Abstract
Objective
The concept of an epileptic network has long been suggested from both animal and human studies of epilepsy. Based on the common observation that the MR spectroscopic imaging measure of NAA/Cr is sensitive to neuronal function and injury, we use this parameter to assess for the presence of a metabolic network in mesial temporal lobe epilepsy (MTLE) patients.
Materials and methods
A multivariate factor analysis is performed with controls and MTLE patients, using NAA/Cr measures from 12 loci: the bilateral hippocampi, thalami, basal ganglia, and insula. The factor analysis determines which and to what extent these loci are metabolically covarying.
Results
We extract two independent factors that explain the data’s variability in control and MTLE patients. In controls, these factors characterize a ‘thalamic’ and ‘dominant subcortical’ function. The MTLE patients also exhibit a ‘thalamic’ factor, in addition to a second factor involving the ipsilateral insula and bilateral basal ganglia.
Conclusions
These data suggest that MTLE patients demonstrate a metabolic network that involves the thalami, also seen in controls. The MTLE patients also display a second set of metabolically covarying regions that may be a manifestation of the epileptic network that characterizes limbic seizure propagation.
Keywords: epilepsy, neuroimaging, seizures
Introduction
The presence of a cerebral network in epilepsy has been suggested by several avenues of human and animal investigation. In patient studies, Benedek and Dlugos (1,2) used fluorodeoxyglucose positron emission tomography (FDG-PET) to demonstrate correlations between various subcortical regions. Similarly, magnetic resonance spectroscopic imaging (MRSI) data have found numerous relationships suggesting a subcortical network in MTLE (3–5). The intracranial EEG commonly shows regions of linked electrical activity with regard to both seizure propagation and inter-ictal activity. In animal studies of limbic seizures, the subcortical network has been shown to participate in seizures, as well as potentially in the process of epileptogenesis (6–8).
The presence and role of such epilepsy networks has gained significant interest with the utilization of electrical stimulation devices that may achieve some seizure control through such a network (as being tested with the SANTE trial, 9). As different epilepsy types may appear with distinctive networks (e.g., generalized vs localization-related epilepsy), this has implications for success of stimulators. From a diagnostic perspective, subcortical involvement may be a feature that might help detect the presence of MTLE in the setting of overlapping neocortical epilepsy.
Recently, methods of BOLD and perfusion MRI have been used in the resting state to statistically define the presence of cerebral networks in healthy brain (10–13). Such non-task-activated studies assess the slow (typically 0.1 Hz) moment-to-moment variability of the gradient echo MRI signal, which is principally influenced by physiological fluctuations in perfusion, blood volume, and metabolism [for review see Auer (10)]. These data, evaluated using temporal correlations between many different pixels, have been used to define the ‘default mode network’. For example, Stein (13) identified resting networks linking the thalami and hippocampi. In epilepsy, Bettus (14) reported that seven out of eight left MTLE patients showed decreased or disrupted functional connectivity in the epileptogenic medial temporal lobe in comparison with control. Similarly, Waites and Pereira (15,16) found impaired functional connectivity ipsilateral to the seizure focus in comparison with control. Thus, for MTLE, the majority of the resting connectivity BOLD studies have identified the ‘epileptic network’ as showing decreased correlations in comparison with control.
A complementary approach to evaluating networks may be considered from MR spectroscopic imaging. Our group has studied MTLE patients, identifying correlations between the epileptogenic hippocampus with several subcortical loci (4,17). These data, however, did not attempt to evaluate how a network may be present across multiple loci (or nodes) within a given individual. It is worthwhile to note some distinctions between multivariate analyses in comparison with pairwise analyses. Pairwise correlations necessarily require selection of two specific nodes to compare and thus apply a bias as to which nodes are the most pertinent. Multiple pairwise correlations do not address how the network nodes function together, as correlations between a given pair of nodes do not identify how this pair would relate to another (third) node. Similarly, while the epileptogenic mesial temporal lobe is clearly a very important node in MTLE, it does not a priori have to be the best correlated with other network nodes. Such a process was reported by Dlugos (2), who found that thalamic FDG uptake did not correlate with mesial temporal lobe FDG uptake (and instead correlated with hippocampal neuronal loss).
