Abstract
Although current breast cancer treatment guidelines limit the use of HER2 blocking agents to tumors with HER2 gene amplification, recent retrospective analyses suggest that a wider group of patients may benefit from this therapy. Utilizing breast cancer cell lines, mouse xenograft models and matched human primary and metastatic tissues, we demonstrate that HER2 is selectively expressed in and regulates self-renewal of the cancer stem cell population in ER+, HER2− luminal breast cancers. Although trastuzumab had no effects on the growth of established luminal breast cancer mouse xenografts, administration after tumor inoculation blocked subsequent tumor growth. HER2 expression is increased in luminal tumors grown in mouse bone xenografts, as well as in bone metastases from breast cancer patients compared to matched primary tumors. Furthermore this increase in HER2 protein expression was not due to gene amplification but rather was mediated by RANK-ligand in the bone microenvironment. These studies suggest that the clinical efficacy of adjuvant trastuzumab may relate to the ability of this agent to target the cancer stem cell population in a process that does not require HER2 gene amplification. Furthermore these studies support a cancer stem cell model in which maximal clinical benefit is achieved when cancer stem cell targeting agents are administered in the adjuvant setting.
Introduction
Approximately 20% of breast cancers display amplification of the HER2 gene, a genotype associated with an aggressive course and poor outcome (1). The development of HER2 targeting agents such as trastuzumab and lapatinib represents one of the greatest achievements in clinical oncology demonstrating the effectiveness of molecularly targeted therapeutics (2). In women with advanced metastatic breast cancer, addition of trastuzumab to cytotoxic chemotherapy increases the response rate, time to tumor progression and survival (2–4). In this setting, the beneficial effect of trastuzumab appears to be limited to breast tumors with HER2 amplification, a finding predicted by pre-clinical data (1, 5–7).
Based on the demonstrated clinical efficacy of HER2 blockade in women with advanced HER2 amplified tumors, inclusion of patients into adjuvant trials has been largely limited to this patient population. These adjuvant trials demonstrated a remarkable 50% reduction in recurrence rate with the addition of trastuzumab to chemotherapy compared to chemotherapy alone (8–12). These results have led to establishment of guidelines for HER2 testing (6, 13).
The conventional wisdom that only patients with HER2-amplified breast tumors would benefit from trastuzumab was challenged by a provocative paper published in the New England Journal of Medicine in 2008, in which, Paik, et al., reanalyzed HER2 expression in tumors from patients on NSABP-B31, one of the pivotal trials that demonstrated the efficacy of adjuvant trastuzumab (13). They reported that 174 cases originally classified as HER2+ actually lacked HER2 gene amplification when reanalyzed in a central laboratory. Surprisingly, these “HER2-negative” patients benefitted as much from adjuvant trastuzumab as did women whose tumors displayed classical HER2 amplification. Although questions have been raised regarding the reliability of HER2 analyses in this study (14), similar results were recently reported by the North Central Group (15), which makes it less likely that these results were due to chance or laboratory error.
The molecular mechanisms that may account for a clinical benefit of HER2 blockade in the adjuvant setting in patients whose tumors do not display classical HER2 amplification are not known. However, we have recently proposed that the clinical efficacy of HER2 blockade in tumors classified as HER2-negative might be explained by the “cancer stem cell hypothesis”. According to this model, many human cancers, including breast cancer are driven by a subpopulation of cells that display stem cell properties (16). We have previously shown that HER2 is an important driver of the cancer stem cell (CSC) population in tumors with HER2 amplification (17, 18). Utilizing breast cancer cell lines, xenograft models, as well as primary and metastatic human breast cancer samples, we now show that HER2 is selectively expressed in the CSC population of luminal ER+ breast cancers in the absence of HER2 gene amplification, and provide evidence that the efficacy of HER2 blocking agents in the adjuvant setting may reflect effects on these cells.
Material and methods
Cell culture and treatment and flow cytometry
MCF7, ZR75-1, BT474, SKBR3 and MDA-MB231 cell lines were purchased from ATCC and maintained in culture conditions according to supplier’s recommendation. The SUM159 cell line was cultured as previously described (19).
Trastuzumab was obtained from the Cancer Center Pharmacy at the University of Michigan.
The Aldefluor assay was carried out as previously described (20) according to manufacturer’s guidelines (StemCell Technologies, Durham, NC).
Flow cytometry analyses and Immunohistochemical (IHC) staining were described in detail in supplementary section.
Tumorsphere assay was performed as previously described (17).
Lentivirus infections have been described in supplementary data.
Mice and xenograft models, treatment and bioluminescence
Details of mouse xenografts and treatment of animals has been given in the supplementary section.
Patient selection
After IRB approval (IRB# HUM00041153), a free-text search of the University of Michigan Department of Pathology database was performed using SNOMED. 19 patients (between 1986 and 2008) were selected based on the following criteria: matched primary breast cancer and bone metastatic tumor with slides and blocks available for study.
HER2 automated quantitative analysis (AQUA©) of matched primary cancer and bone metastasis
The AQUA© system (HistoRx, New Haven, Connecticut) was used for the automated image acquisition and analysis as described in the supplementary data.
All statistical analysis has been performed as explained in the supplemental data.
