Abstract
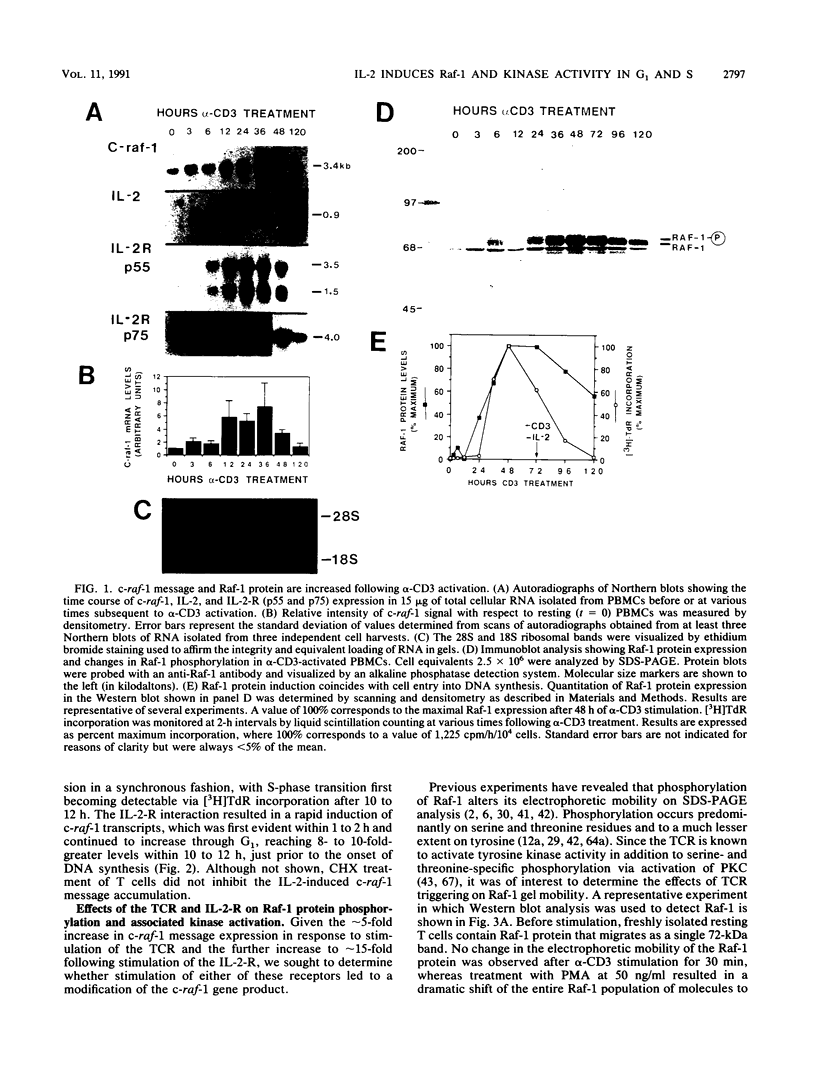
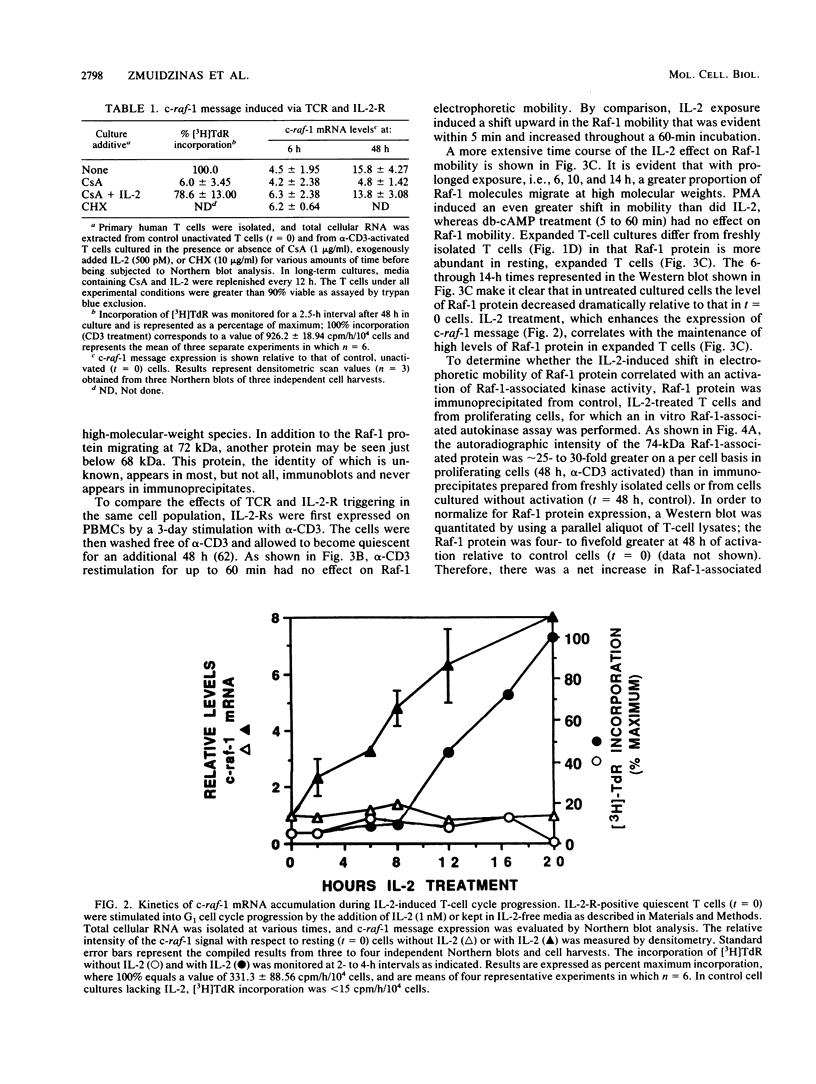
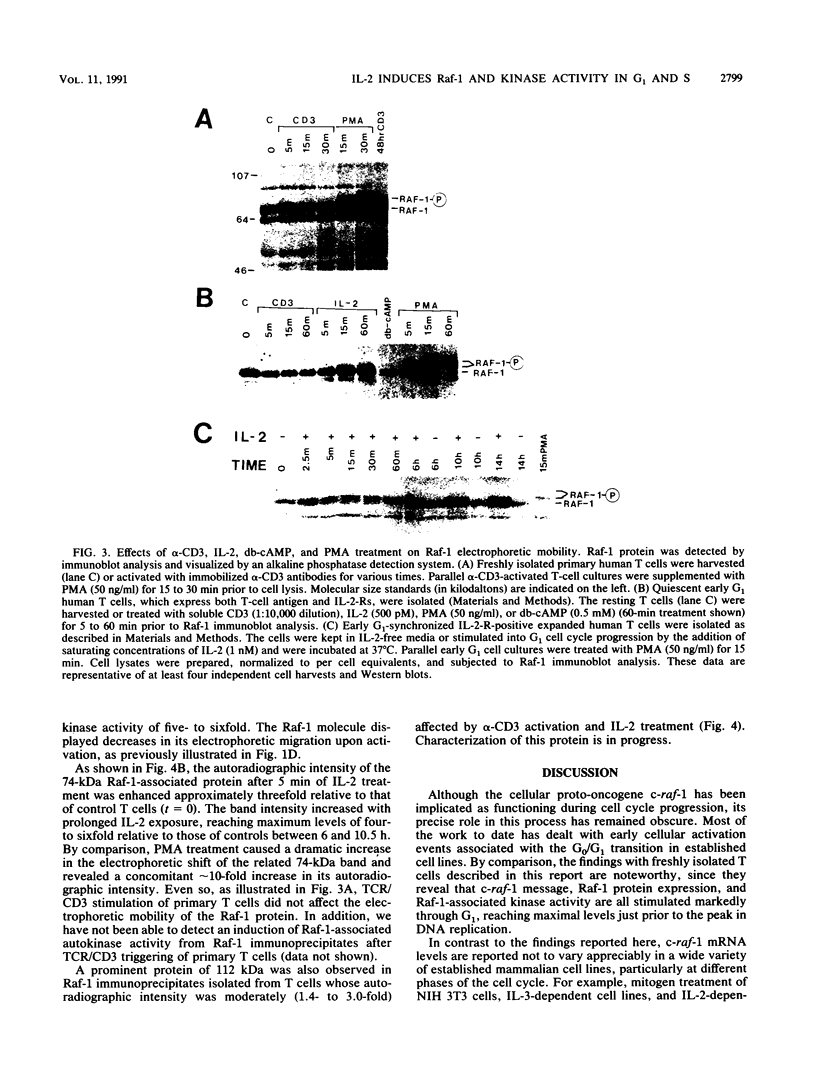
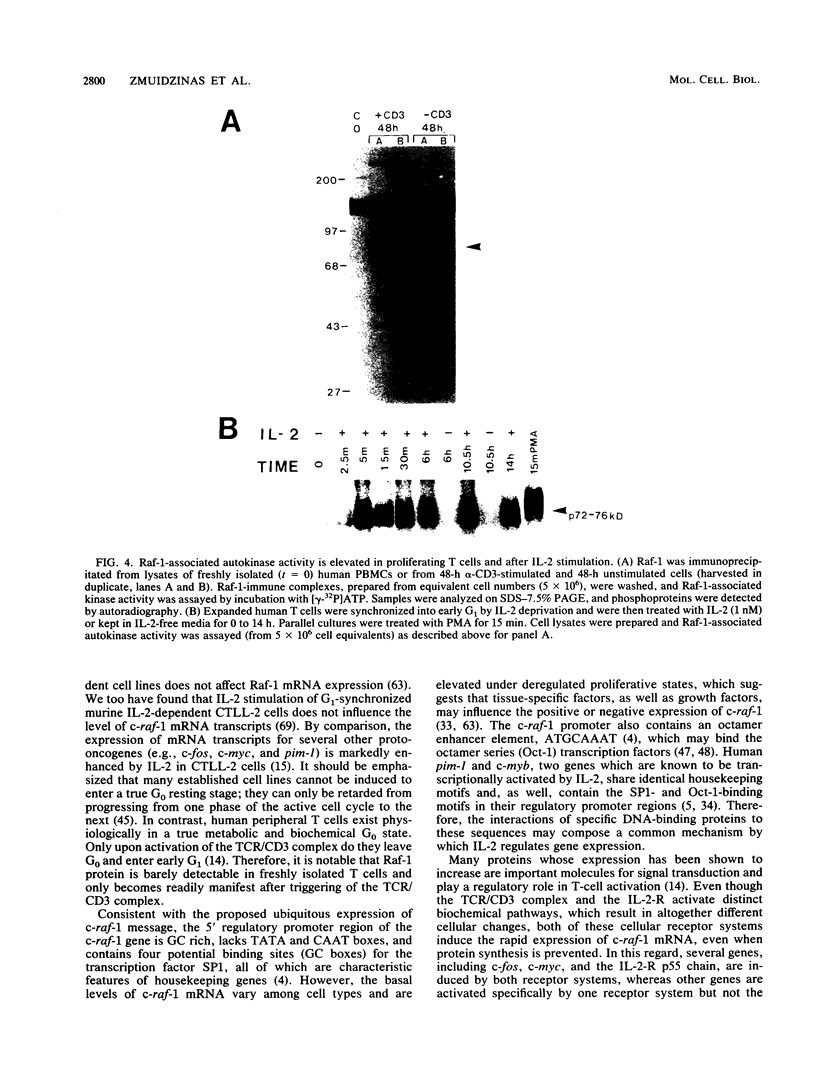
To gain further insight into the role of Raf-1 in normal cell growth, c-raf-1 mRNA expression, Raf-1 protein production, and Raf-1-associated kinase activity in normal human T cells were analyzed. In contrast to the constitutive expression of Raf-1 in continuously proliferating cell lines, c-raf-1 mRNA and Raf-1 protein levels were barely detectable in freshly isolated G0 T lymphocytes. Previous work with fibroblasts has suggested that Raf-1 plays a signaling role in the G0-G1 phase transition. In T cells, triggering via the T-cell antigen receptor (TCR)-CD3 complex (TCR/CD3) resulted in an approximately fourfold increase in c-raf-1 mRNA. In addition, the promotion of G1 progression by interleukin 2 (IL-2) was associated with a 5- to 10-fold immediate/early induction of c-raf-1 mRNA, resulting in up to a 12-fold increase in Raf-1 protein expression. TCR/CD3 activation did not alter the phosphorylation state of Raf-1, whereas interleukin 2 receptor stimulation resulted in a rapid increase in the phosphorylation state of a subpopulation of Raf-1 molecules progressively increasing throughout G1. These findings were complemented by assays for Raf-1-associated kinase activity which revealed a gradual accumulation of serine and threonine autokinase activity in Raf-1 immunoprecipitates during G1, which remained elevated throughout DNA replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asao H., Takeshita T., Nakamura M., Nagata K., Sugamura K. Interleukin 2 (IL-2)-induced tyrosine phosphorylation of IL-2 receptor p75. J Exp Med. 1990 Mar 1;171(3):637–644. doi: 10.1084/jem.171.