In this report, we use a multivariate analysis on metabolic imaging data of NAA/Cr to evaluate for the presence of networks. As reported by many groups, this MRSI measure has been identified as an assessment of neuronal mitochondrial injury and found to consistently identify regional abnormalities in epilepsy (4,16–20). From this interpretation, we hypothesize that a multivariate analysis will identify networks of metabolically coherent regional function and dysfunction. We use measurements of NAA/Cr from 12 key loci likely to participate in limbic seizures: bilateral anterior and posterior hippocampus, thalamus, and bilateral basal ganglia and insula. The multivariate analysis used is a factor analysis (for review of statistical methods, see 21, 22). This approach was selected because we wanted to better understand how various brain loci (or ‘nodes’) metabolically covary and to what extent they covary. We use data from n = 20 controls and n = 26 MTLE patients. As a post hoc test of how the MTLE seizure network may help understand neocortical epilepsy, we also evaluated n = 12 neocortical epilepsy patients, examining whether the network could partition this group into those with or without MTLE-associated pathology.
Methods
Clinical
Patients were recruited from the Yale Epilepsy Program and the NYU Comprehensive Epilepsy Center, all patients undergoing surgical evaluation. Clinical descriptions of the MTLE and neocortical patients are shown in Tables 1 and 2A, B. The n = 26 MTLE patients (14F; mean age, 39 ± 12) were included in this study if their clinical history was highly characteristic (stereotyped semiology; febrile seizures as a child, or idiopathic), or either had intracranial evidence for medial temporal onset or had class I or 2 ILAE outcomes after anteromedial temporal lobe resection. For this group, patients who had recurrent post-operative seizures on medications were not included. All patients in the MTLE group had hippocampal atrophy on conventional MR imaging. N = 20 healthy volunteers (10F; mean age, 34 ± 10 years, all right-handed) were studied as controls. For the neocortical group (n = 12), data from patients who had complete available limbic system studies were analyzed. These were patients who generally had temporal, frontal– temporal or parietal–temporal neocortical seizure onset. All these patients underwent intracranial EEG monitoring for seizure localization with determination of seizure onset ascertained by consensus conference.
Table 1.
Epilepsy patient data: MTLE patients
| Age | Gender | Years of epilepsy at evaluation | MRI | Pathology, if available | ILAE outcome at 1 year post-operatively | |
|---|---|---|---|---|---|---|
| 1 | 36 | M | 31 | R HA | HS + heterotopic n | 1 |
| 2 | 52 | F | 44 | R HA | HS | 1 |
| 3 | 51 | M | 49 | R HA | HS | 1 |
| 4 | 40 | F | 11 | R HA | HS | 1 |
| 5 | 39 | F | 9 | L HA | HS + heterotopic n | 1 |
| 6 | 27 | M | 23 | L HA | NA | 1 |
| 7 | 63 | M | 57 | L HA | HS + microcortical dysplasia | 2 |
| 8 | 33 | F | 25 | R HA | NA | 1 |
| 9 | 36 | F | 25 | R HA | NA | 1 |
| 10 | 31 | M | 22 | R HA | HS + rare heterotopic n | 2 |
| 11 | 38 | F | 3 | L HA | NA | ND (verbal function) |
| 12 | 50 | F | 10 | R HA | HS | 1 |
| 13 | 61 | F | 52 | L HA | HS + rare heterotopic n | 1 |
| 14 | 37 | F | 18 | L HA | NA | 1 |
| 15 | 31 | M | 27 | R HA | NA | 1 |
| 16 | 38 | M | 26 | L HA | NA | 1 |
| 17 | 26 | M | 17 | L HA | HS + heterotopic n | 1 |
| 18 | 30 | F | 30.5 | R HA | HS | 1 |
| 19 | 46 | F | 41 | L HA | HS + heterotopic n | 1 |
| 20 | 58 | M | 48 | L HA | HS | 1 |
| 21 | 18 | F | 3 | R HA | NA | 1 |
| 22 | 45 | F | 39 | L HA | HS | 1 |
| 23 | 51 | M | 42 | B HA | HS + heterotopic n | 2 |
| 24 | 20 | M | 12 | R HA | HS | 1 |
| 25 | 27 | M | 20 | B HA | NA | ND- (verbal function) |
| 26 | 42 | F | 20 | R HA | NA | 1 |
HA, hippocampal atrophy; HS, hippocampal sclerosis; MTLE, mesial temporal lobe epilepsy; ND, not done; NA, not available.
Table 2.