RESULTS
Expression of HER2 correlates with the CSC marker aldehyde dehydrogenase (ALDH) in luminal breast cancer cell lines
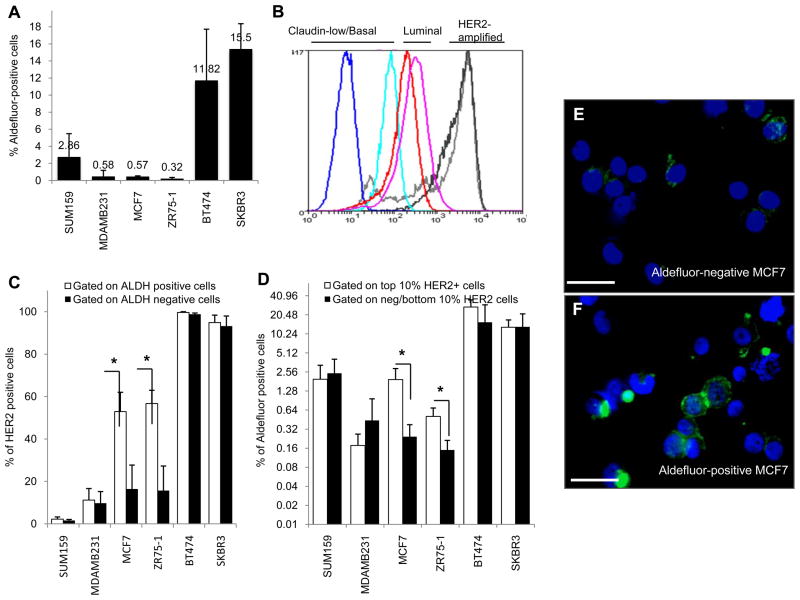
We have previously demonstrated that normal and malignant breast CSCs are characterized by aldehyde dehydrogenase (ALDH) expression (20). Furthermore, established breast cancer cell lines demonstrate a hierarchical organization in which a subpopulation of ALDH expressing cells are enriched for tumor initiating characteristics (21). As assessed by Aldefluor assay, MCF7 and ZR75-1 luminal breast cancer cell lines express the lowest level of ALDH (Figure 1A), while BT474 and SKBR3, HER2-amplified cell lines express the highest levels of ALDH. SUM159, and MDA-MB231, basal/claudin-low cell lines, express an intermediate level of ALDH consistent with previous reports (17, 21). The HER2-amplified BT474 and SKBR3 cell lines expressed the highest levels of HER2, the basal/claudin-low SUM159 and MDA-MB231 cell lines expressed the lowest levels of HER2 and, the luminal estrogen receptor-positive cell lines MCF7 and ZR75-1 expressed an intermediate levels of HER2 (Figure 1B). We utilized fluorescence activated cell sorting (FACS) to determine the relationship between HER2 and ALDH expression at the individual cell level. In MCF7 and ZR75-1 luminal cell lines, the level of HER2 expression was considerably lower than in the HER2 amplified cell lines. However in these cells, HER2 expression was increased 2–3 fold in Aldefluor-positive as compared to Aldefluor-negative cells (Figure 1C)(p=0.012 and 0.047 for MCF7 and ZR75-1, respectively). This association was seen when cells were first gated on Aldefluor-positive and Aldefluor-negative cell populations or, conversely when high and low HER2 expressing cells were separated and assessed for ALDH expression (Figure 1C–D, Supplementary 1A–B) (p=0.038 and 0.033 for MCF7 and ZR75-1, respectively). In contrast, there was no association between HER2 and ALDH expression in SUM159 and MDA-MB231 basal/claudin-low cell lines (Figure 1C,D). Immunofluorescence using anti-HER2 antibody in Aldefluor-positive and Aldefluor-negative cells confirmed the concordance between HER2 and ALDH expression in luminal MCF7 cells (Figure 1E–F).
Figure 1.
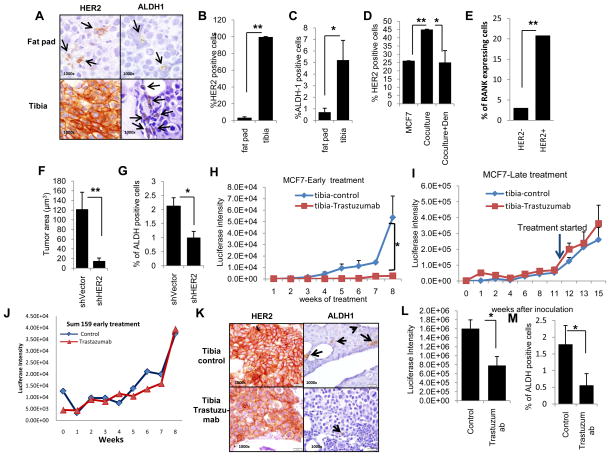
High HER2 protein expression correlates with increased levels of ALDH activity in luminal breast cancer cell lines. A, The percentages of ALDH positivity as assessed by the Aldefluor assay in basal/claudin-low (SUM159, MDA-MB231), luminal (MCF7, ZR75-1) and HER2-amplified (BT474, SKBR3) cells. B, HER2 expression by FACS of the same cell lines demonstrated low HER2 expression (basal/claudin-low (dark and light blue)), moderate HER2 protein (luminal (red and pink)) and high levels of HER2 expression (amplified (light and dark grey)). C, FACS analysis of Aldefluor positive and negative cells demonstrated enrichment for HER2 expression in Aldefluor positive cells. D, FACS analysis of the top 10% of HER2-positive and bottom 10% HER2-negative cells demonstrated enrichment for Aldefluor positivity within HER2-positive cells. Increased HER2 expression correlated with increased Aldefluor activity where * is denoted, p<0.05. E–F, Immunofluorescence demonstrating increased HER2 expression in Aldefluor-positive compared to Aldefluor-negative MCF7 cells. Scale bar 50μm.