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M., Sabatini D. M., App H., Rapp U. R., Stanley E. R. Colony stimulating factor-1 (CSF-1) stimulates temperature dependent phosphorylation and activation of the RAF-1 proto-oncogene product. EMBO J. 1990 Nov;9(11):3649–3657. doi: 10.1002/j.1460-2075.1990.tb07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Luong E., Samelson L. E., Klausner R. D. The T cell antigen receptor zeta chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988 Dec 5;263(34):18225–18230. [PubMed] [Google Scholar]
- Beck T. W., Brennscheidt U., Sithanandam G., Cleveland J., Rapp U. R. Molecular organization of the human Raf-1 promoter region. Mol Cell Biol. 1990 Jul;10(7):3325–3333. doi: 10.1128/mcb.10.7.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender T. P., Kuehl W. M. Murine myb protooncogene mRNA: cDNA sequence and evidence for 5' heterogeneity. Proc Natl Acad Sci U S A. 1986 May;83(10):3204–3208. doi: 10.1073/pnas.83.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Haupt D. M., App H., Rapp U. R. Insulin activates the Raf-1 protein kinase. J Biol Chem. 1990 Jul 25;265(21):12131–12134. [PubMed] [Google Scholar]
- Bonner T. I., Oppermann H., Seeburg P., Kerby S. B., Gunnell M. A., Young A. C., Rapp U. R. The complete coding sequence of the human raf oncogene and the corresponding structure of the c-raf-1 gene. Nucleic Acids Res. 1986 Jan 24;14(2):1009–1015. doi: 10.1093/nar/14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. P., Clark-Lewis I., Rapp U. R., May W. S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990 Nov 15;265(32):19812–19817. [PubMed] [Google Scholar]
- Cleveland J. L., Rapp U. R., Farrar W. L. Role of c-myc and other genes in interleukin 2 regulated CT6 T lymphocytes and their malignant variants. J Immunol. 1987 May 15;138(10):3495–3504. [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Dautry F., Weil D., Yu J., Dautry-Varsat A. Regulation of pim and myb mRNA accumulation by interleukin 2 and interleukin 3 in murine hematopoietic cell lines. J Biol Chem. 1988 Nov 25;263(33):17615–17620. [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., DeCaprio J. A., Freedman A., Kanakura Y., Nakamura M., Ernst T. J., Livingston D. M., Griffin J. D. Expression and state of phosphorylation of the retinoblastoma susceptibility gene product in cycling and noncycling human hematopoietic cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2770–2774. doi: 10.1073/pnas.87.