(A) Epilepsy patient data: Neocortical patients. (B) 2 × 2 Contingency Table: Prediction Groups for Neocortical Epilepsy Patients
| Age | Gender | Years of epilepsy | MRI | Surgical localization | Pathology, if available | Prediction Group** | Outcome (ILAE) | |
|---|---|---|---|---|---|---|---|---|
| (A) | ||||||||
| 27 | 52 | M | 8 | L HA | L temporal, medial | HS | A | 4 |
| 28 | 44 | M | 19 | Normal | R temporal, lateral | NA | C | NSP |
| 29 | 23 | M | 13 | Normal | R temporal, medial + lateral | Heterotopic neurons, HS | A | 1 |
| 30 | 36 | F | 7 | Normal | R temporal, lateral | Non-specific gliosis | D | 2 |
| 31 | 38 | F | 26 | Normal | R parietal-frontal | Heterotopic neurons | D | 1 |
| 32 | 53 | M | 2 | Normal | L frontal | N/A | D | NSP |
| 33 | 37 | M | 25 | Normal | L frontal | Heterotopic neurons | D | 1 |
| 34 | 22 | M | 6 | R temporal-uncal tumor | R temporal, medial and tumor | Glioma, no hippocampal sclerosis | B | 1 |
| 35 | 28 | M | 11 | L parietal-temporal encephalomalacia; L HA | L posterior temporal (neocortical)-parietal; partial frontal; multiple subpial transections | Reactive astrocytosis | D | 4 |
| 36 | 28 | F | 17 | Normal | L temporal-parietal | N/A | D | NSP |
| 37 | 30 | F | 23 | L HA, h/o childhood fronto-parietal ganglioglioma resection | L temporal, medial+ lateral | Atypical neurons, HS | A | 1 |
| 38 | 33 | M | 20 | R HA, occipital lesional | R temporal, medial and occipital | Gliosis, HS | A | 4 |
| (B) | ||||||||
| Prediction Groups** | MRSI + MTLE | MRSI 3 MTLE | ||||||
| Planned/completed surgery MTLE ± neocortical resection | A = 4 | B = 1 | ||||||
| Planned/completed surgery neocortical resection only | C = 1 | D = 6 | ||||||
Metabolic imaging
1H spectroscopic imaging
All data were acquired using a whole-body 4T Varian Inova imaging spectrometer (Varian-Agilent Palo Alto, CA, USA) with a volume 1H TEM coil. Scout images were acquired with an inversion recovery gradient echo. The spectroscopic data were collected using a modified LASER sequence with 10-mm thickness over a defined region of interest (typically 80 × 100 mm2 for the hippocampi, 100 × 120 mm2 basal ganglia, etc.) with 24 × 24 steps of phase encoding (FOV192 × 192mm, voxel size 0.64cc), TR/TE 2 s/72 ms (4). The triply obliqued hippocampal data were symmetrically angulated along the planum temporale defined from off-sagittal images. The other data were acquired with the spectroscopic plane angulated parallel to the anterior– posterior commissure line at the level of the thalamus. At this position, voxels from the basal ganglia, thalami and insulae are included. The hippocampal and subcortical studies were performed in two different imaging sessions.
Voxel reconstruction and selection
To provide reproducible selection criteria across all studies, the hippocampal and other gray matter voxels were reconstructed using a voxel-shifting method (Fig. 1 shows examples). The reconstructed spectroscopic voxels were defined from the structural images using anatomically defined criteria (4). Because the locations of the spectroscopic imaging voxels were determined by the anatomic criteria and voxel-shifting methods rather than standard grid reconstruction, the voxels acquired for different loci may overlap spatially. The spatial overlap was assessed for the 12 loci considered and ascertained to be <3%, meaning that all measurements are independent and statistically comparable.
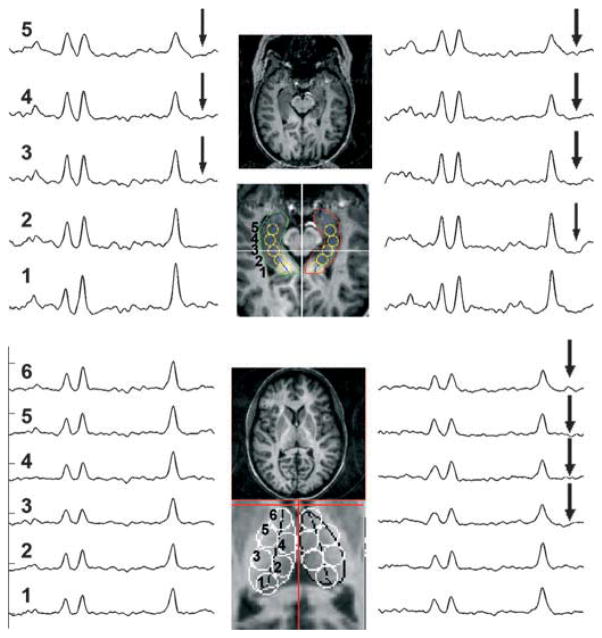
Figure 1.