Trastuzumab reduces the CSC population of luminal breast cancer cells
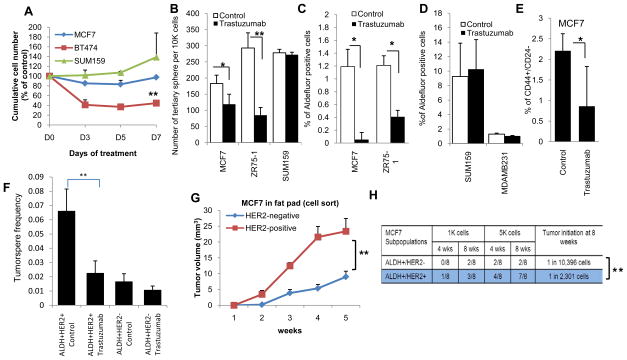
To determine the functional role of HER2, we assessed the effects of the HER2-blocking antibody trastuzumab on cell growth in vitro. As previously reported, under standard tissue culture conditions (22), the effects of trastuzumab on inhibiting cell growth were limited to cells that displayed HER2-amplification (Figure 2A). While cell growth in tissue culture under attached conditions largely reflects proliferation of bulk cell populations, formation and serial passage of tumorspheres is a validated in vitro surrogate assay for CSCs (23). In contrast to the absence of effects in standard culture conditions, trastuzumab significantly reduced tertiary tumorsphere formation of luminal MCF7 and ZR75-1 mammary carcinoma cells (p<0.05, p=0.003 respectively) but had no effect on tumorsphere formation in basal/claudin-low SUM159 cells (Figure 2B, Supplementary 2A,B). The effects of trastuzumab on the CSC population were further assessed utilizing the Aldefluor assay. Trastuzumab significantly reduced the percent of Aldefluor-positive cells in luminal MCF7 and ZR75-1 cells (Figure 2C, Supplementary 3A) but had no effect on the Aldefluor-positive populations in basal/claudin-low SUM159 or MDA-MB231 cells (Figure 2D). Breast CSCs also have been characterized as having the phenotype CD44+/CD24- as assessed by flow cytometry (24). Trastuzumab significantly reduced the CD44+/CD24− population (p=0.025) in luminal MCF7 cells (Figure 2E, Supplementary 3B). ZR75-1 cells lack a CD44+/CD24− population while SUM159 cells are all CD44+/CD24− precluding the use of those CSC markers in these cell lines. To further investigate the role of HER2 in driving the CSC population, we utilized flow cytometry to fractionate HER2+ and HER2− cells within the ALDH-positive population. ALDH+HER2+ cells showed significantly greater tumorsphere forming capacity than ALDH+HER2− cells, an effect that was inhibited by trastuzumab (Figure 2F). We also knocked down HER2 expression in MCF7 cells (Supplementary 4A) and demonstrated that this abrogated tumorsphere formation, furthermore trastuzumab had no effect in HER2 knockdown cells (Supplementary 4B).
Figure 2.
Trastuzumab targets CSCs in luminal breast cancer cells. A, MTT assay in 2-D culture demonstrated inhibition of cell growth by trastuzumab in BT474, HER2 amplified but not in MCF7 or SUM159 cells that do not display HER2 amplification. B, Tertiary tumorsphere formation of MCF7, ZR75-1 and SUM159 cells was analyzed after treatment of primary spheres with trastuzumab (at 21μg/ml) and two serial passages were performed in the absence of trastuzumab. Trastuzumab treatment significantly reduced the number of tertiary tumorspheres of luminal MCF7 and ZR75-1 cells but had no effect on basal/claudin-low Sum159 cells. C, Trastuzumab reduced the percent of Aldefluor-positive cells in luminal cell lines, D, but had no effect on basal/claudin-low cell lines. E, Trastuzumab significantly reduced the proportion of CD44+/CD24− in MCF7 cells. F, Trastuzumab significantly reduced the sphere formation in ALDH+HER2+ but had no significant effect onALDH+HER2− cells. G, MCF7 HER2-positive and HER2-negative cell populations were FACS sorted and 10,000 cells were then injected into mammary fat pads of NOD-SCID mice. HER2-positive cells produced larger tumors compared to HER2 negative cells as assessed by luciferase imaging. H, HER2 expression in ALDH-positive MCF7 cells demonstrated a significantly higher frequency of tumor initiating cells as compared to the ALDH-positive MCF7 cells without HER2 expression. (*p<0.05, **p<0.01).
