeberényi J., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol. 1990 Oct;10(10):5314–5323. doi: 10.1128/mcb.10.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases. Mol Cell Biol. 1992 May;12(5):2315–2321. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E., Centrella M., Burch W., McCarthy T. L. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989 Jan;83(1):60–65. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Dumont J. E., Lamy F., Roger P., Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev. 1992 Jul;72(3):667–697. doi: 10.1152/physrev.1992.72.3.667. [DOI] [PubMed] [Google Scholar]
- Farnsworth C. L., Feig L. A. Dominant inhibitory mutations in the Mg(2+)-binding site of RasH prevent its activation by GTP. Mol Cell Biol. 1991 Oct;11(10):4822–4829. doi: 10.1128/mcb.11.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Gross M., Sweet R. W., Sathe G., Yokoyama S., Fasano O., Goldfarb M., Wigler M., Rosenberg M. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985 May;5(5):1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Gallego C., Johnson G. L. Mitogenic pathways regulated by G protein oncogenes. Mol Biol Cell. 1992 Jan;3(1):123–128. doi: 10.1091/mbc.3.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Robbins K. C. Activation of transforming G protein-coupled receptors induces rapid tyrosine phosphorylation of cellular proteins, including p125FAK and the p130 v-src substrate. Biochem Biophys Res Commun. 1992 Oct 15;188(1):155–161. doi: 10.1016/0006-291x(92)92363-3. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Pitcher J. A., Luttrell D. K., Linder M. E., Kurose H., Parsons S. J., Caron M. G., Lefkowitz R. J. Tyrosine phosphorylation of G protein alpha subunits by pp60c-src. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. N., Erdreich L. S., Paynter O. E., Roberts P. A., Rosenthal S. L., Wilkinson C. F. Thyroid follicular cell carcinogenesis. Fundam Appl Toxicol. 1989 May;12(4):629–697. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Javaux F., Donda A., Vassart G., Christophe D. Cloning and sequence analysis of TFE, a helix-loop-helix transcription factor able to recognize the thyroglobulin gene promoter in vitro. Nucleic Acids Res. 1991 Mar 11;19(5):1121–1127. doi: 10.1093/nar/19.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMorte V. J., Goldsmith P. K., Spiegel A. M., Meinkoth J. L., Feramisco J. R. Inhibition of DNA synthesis in living cells by microinjection of Gi2 antibodies. J Biol Chem. 1992 Jan 15;267(2):691–694. [PubMed] [Google Scholar]
- Lemoine N. R., Staddon S., Bond J., Wyllie F. S., Shaw J. J., Wynford-Thomas D. Partial transformation of human thyroid epithelial cells by mutant Ha-ras oncogene. Oncogene. 1990 Dec;5(12):1833–1837. [PubMed] [Google Scholar]
- Letterio J. J., Coughlin S. R., Williams L. T. Pertussis toxin-sensitive pathway in the stimulation of c-myc expression and DNA synthesis by bombesin. Science. 1986 Nov 28;234(4780):1117–1119. doi: 10.1126/science.3465038. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Martin G. A., Viskochil D., Bollag G., McCabe P. C., Crosier W. J., Haubruck H., Conroy L., Clark R., O'Connell P., Cawthon R. M. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990 Nov 16;63(4):843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989 Mar;124(3):1247–1253. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- Meinkoth J. L., Dela Cruz J., Burrow G. N. TSH, IGF-1 and activated ras protein induce DNA synthesis in cultured thyroid cells. Thyroidology. 1991 Dec;3(3):103–107. [PubMed] [Google Scholar]
- Meinkoth J. L., Goldsmith P. K., Spiegel A. M., Feramisco J. R., Burrow G. N. Inhibition of thyrotropin-induced DNA synthesis in thyroid follicular cells by microinjection of an antibody to the stimulatory G protein of adenylate cyclase, Gs. J Biol Chem. 1992 Jul 5;267(19):13239–13245. [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Namba H., Rubin S. A., Fagin J. A. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990 Oct;4(10):1474–1479. doi: 10.1210/mend-4-10-1474. [DOI] [PubMed] [Google Scholar]
- Roy S. K., Greenwald G. S. Mediation of follicle-stimulating hormone action on follicular deoxyribonucleic acid synthesis by epidermal growth factor. Endocrinology. 1991 Oct;129(4):1903–1908. doi: 10.1210/endo-129-4-1903. [DOI] [PubMed] [Google Scholar]
- Shenker A., Goldsmith P., Unson C. G., Spiegel A. M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 1991 May 15;266(14):9309–9313. [PubMed] [Google Scholar]
- Stacey D. W., Feig L. A., Gibbs J. B. Dominant inhibitory Ras mutants selectively inhibit the activity of either cellular or oncogenic Ras. Mol Cell Biol. 1991 Aug;11(8):4053–4064. doi: 10.1128/mcb.11.8.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Suarez H. G., du Villard J. A., Caillou B., Schlumberger M., Parmentier C., Monier R. gsp mutations in human thyroid tumours. Oncogene. 1991 Apr;6(4):677–679. [PubMed] [Google Scholar]
- Suarez H. G., du Villard J. A., Severino M., Caillou B., Schlumberger M., Tubiana M., Parmentier C., Monier R. Presence of mutations in all three ras genes in human thyroid tumors. Oncogene. 1990 Apr;5(4):565–570. [PubMed] [Google Scholar]
- Szeberényi J., Cai H., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990 Oct;10(10):5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Conti M., Prokop C., Van Wyk J. J., Earp H. S., 3rd Thyrotropin and insulin-like growth factor I regulation of tyrosine phosphorylation in FRTL-5 cells. Interaction between cAMP-dependent and growth factor-dependent signal transduction. J Biol Chem. 1991 Apr 25;266(12):7834–7841. [PubMed] [Google Scholar]
- Thomas J., Van Patten S. M., Howard P., Day K. H., Mitchell R. D., Sosnick T., Trewhella J., Walsh D. A., Maurer R. A. Expression in Escherichia coli and characterization of the heat-stable inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1991 Jun 15;266(17):10906–10911. [PubMed] [Google Scholar]
- Thorburn A., Thorburn J., Chen S. Y., Powers S., Shubeita H. E., Feramisco J. R., Chien K. R. HRas-dependent pathways can activate morphological and genetic markers of cardiac muscle cell hypertrophy. J Biol Chem. 1993 Jan 25;268(3):2244–2249. [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Tramontano D., Cushing G. W., Moses A. C., Ingbar S. H. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology. 1986 Aug;119(2):940–942. doi: 10.1210/endo-119-2-940. [DOI] [PubMed] [Google Scholar]
- Williams D. W., Williams E. D., Wynford-Thomas D. Evidence for autocrine production of IGF-1 in human thyroid adenomas. Mol Cell Endocrinol. 1989 Jan;61(1):139–143. doi: 10.1016/0303-7207(89)90199-8. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. A cytosolic protein catalyzes the release of GDP from p21ras. Science. 1990 Apr 6;248(4951):67–69. doi: 10.1126/science.2181667. [DOI] [PubMed] [Google Scholar]
- Xu G. F., Lin B., Tanaka K., Dunn D., Wood D., Gesteland R., White R., Weiss R., Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990 Nov 16;63(4):835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Hordijk P. L., Medema R. H., Bos J. L., Moolenaar W. H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]