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Accessory cell independent proliferation of human T4 cells stimulated by immobilized monoclonal antibodies to CD3. J Immunol. 1987 Mar 15;138(6):1660–1666. [PubMed] [Google Scholar]
- Gullberg M., Smith K. A. Regulation of T cell autocrine growth. T4+ cells become refractory to interleukin 2. J Exp Med. 1986 Feb 1;163(2):270–284. doi: 10.1084/jem.163.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Huleihel M., Cleveland J. L., Kolch W., Beck T. W., Lloyd P., Pawson T., Rapp U. R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990 Jun;10(6):2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Smith K. A., Fornace A. J., Jr, Comeau C. M., Wiskocil R. L., Crabtree G. R. T-cell growth factor: complete nucleotide sequence and organization of the gene in normal and malignant cells. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1634–1638. doi: 10.1073/pnas.81.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Wood K. W., Mamon H. J., Okuda K., Roberts T. M., Griffin J. D. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce rapid phosphorylation and activation of the proto-oncogene Raf-1 in a human factor-dependent myeloid cell line. Blood. 1991 Jan 15;77(2):243–248. [PubMed] [Google Scholar]
- Kovacina K. S., Yonezawa K., Brautigan D. L., Tonks N. K., Rapp U. R., Roth R. A. Insulin activates the kinase activity of the Raf-1 proto-oncogene by increasing its serine phosphorylation. J Biol Chem. 1990 Jul 25;265(21):12115–12118. [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Levi A., Eldridge J. D., Paterson B. M. Molecular cloning of a gene sequence regulated by nerve growth factor. Science. 1985 Jul 26;229(4711):393–395. doi: 10.1126/science.3839317. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Pfeifer A., Mann D. L., Harris C. C., Berman R., Pert C. B. raf protooncogene expression in neural and immune tissues. Adv Biochem Psychopharmacol. 1988;44:45–55. [PubMed] [Google Scholar]