HER2 drives the cancer initiating population in luminal breast cancer xenografts
To determine the relationship between HER2 expression and tumor initiating capacity, MCF7 cells were sorted for HER2 expression and injected into the mammary fat pads of NOD/SCID mice. The median time to develop palpable tumors following injection of 1×104 cells was significantly less in HER2 expressing than in HER2 non-expressing cells (Figure 2G, p=0.05). Furthermore, tumor growth rate and size was significantly greater in HER2-positive as compared to HER2-negative cells (Figure 2G). We have previously reported that aldehyde dehydrogenase expressing cells are enriched for tumor initiating properties (21). In order to determine whether expression of HER2 in these Aldefluor-positive cells further enhances tumor initiation, we sorted Aldefluor+HER2+ and Aldefluor+HER2- cell populations by FACS and implanted serial dilutions of these cells into the mammary fat pads of NOD/SCID mice (24). Interestingly, within the Aldefluor-positive population the frequency of tumor initiation was more than four-fold higher in HER2 expressing compared to HER2 low cells (Figure 2H, p=0.009).
Effect of trastuzumab on the growth of luminal breast cancer xenografts is dependent upon the timing of administration
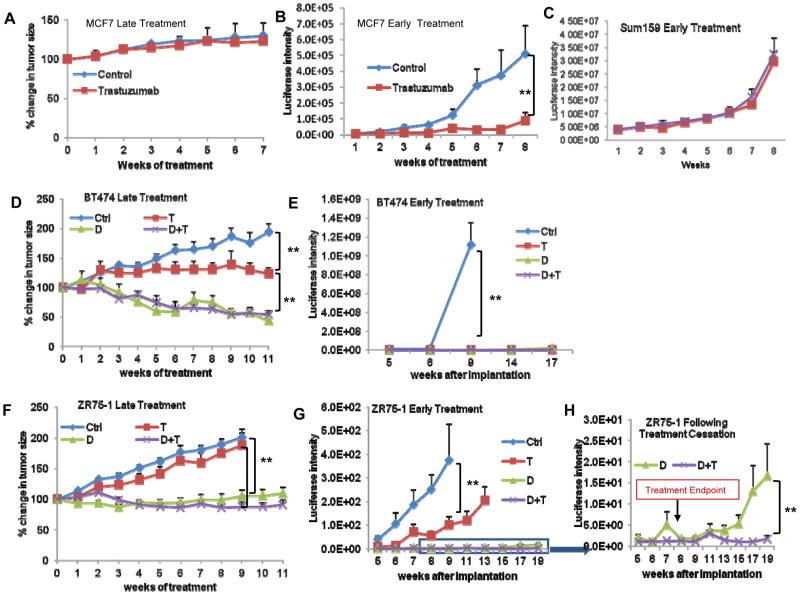
We utilized the HER2 blocking antibody trastuzumab to determine the functional role of HER2 in tumor growth in mouse xenografts. Cancer stem cell models predict that in advanced cancers, stem cell targeting agents would have little effect on tumor shrinkage since these cells constitute only a small fraction of the total cell population. In contrast, growth of tumors from microscopic disease at primary or metastatic sites depends on cancer stem cells which have unique self-renewal capacity compared to bulk tumor populations (16). We therefore compared the effects of trastuzumab administered immediately after tumor inoculation (early treatment) to administration after the establishment of measurable tumors (late treatment). Although trastuzumab had little effect on the growth of established luminal MCF7 and ZR75-1 xenografts (Figure 3A,F), it significantly blocked tumor growth when treatments were initiated immediately after tumor inoculation (Figure 3B,G). In contrast, early trastuzumab treatment had no effect on basal/claudin-low Sum159 tumor growth demonstrating the specificity of trastuzumab for luminal breast CSCs (Figure 3C).
Figure 3.
Effects of trastuzumab on mouse tumor xenograft depend on the timing of administration. Luciferase expressing MCF7, BT474 and ZR75-1 cells were grown in mouse fat pads, and tumor size was measured with calipers or determined by luciferase intensity. In the late treatments, control (Ctrl), trastuzumab (T) and/or docetaxel (D) were administered over 6 weeks starting at 4 weeks post inoculation. In early treatments, the treatment started on the day of inoculation. A–B, MCF7 tumor xenografts were inhibited when trastuzumab treatment was administered early but not late. C, The Sum159 cells (basal/claudin-low breast cancer cells) showed no difference between control animals and trastuzumab treated even when treatments initiated at early time. D–E, Both trastuzumab and docetaxel inhibited growth of BT474 xenografts when administered in late or early setting. F–G, Trastuzumab inhibited ZR75-1 tumor xenograft growth when administered early but not at late setting. H, Following the cessation of treatment, tumors recurred in mice treated with docetaxel alone but did not recur in those treated with docetaxel plus trastuzumab. (**p<0.01).