Example data from patient showing scouts and spectra from the medial temporal lobes and thalamus. For the temporal lobe data, two spectra are summed for the anterior (spectra no. 4,5), posterior (spectra no. 2,3) temporal lobe loci used in the Factor Analysis. For the thalamic data, three spectra are summed for the analysis, anterior (spectra no. 4,5,6) and posterior (spectra no. 1,2,3). The arrows indicate spectra where the NAA/Cr values are abnormally low.
Analysis
All NAA/Cr data were initially evaluated by unpaired t-test comparing MTLE with control, taking P < 0.05 for significant differences. This was separately performed with data from each locus (Table 3).
Table 3.
Regional differences between control and mesial temporal lobe epilepsy (MTLE) of NAA/Cr
| Basal ganglia
|
Thalamus
|
Hippocampus
|
Insula
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ipsi | Contra | Ipsi
|
Contra
|
Ipsi
|
Contra
|
Ipsi** | Contra* | |||||
| Post** | Ant* | Post | Ant | Post* | Ant** | Post | Ant | |||||
| MTLE | 1.41 ± 0.16 | 1.39 ± 0.13 | 1.35 ± 0.16 | 1.49 ± 0.15 | 1.45 ± 0.16 | 1.54 ± 0.14 | 1.15 ± 0.16 | 1.05 ± 0.18 | 1.23 ± 0.19 | 1.20 ± 0.26 | 1.25 ± 0.13 | 1.28 ± 0.15 |
| Control | 1.37 ± 0.09 | 1.38 ± 0.14 | 1.53 ± 0.20 | 1.63 ± 0.24 | 1.44 ± 0.15 | 1.55 ± 0.19 | 1.26 ± 0.14 | 1.22 ± 0.14 | 1.25 ± 0.16 | 1.26 ± 0.17 | 1.37 ± 0.11 | 1.37 ± 0.13 |
Values in boldface are significantly different between MTLE and control,
P < 0.005;
P < 0.05. For the control group, ipsi refers to left.
A factor analysis was used to examine how the NAA/Cr values at the 12 loci covary. In both healthy and epilepsy brain, we may anticipate that the metabolic function in one brain region will correlate with the metabolic function in (multiple) other regions, particularly if these regions contribute to a shared neurophysiologic function. For example, if we observed a dominant sided brain network of the temporal lobe and multiple thalamic regions, we could interpret this as characterizing a ‘subcortical language function’. As a result, because these regions are functioning together, these regional NAA/Cr values for any given single volunteer would share common demands for energy and demonstrate coherent changes (increases or decreases) from the group’s mean values of NAA/Cr. More quantitatively, this analysis seeks to define which loci (and how much for each locus) are metabolically covarying by extracting from the patient and locus data set a series of factors F that account for the common metabolic variance. The first factor F1 describes that linear combination of 12 loci that describes the largest metabolic covariance among the loci. After removal of the variance due to factor F1, the second factor F2 describes that linear combination of loci describes the remaining largest metabolic covariance among the loci. Because the metabolic variation for any given locus (i.e., above and below the mean for that locus) may actually be the consequence of multiple physiological processes (after all, these limbic regions are not thought to be highly specific to a single cognitive or neurological function), it is possible that each locus may contribute to more than one factor Fj. Two factors were determined in this analysis as determined from the chi-square statistic, with the resulting factor interpretable in terms of their ‘networked’ neurophysiological function. In the control group, such factors will represent ‘normal’ neurophysiologic function, while in MTLE, factors will likely characterize both ‘normal’ and pathophysiologic function.
The most important result of the factor analysis is the profile, or ‘loadings’ of each locus toward any factor Fj. In our data, for each factor Fj, there are 12 loading values, denoted mi,j, where i ranges from 1 to 12. These loadings mi,j are the Pearson correlation values that locus i has with factor Fj. To assess the value of the loadings, we use the criteria of Hair (23), where the magnitude of loadings >0.6 are regarded as large, <0.4 as small. It should be recognized however that these criteria are not rigid, and that lesser loadings can be informative (21, 22).