- Meeker T. C., Nagarajan L., ar-Rushdi A., Rovera G., Huebner K., Croce C. M. Characterization of the human PIM-1 gene: a putative proto-oncogene coding for a tissue specific member of the protein kinase family. Oncogene Res. 1987 Jun;1(1):87–101. [PubMed] [Google Scholar]
- Merida I., Gaulton G. N. Protein tyrosine phosphorylation associated with activation of the interleukin 2 receptor. J Biol Chem. 1990 Apr 5;265(10):5690–5694. [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Interleukin 2-induced lymphocyte proliferation is independent of increases in cytosolic-free calcium concentrations. J Immunol. 1985 Apr;134(4):2431–2435. [PubMed] [Google Scholar]
- Mills G. B., Girard P., Grinstein S., Gelfand E. W. Interleukin-2 induces proliferation of T lymphocyte mutants lacking protein kinase C. Cell. 1988 Oct 7;55(1):91–100. doi: 10.1016/0092-8674(88)90012-8. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Stewart D. J., Mellors A., Gelfand E. W. Interleukin 2 does not induce phosphatidylinositol hydrolysis in activated T cells. J Immunol. 1986 Apr 15;136(8):3019–3024. [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Turley R. B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986 Mar;153(2):267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Prystowsky M. B., Nowell P. C. Regulation of gene expression in lectin-stimulated or lymphokine-stimulated T lymphocytes. Effects of cyclosporine. Transplantation. 1988 Aug;46(2 Suppl):85S–89S. doi: 10.1097/00007890-198808001-00016. [DOI] [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Saltzman E. M., White K., Casnellie J. E. Stimulation of the antigen and interleukin-2 receptors on T lymphocytes activates distinct tyrosine protein kinases. J Biol Chem. 1990 Jun 15;265(17):10138–10142. [PubMed] [Google Scholar]