In order to simulate the standard treatment regimen in women in the early (adjuvant) versus advanced disease settings, we determined the effect of trastuzumab, the cytotoxic chemotherapy docetaxel, or both on the growth of HER2 amplified BT474 or luminal ZR75-1 tumor xenografts. Administration of trastuzumab after tumors were established (late treatment) significantly reduced the growth of HER2-amplified tumors (Figure 3E) but had no significant effect on the growth of luminal MCF7 and ZR75-1 xenografts (Figure 3A,F). When administered after tumors were established, the cytotoxic chemotherapy docetaxel reduced tumor growth of both xenografts (Figure 3D,F). In contrast, when administration was begun in the early setting, trastuzumab significantly reduced growth of HER2-amplified BT474 (Figure 3E) as well as luminal MCF7 and ZR75-1 cells (Figure 3B,G). Although ZR75-1 luminal tumors recurred following cessation of treatment with docetaxel alone, addition of trastuzumab to docetaxel in the early setting completely prevented tumor growth following cessation of therapy (Figure 3H). Together, these experiments suggest that in HER2 amplified cells, trastuzumab has a significant effect on tumor growth when administered in either the advanced or early settings. In contrast in HER2 non-amplified luminal tumors, trastuzumab only has a significant beneficial effect when it is administered in the early (adjuvant) setting.
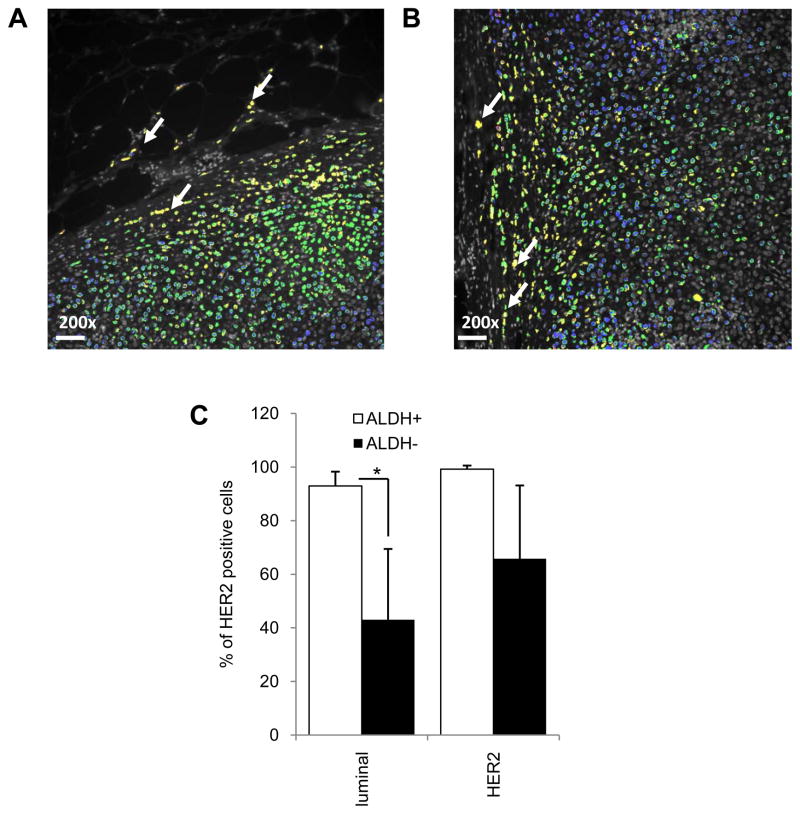
HER2 and ALDH1 are co-expressed in cells at the invasive front of human luminal breast cancers
We utilized automated quantitative immunofluorescence analysis (AQUA®) to correlate HER2 and ALDH expression at the individual cell level in primary human breast cancer tissues (25). Tumor cells were distinguished from stroma by their expression of CK8, an epithelial marker. In luminal tumors without HER2 amplification, HER2-positive/ALDH1-positive tumor cells were preferentially found at the tumor invasive front (Figure 4A,B, yellow cells white arrow). AQUA analysis and a computer algorithm were then utilized to quantitatively correlate HER2 and ALDH1 expression in individual cells. This algorithm permitted calculation of the percentage of HER2 expressing cells within the ALDH1-expressing cell population or the percentage of ALDH1 expressing cells in the top and bottom 10% of HER2-expressing cells. As was the case in breast cancer cell lines, in primary luminal breast tumors, there was a significant association between expression of HER2 and ALDH1 in these cells (Figure 4C, p<0.05). Within the ALDH-positive cells more than 90% of cells displayed HER2 expression while only 40% of ALDH-negative cells expressed HER2 in primary luminal tumors. In contrast, in HER2 amplified tumors, HER2 expression was more uniformly expressed (Supplementary 4C) and there was no significant difference in HER2 expression in ALDH+ versus ALDH−cells (Figure 4C).
Figure 4.
HER2 and ALDH are co-expressed in primary luminal breast tumor cells. A–B, Cells co-expressing HER2 (green) and ALDH1 (red) in human breast carcinoma cells identified by cytokeratin 8 (blue) are located (yellow; white arrows) at invasive edges of primary mammary carcinomas in FFPE sections. C, Shown here are average HER2-positive cells in ALDH1-positive versus -negative populations analyzed by AQUA. Scale bar 50μm, as the original images were taken at 200X magnification. (*p<0.05, **p<0.01).