The factor analysis was performed with the control and MTLE groups separately. This is important to consider, because as a result for the two groups, the calculated factors F are based on the variability within a given group. While this is necessary to clearly define the limbic network seen in MTLE in parallel to that seen in controls, it should be noted that a quantitative comparison between these two groups’ factors is not feasible. For example, the metabolic function of the ipsilateral anterior MTL in the MTLE group is significantly lower than that in control. However, because the factor analysis evaluates the variation within each group, this group offset seen in the seizure onset zone is not a part of the resulting factors. Our goal in this analysis remains to define the metabolic network within MTLE. For simple consideration of the ipsilateral MTL itself, the straightforward t-tests between MTLE and control clearly detect this significant difference (Table 3).
Results
In these regions, several loci are significantly different by two-tailed t-tests between the MTLE and control group (Table 3). These loci are the anterior, posterior loci of the ipsilateral hippocampus, and the anterior, posterior loci of the ipsilateral thalamus, and finally, the bilateral insula (example, Fig. 1).
Fig. 2 shows bar plots of the loadings for the factor analysis from the control and MTLE groups. It is re-emphasized that the loadings shown in Fig. 2 indicate the correlation values (Pearson R value) of that locus with its corresponding factor. For example, in controls, the loading (0.78) of the dominant (left) posterior thalamus with factor no. 1 is the correlation coefficient (or R value) of factor no. 1 with the dominant (left) posterior thalamus. In controls, it is clear that all of the thalamic loci are highly covarying and factor no. 1 extracts this covariance. The non-dominant (right) posterior medial temporal lobe also covaries well with factor no. 1 in controls. Together, factor no. 1 can be interpreted as a ‘thalamic’ factor. Factor no. 2 in the controls demonstrates covariance of the dominant (left) posterior hippocampus with the dominant (left) posterior thalamus with lesser involvement from the dominant (left) insula, which may be described as a ‘dominant subcortical’ factor.
Figure 2.
Coefficient loadings for factors no. 1 -and no. 2 are shown from the control (top, open bars ) and mesial temporal lobe epilepsy (MTLE) (bottom, shaded bars) groups. The loci are indicated along the x-axis. The heavy black lines segregate the dominant or ipsi (left panel) from non-dominant or contra (right panel) loci. Twelve loci: basal ganglia, thalamus (posterior, anterior), hippocampus (posterior, anterior) and insula are shown for each hemisphere. Asterisks indicate the significant loadings using a threshold value of ±0.6. In the MTLE group, factor 1 had a loading factor of 0.55 from the epileptogenic, i.e., ipsilateral, anterior hippocampus (asterisk in parentheses).
In the MTLE group, similar to controls, the thalamic loci are strongly covarying in factor no. 1. There is some covariance of this ‘thalamic’ factor no. 1 with the ipsilateral anterior medial temporal lobe, at 0.55. In contrast, factor no. 2 displays large loadings for the ipsilateral insula and bilateral basal ganglia. As the ipsilateral insula and basal ganglia may be considered to be loci participating in the seizure spread, we consider factor no. 2 in the MTLE group as a ‘seizure network’ factor.
To demonstrate some of the less anticipated relationships seen from the factor analysis, we assessed the data by simple Pearson correlations. In particular, if we interpret Factor no. 2 from the MTLE group as a ‘seizure network’ factor, we may predict that the ipsilateral insula and basal ganglia would be potentially directly covarying as both may be defined as possible sites of seizure spread from the ipsilateral hippocampus. As shown in Fig. 3, these two loci correlated with R = +0.71 (P < 0.00004) in the MTLE group, this correlation being non-significant in the control group. While it may not always be evident to a priori consider which and how such regions are linked, the factor analysis helps understand the observed relationships between such regions.
Figure 3.
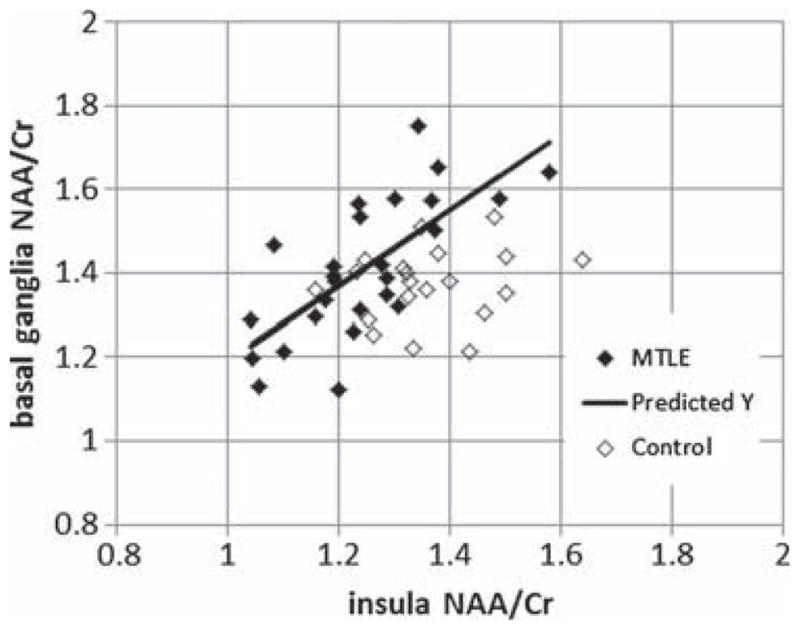
Pearson correlation between the ipsilateral insula and ipsilateral basal ganglia. Filled symbols, mesial temporal lobe epilepsy(MTLE); open symbols control. The MTLE group regression is significant with R = 0.71, P < 0.001. The control group regression is not significant.