- Schultz A. M., Copeland T. D., Mark G. E., Rapp U. R., Oroszlan S. Detection of the myristylated gag-raf transforming protein with raf-specific antipeptide sera. Virology. 1985 Oct 15;146(1):78–89. doi: 10.1016/0042-6822(85)90054-6. [DOI] [PubMed] [Google Scholar]
- Siegel J. N., Klausner R. D., Rapp U. R., Samelson L. E. T cell antigen receptor engagement stimulates c-raf phosphorylation and induces c-raf-associated kinase activity via a protein kinase C-dependent pathway. J Biol Chem. 1990 Oct 25;265(30):18472–18480. [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Heidecker G., Rapp U. R., Kung H. F. Induction of transformation and DNA synthesis after microinjection of raf proteins. Mol Cell Biol. 1990 Jul;10(7):3828–3833. doi: 10.1128/mcb.10.7.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton V. P., Jr, Nichols D. W., Laudano A. P., Cooper G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989 Feb;9(2):639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. B., Smith K. A. Interleukin-2 induction of T-cell G1 progression and c-myb expression. Science. 1986 Jul 11;233(4760):203–206. doi: 10.1126/science.3523754. [DOI] [PubMed] [Google Scholar]
- Storm S. M., Cleveland J. L., Rapp U. R. Expression of raf family proto-oncogenes in normal mouse tissues. Oncogene. 1990 Mar;5(3):345–351. [PubMed] [Google Scholar]
- Tahira T., Ochiai M., Hayashi K., Nagao M., Sugimura T. Activation of human c-raf-1 by replacing the N-terminal region with different sequences. Nucleic Acids Res. 1987 Jun 25;15(12):4809–4820. doi: 10.1093/nar/15.12.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Zmuidzinas A., Gould G. W., Yager J. D. Expression of c-raf-1 and A-raf-1 during differentiation of 3T3-L1 preadipocyte fibroblasts into adipocytes. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1180–1187. doi: 10.1016/0006-291x(89)90798-5. [DOI] [PubMed] [Google Scholar]