The bone microenvironment induces HER2 protein expression in luminal tumor cells
Bone represents the most frequent site for metastasis of human tumors and luminal breast cancers are the most frequent subtype that metastasizes to bone (26). Although a number of factors have been postulated to play a role in facilitating the metastasis and growth of breast cancers in the bone microenvironment, the role of HER2 in this setting is poorly understood. To stimulate the bone microenvironment, we injected luciferase labeled MCF7 cells directly into mouse tibias and assessed tumor growth by light emission. Whereas MCF7 cells in the mouse mammary fat pad required estrogen supplementation for tumor growth, MCF7 cells in the tibia grew without estrogen supplementation. As assessed by IHC, HER2 expression was significantly upregulated in MCF7 cells grown within the bone microenvironment compared to cells grown in the mammary fat pad (Figure 5A,B). This increased expression was not due to HER2 gene amplification as assessed by fluorescence in situ hybridization (FISH) (data not shown), but was accompanied by an increase in the percent of ALDH1 expressing cells (Figure 5A,C).
Figure 5.
HER2 and ALDH1 expression are increased in MCF7 cells growing in the bone microenvironment. A, Representative images demonstrate an increase in HER2 and ALDH1 expression in MCF7 cells grown in mouse tibia compared to mammary fat pads. B–C, Quantitation of HER2 expression and ALDH1 expression was evaluated by counting positive cells by IHC. D, MCF7 cells co-cultured in vitro with human osteoblasts expressed increased levels of HER2 and this effect is inhibited by denosumab. E, HER2 and RANK expression in MCF7 cells was analyzed and the percent RANK expressing cells was assessed for the top 10% and bottom 10% of HER2 expressing cells. F, MCF7 cells with HER2 knockdown generated smaller tumors with G, fewer ALDH1-positive cells compared to tumors generated from MCF7 control cells as determined by IHC. H, Trastuzumab effectively inhibited luciferase expressing MCF7 cell growth as measured by luciferase expression in mouse tibias when administered in early setting but I, had no effect on established tumors in tibia (late setting, arrow indicates the initiation of trastuzumab). J, Trastuzumab had no effect on basal/claudin-low Sum159 cells introduced into tibia even when administered in the early setting. K–L–M, Trastuzumab reduced the expression of HER2 and ALDH1 in MCF7 cells grown in mouse tibia and L, resulted in smaller tumors with M, fewer ALDH1 positive cells than tumors generated in the absence of trastuzumab treatment. (*p<0.05, **p<0.01).
To simulate the bone microenvironment in vitro, we co-cultured DsRed labeled MCF7 cells with human osteoblasts (27). HER2 expression in MCF7 cells was significantly increased when the cells were co-cultured with human osteoblasts compared to culture in the absence of osteoblasts, an effect that was blocked by the RANK-L inhibitor denosumab (Figure 5D, p<0.01, Supplementary 5A).. Recombinant RANKL increased primary and secondary tumorpshere forming capacity of MCF7 cells (Supplementary 5B). Utilizing FACS, we also determined that HER2 and RANK receptors are co-expressed. The top 10% of HER2 expressing MCF7 cells contained a significantly higher proportion of RANK receptor expression than did the bottom 10% (HER2-negative) of MCF7 cells (p=0.0029) (Figure 5E, Supplementary 3C). These results suggest that RANK-L produced by osteoblasts in the bone microenvironment (28) may induce the expression of HER2 in RANK expressing luminal breast cancer cells.
HER2 drives the CSC population and is necessary for maintaining tumor growth in the bone microenvironment
To determine the functional role of HER2 in the growth of breast cancer cells in the bone microenvironment, we knocked down HER2 expression with an shRNA lentivirus (Supplementary 4A). This intervention significantly inhibited growth of MCF7 in mouse tibias (Figure 5F, p=0.004) and the small tumors that grew expressed lower levels of ALDH1 than controls (Figure 5G, p=0.05). We next examined the effects of systematically administered trastuzumab on tumor growth in the tibia model. As was the case in the mammary fat pad, trastuzumab effects on bone lesions were dependent on the timing of administration. When trastuzumab administration was initiated immediately after tumor inoculation (early), tumor growth was almost completely blocked (Figure 5H). In contrast, trastuzumab had little effect on the growth of established (late) tumors (Figure 5I). In contrast to the growth suppressing effect of trastuzumab administered in the early setting in luminal tumors, early trastuzumab treatment had no effect on growth of the basal/claudin-low Sum159 implanted into mouse tibia (Figure 5J). This provides further evidence for the specificity of early (adjuvant) trastuzumab for luminal breast cancer cells. Trastuzumab treatment significantly reduced the HER2 expression in luminal breast cancer cells in the mouse tibia (Figure 5K) resulting in decreased tumor area (Figure 5L) and the percent of ALDH1 expressing cells in resultant tumors (Figures 5K,M). We previously demonstrated that CSCs are regulated by Akt through activation of the Wnt/β-catenin pathway (19). To test whether HER2 upregulation in the bone microenvironment drives Wnt/β-catenin signaling, we assessed nuclear localization of β-catenin in MCF7 xenografts grown in mouse tibia. There was significantly higher nuclear localization of β-catenin in bone tumors in control mice compared to the bone tumors in trastuzumab treated mice (Supplementary 5D).