Linear discriminant analysis for neocortical epilepsy
As the question of MTL involvement frequently arises in the evaluation of neocortical epilepsy of temporal or frontal lobe origin, we considered whether the presence (or absence) of a metabolic limbic MTL network may be informative for an additional n = 12 neocortical (‘unknown’, Table 2A) group, all of whom underwent intracranial EEG monitoring for the identification of seizure onset. We performed a post hoc linear discriminant analysis with this n = 12 neocortical patients using the MTLE and control data as two well-classified groups. This analysis uses a Gaussian model to classify new patient values as either exhibiting the metabolic features consistent with the limbic MTLE network (‘MTLE-like’) or not (‘non-MTLE’). This classification was evaluated in a 2 × 2 contingency table with regard to planned surgical outcome, whether the planned surgery would or would not include a medial temporal lobectomy.
The classifications are shown in Table 2A (‘Prediction’ column) and in a 2 × 2 contingency Table 2B. Altogether, 10 of 12 cases classified correctly, with n = 4 (‘group A’) predicted by the MRSI network to exhibit the MTLE pattern, and n = 6 (‘group D’) predicted to not exhibit the MTLE pattern. Many patients with frontal, temporal neocortical or parietal onset can have semiologies that are unclear, and group D (no. 30–33,35,36) was successfully shown not to have limbic metabolic involvement. One of the five cases (patient no. 34, ‘group B’) who ultimately underwent medial temporal lobectomy was predicted by the MRSI network not to exhibit the MTLE pattern; notably, in this patient, the MTL surgery was being performed for tumor resection rather than epilepsy and showed negative pathology for hippocampal sclerosis. Notably, patients no. 29 and no. 37 both with lateral and medial temporal lobe involvement were successfully classified as ‘group A’, exhibiting limbic MRSI metabolic involvement. These two patients, being ‘dual pathology’ cases, show the utility of understanding the limbic MTLE network, especially as patient no. 29 was MRI negative. There was one discordant patient (no. 28, ‘group C’) who was predicted by the MRSI network to exhibit MTLE involvement; however, surgery was ultimately not performed in this patient because of cognitive function.
Discussion
Factor and linear discriminant analysis
The present data agree with the literature in the significant depression of NAA/Cr in the ipsilateral hippocampus, thalamus and insula (4,17–20). The factor analysis demonstrated that in both control and MTLE brain, the thalamic loci are metabolically highly covarying. Factor no. 1 in control brain also displays dependence from the non-dominant (right) posterior hippocampus, while in MTLE, factor no. 1 shows covariance with the epileptogenic anterior hippocampus. This suggests that the MTLE brain shows a similar thalamic network as in control; however, it is influenced by the ipsilateral hippocampus.
Factor no. 2 of control brain shows covariance between the dominant (left) posterior hippocampus and dominant (left) posterior thalamus. This is not seen in MTLE, where factor no. 2 is heavily loaded by the ipsilateral insula and bilateral basal ganglia. It is possible that these two latter regions are relatively proximal seizure propagation regions from the ipsilateral hippocampus and thus show correlated levels of metabolic injury (also see Fig. 3). However, there is no substantial covariance between the ipsilateral hippocampus with factor no. 2. This may be similar to that reported by our previous 31P data and Dlugos (2), where the extent of injury measured in the region of seizure onset was nonlinearly related to surrounding tissue.