HER2 expression is increased in bone metastasis of luminal breast cancers compared to primary tumors in matched patient samples
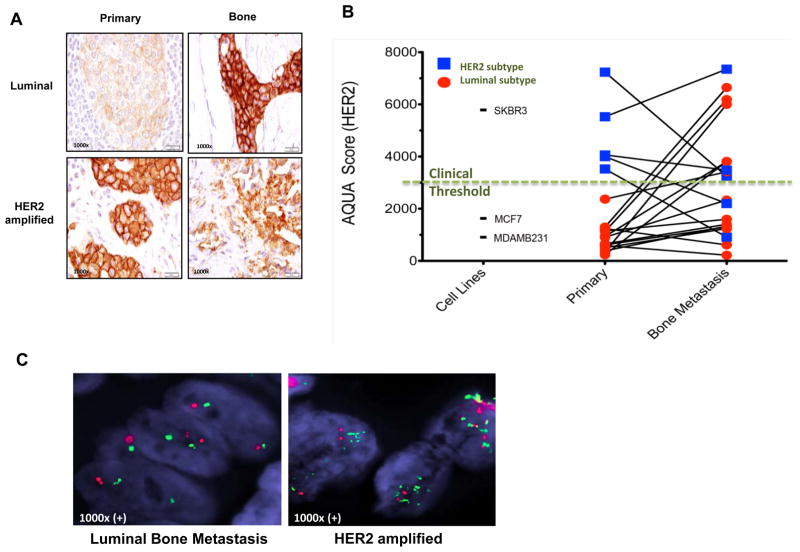
We assessed HER2 levels in matched primary and bone metastasis in 19 breast cancer patients using IHC and AQUA® analysis. In 12 out of 14 (87%) of luminal tumors classified as “HER2-negative” by classical criteria HER2 protein expression was significantly higher in bone metastases compared to matched primary tumors from the same patients (Figures 6A–B, Supplementary 6). Interestingly HER2 levels as determined by AQUA were above the clinical positive threshold in 6 out of 14 bone metastases of luminal tumors even though primary tumors from these patients were HER2-negative by AQUA (6B). Furthermore, none of these HER2 expressing bone metastases displayed HER2 gene amplification as determined by FISH (Figure 6C, Supplementary 6A). In contrast, there was no significant increase in HER2 expression between primary tumor and bone metastasis in five patients with HER2 amplified breast tumors. In fact, three of these patients who received trastuzumab treatment had a significant decrease in HER2 protein expression in bone metastases compared to their primary tumors (Figure 6B, Supplementary Figure 7). The fact that we did not detect increased HER2 expression in bone metastases of HER2 amplified breast tumors compared to their matched primary tumors suggest that the findings in luminal tumors are not an artifact of bone fixation as has been previously suggested (29). Furthermore, the absence of HER2 gene amplification in these metastases suggests that the bone microenvironment induces the expression of HER2 in luminal breast cancer cells metastatic to that site (Figure 7A).
Figure 6.
HER2 expression is increased in bone metastases compared to matched primary tumors in luminal breast cancers. A, HER2 expression by IHC score was examined in bone metastases as compared to matched breast primary tumor in patients with luminal and HER2 amplified breast cancers (additional examples in Supplementary Figure 6). B, HER2 expression was quantitated by AQUA score of 19 matched bone metastasis and primary breast cancers. 12 of 14 (87%) luminal breast cancers (red) demonstrated significant increase in HER2 expression in bone metastasis compared to matched primary breast tumors (95% confidence interval: 57–98%). All cases of HER2 intrinsic subtype had high score except for two patients that received trastuzumab treatment (blue line falls below the clinical threshold line). Cell lines MDA-MB231 (basal/claudin-low), MCF7 (luminal) and SKBR3 (HER2 amplified) were used to determine the high and low levels of HER2. C, Fluorescence in situ hybridization (FISH) assay for HER2 amplification shows two normal copies of the HER2 gene in representative bone metastasis sample of a patient with luminal breast cancer, while bone metastasis sample from a patient with HER2-amplified primary tumor as a positive control show multiple copies of the HER2 gene.
Figure 7.
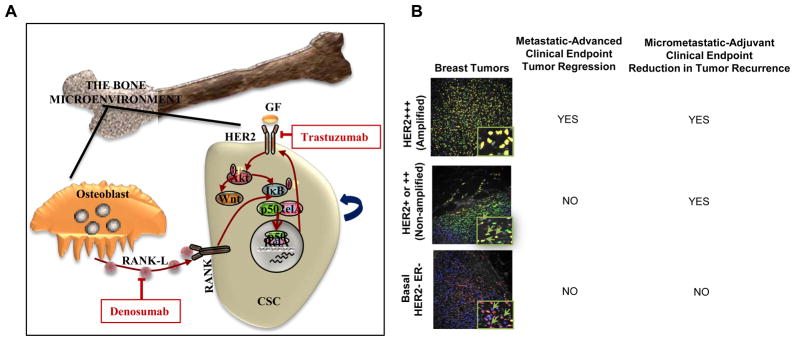
Regulation of breast CSCs by the bone microenvironment. A, Osteoblasts generated RANK-L activates the RANK receptor on breast CSCs activating NF-κB and HER2 expression. HER2 signaling further activates NF-κB generating a positive feedback loop. B, Clinical benefit of trastuzumab in advance setting as assessed by tumor regression is limited to tumors with HER2 gene amplification where HER2 is expressed in both bulk and CSC populations. In contrast, in the adjuvant setting, the clinical benefit of trastuzumab extends to luminal breast cancers in which HER2 is selectively expressed in the CSC population at sites of bone micrometastases. Tumors that are truly negative for HER2 do not benefit from trastuzumab in either setting.