Based solely on the pattern of dysfunction seen in the 12 limbic loci, a linear discriminant analysis was able to segregate 10/12 neocortical epilepsy patients with or without MTL (dual pathology) involvement. In this analysis, from the two ‘mis-classified’ patients, one (no. 34) had MTL surgery for tumor rather than epilepsy, and the other patient (no. 28) did not undergo surgery because of cognitive function. As the sample numbers are small, this discriminant analysis is clearly not meant to be definitive. However, evaluation of a Fisher’s exact test shows that the detection of the MTLE-like network is significantly (P < 0.05) linked with the presence of dual pathology as defined by surgical intervention, with 4/5 patients accurately detected.
Meaning of a metabolic network in control and epilepsy
Given that the NAA measures have been found to be informative for both healthy and diseased brain (24–27), it is not surprising that the present data argue for regionally covarying areas of mitochondrial function in healthy and MTLE brain. These networks may be considered in the context of recent resting state connectivity studies. While the present data – based on metabolic function – do not require direct neurotransmission linkage, resting state BOLD connectivity data have shown that in healthy brain, the thalami are well interlinked with extension into the hippocampi (13). It is likely that in healthy brain, the observed factors characterize a common ‘idling network’ of coherent metabolic activity.
In comparison, many of the connectivity studies in MTLE have reported decreased resting connectivity (14–16, 28). As metabolic need intrinsically contributes to the neurovascular coupling that underlies the connectivity, it is not surprising that the MTLE patient displays decreased metabolic function. The metabolic regional covariances of factor no. 1 in MTLE likely reflects both neuronal dysfunction and injury in addition to normal metabolic coupling of the ‘idling thalamic network’ and thus explains why factor no. 1 in MTLE is similar to control factor no. 1. The correlation of MTLE factor no. 1 with the ipsilateral anterior hippocampus (loading, 0.55) is possibly related to seizure linked injury and metabolism, given the significant decreases in the thalamic loci and hippocampus (Table 3). However, the ipsilateral anterior hippocampus did not correlate with MTLE factor no. 2. Given what we know about the ipsilateral insula and basal ganglia, factor no. 2 in MTLE most likely characterizes injury linked with seizure spread and suggests down-stream coherence of metabolic injury.
With the participation of the MTLE network in seizure propagation and/or genesis, it is of interest to consider how this network evolves post-operatively. Clearly, anterior medial temporal lobe surgery is highly successful for the reduction of seizure frequency and severity (29–31). However, we do not know whether the network participates in the unfortunately relatively common longer-term recurrence of seizures in MTLE, with ~25% of MTLE patients eventually recurring within 2 years after resection (29). We anticipate that understanding the epileptic network will be constructive for multiple reasons: for possibly helping to detect dual pathology, for better utilization and implementation of brain stimulator devices, and finally to better understand its potential participation in long-term seizure recurrence.
Acknowledgments
We are grateful for the participation of our colleagues, including Drs RH Mattson, H Blumenfeld, E Fertig, H. Zaveri, O. Devinsky and others who have studied epilepsy and contributed patients to our study. We gratefully acknowledge support from NIH R01-EB011639, R21-AT002984, R01-NS054038 and the Swebilius Foundation.
Footnotes
Conflict of interest and sources of funding
There are no conflict of interests in the preparation of this work.
References
- 1.Benedek K, Juhász C, Muzik O, Chugani DC, Chugani HT. Metabolic changes of subcortical structures in intractable focal epilepsy. Epilepsia. 2004;45:1100–5. doi: 10.1111/j.0013-9580.2004.43303.x. [DOI] [PubMed] [Google Scholar]
- 2.Dlugos DJ, Jaggi J, O’Connor WM, et al. Hippocampal cell density and subcortical metabolism in temporal lobe epilepsy. Epilepsia. 1999;40:408–13. doi: 10.1111/j.1528-1157.1999.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 3.Pan JW, Kim JH, Cohen-Gadol A, Pan C, Spencer DD, Hetherington HP. Regional energetic dysfunction in hippocampal epilepsy. Acta Neurol Scand. 2005;111:218–24. doi: 10.1111/j.1600-0404.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 4.Hetherington HP, Kuzniecky RI, Vives K, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology. 2007;69:2256–65. doi: 10.1212/01.wnl.0000286945.21270.6d. [DOI] [PubMed] [Google Scholar]
- 5.Fojtiková D, Brázdil M, Skoch A, et al. MRS of the thalamus in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Epileptic Disord. 2007;9(Suppl 1):S59–67. doi: 10.1684/epd.2007.0148. [DOI] [PubMed] [Google Scholar]
- 6.Bertram EH, Zhang D, Williamson JM. Multiple roles of midline dorsal thalamic nuclei in induction and spread of limbic seizures. Epilepsia. 2008;49:256–68. doi: 10.1111/j.1528-1167.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- 7.Bouilleret V, Boyet S, Marescaux C, Nehlig A. Mapping of the progressive metabolic changes occurring during the development of hippocampal sclerosis in a model of mesial temporal lobe epilepsy. Brain Res. 2000;852:255–62. doi: 10.1016/s0006-8993(99)02092-2. [DOI] [PubMed] [Google Scholar]
- 8.Maggio R, Lanaud P, Grayson DR, Gale K. Expression of c-fos mRNA following seizures evoked from an epileptogenic site in the deep prepiriform cortex: regional distribution in brain as shown by in situ hybridization. Exp Neurol. 1993;119:11–9. doi: 10.1006/exnr.1993.1002. [DOI] [PubMed] [Google Scholar]
- 9.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. Epub 2010 Mar 17. [DOI] [PubMed] [Google Scholar]
- 10.Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26:1055–64. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 11.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–67. doi: 10.1016/j.neuroimage.2005.08.035. Epub 2005 Nov 2. [DOI] [PubMed] [Google Scholar]
- 12.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 13.Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MRI. Am J Neuroradiol. 2000;21:1397–401. [PMC free article] [PubMed] [Google Scholar]
- 14.Bettus G, Guedj E, Joyeux F, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–91. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- 16.Pereira FR, Alessio A, Sercheli MS, et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetherington HP, Pan JW, Chu WJ, Mason GF, Newcomer BR. Biological and clinical MRS at ultra-high field. NMR Biomed. 1997;10:360–71. doi: 10.1002/(sici)1099-1492(199712)10:8<360::aid-nbm477>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Bernasconi A, Tasch E, Cendes F, Li LM, Arnold DL. Proton magnetic resonance spectroscopic imaging suggests progressive neuronal damage in human temporal lobe epilepsy. Prog Brain Res. 2002;135:297–304. doi: 10.1016/S0079-6123(02)35027-1. [DOI] [PubMed] [Google Scholar]
- 19.Vermathen P, Ende G, Laxer KD, Knowlton RC, Matson GB, Weiner MW. Hippocampal N-acetylaspartate in neocortical epilepsy and mesial temporal lobe epilepsy. Ann Neurol. 1997;42:194–9. doi: 10.1002/ana.410420210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller SG, Laxer KD, Barakos J, et al. Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia. 2010;51:1436–45. doi: 10.1111/j.1528-1167.2009.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JO, Mueller CW. Introduction to factor analysis. Newbury Park: Sage Publications Inc; 1978. [Google Scholar]
- 22.Harmon H. Modern factor analysis. Chicago: University of Chicago Press; 1976. pp. 278–99. [Google Scholar]
- 23.Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate data analysis. 5. Upper Saddle River, NJ: Prentice Hall; 1998. [Google Scholar]
- 24.Jung RE, Yeo RA, Chiulli SJ, et al. Biochemical markers of cognition: a proton MR spectroscopy study of normal human brain. NeuroReport. 1999;10:3327–31. doi: 10.1097/00001756-199911080-00014. [DOI] [PubMed] [Google Scholar]
- 25.Kuzniecky R, Hugg J, Hetherington H, et al. Predictive value of 1H MRSI for outcome in temporal lobectomy. Neurology. 1999;53:694–8. doi: 10.1212/wnl.53.4.694. [DOI] [PubMed] [Google Scholar]
- 26.Mory SB, Li LM, Guerreiro CA, Cendes F. Thalamic dysfunction in juvenile myoclonic epilepsy: a proton MRS study. Epilepsia. 2003;44:1402–5. doi: 10.1046/j.1528-1157.2003.67702.x. [DOI] [PubMed] [Google Scholar]
- 27.Pan JW, Takahashi K. Interdependence of N-acetyl aspartate and high-energy phosphates in healthy human brain. Ann Neurol. 2005;57:92–7. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- 28.Bettus G, Ranjeva JP, Wendling F, et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS ONE. 2011;6:e20071. doi: 10.1371/journal.pone.0020071. Epub 2011 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65:912–8. doi: 10.1212/01.wnl.0000176055.45774.71. [DOI] [PubMed] [Google Scholar]
- 30.Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2008;49:1308–16. doi: 10.1111/j.1528-1167.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz TH, Jeha L, Tanner A, Bingaman W, Sperling MR. Late seizures in patients initially seizure free after epilepsy surgery. Epilepsia. 2006;47:567–73. doi: 10.1111/j.1528-1167.2006.00469.x. [DOI] [PubMed] [Google Scholar]