DISCUSSION
The cancer stem cell hypothesis posits that many tumors, including human breast cancer, are hierarchically organized and driven by a cellular subcomponent that displays stem cell properties (30). These cells drive tumor growth and metastasis and by virtue of their relative resistance to traditional therapies such as cytotoxic chemotherapy and radiation may contribute to tumor recurrence (19, 31–34). We have previously demonstrated that HER2 is an important regulator of breast CSCs in HER2-amplified breast tumors where it regulates CSC self-renewal through activation of the Akt-Wnt signaling pathway (17, 19).
Our studies suggest that contrary to the dichotomous model utilized clinically, HER2 expression in breast tumors follows a distribution related to molecular sub-type with luminal tumors expressing an intermediate level of HER2 compared to basal/claudin-low (low) and HER2-amplified (high) tumors.
The development of technologies such as AQUA® quantitative immunofluorescence assay, have enabled assessment of HER2 expression as a continuous variable. In addition to inter-tumor heterogeneity, we observed intra-tumor heterogeneity of HER2 expression in both HER2-amplified and non-amplified luminal breast cancer cell lines as well as in human primary and metastatic breast tissues. In luminal cell lines defined as “HER2-negative” by classical criteria, HER2 is preferentially expressed in the CSC population. These observations extend previous studies (35, 36) linking HER2 expression and CSC phenotypes in cell lines.
We determined the effect of trastuzumab on tumor growth in HER2-amplified and non-amplified cell lines under standard culture conditions in vitro, as well as by CSC assays. Although it had no discernable effect on bulk tumor populations, trastuzumab was able to target the CSC populations in MCF7 and ZR75-1 luminal cell lines, as assessed by tumorsphere formation and by reduction in cells expressing the CSC markers ALDH and CD44+/CD24−. This effect was not seen in basal/claudin-low cell lines. Knockdown of HER2 recapitulated the effect of trastuzumab in tumorsphere formation confirming the specificity of trastuzumab in targeting HER2.
The important role of HER2 in the regulation of CSCs in luminal tumors was confirmed using mouse tumor xenografts. HER2 expressing MCF7 cells had significantly greater tumor initiating capacity than HER2 non-expressing cells. We found that the effects of trastuzumab were highly dependent on the timing of administration. When trastuzumab treatments were begun after palpable tumors had been established (late treatments) trastuzumab beneficial effects were limited to HER2-amplified tumors. However, when treatment was started immediately (early treatment) after inoculation, trastuzumab significantly reduced the growth of both luminal ZR75-1 and MCF7 cells, as well as the HER2-amplified BT474 tumors. In contrast, early trastuzumab treatment had no effect on the basal/claudin-low Sum159 tumor growth in fat pads or in the tibia model suggesting a specificity of this treatment for luminal tumors. Furthermore, the combination of adjuvant trastuzumab and cytotoxic chemotherapy, but not cytotoxic chemotherapy alone completely prevented tumor growth in these luminal tumors even after cessation of treatment. These results emphasize the pivotal role of CSCs in mediating tumor recurrence following adjuvant therapy, an important prediction of the CSC hypothesis (16).
Taken together, these results provide a potential biological explanation (illustrated in Figure 7B) for the unexpected findings from two separate prospective randomized trials that suggest that the benefit of adjuvant trastuzumab in women with HER2-negative cancers as assessed retrospectively is roughly the same as those with HER2-positive cancers (13, 15). Our data provide a biological rationale supporting the ongoing prospective randomized clinical trial currently being conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP, Protocol B47), which is designed to evaluate the clinical benefit for adjuvant trastuzumab in women with breast cancers that are HER2-1+ or -2+ by IHC and without HER2 gene amplification. Our findings are further supported by a recent neoadjuvant trastuzumab study, in which 89% of women with breast tumors that were classified as HER2-negative by classical criteria had circulating tumor cells (CTC) that expressed HER2. Furthermore, in these patients neoadjuvant trastuzumab treatment significantly reduced the HER2-expressing CTC population, an effect associated with a significant increase in disease free survival (29).
Our studies also demonstrate that HER2 expression is regulated by the tumor microenvironment. Previous studies examining discordance between HER2 expression in primary and metastatic tumors have suggested a discordance rate of 8–50% (14) including loss of HER2-amplification following trastuzumab-based neoadjuvant therapy (37, 38). We demonstrate that MCF7 cells growing in mouse tibias express higher levels of HER2 compared to those growing in the mammary fat pad. Increased HER2 expression in bone metastases was not due to gene amplification as assessed by FISH, suggesting that HER2 expression is regulated by the bone microenvironment. We also provide evidence that osteoblast generated RANKL may play a role in this regulation. RANK mediated NF-κB signaling has been previously demonstrated to be able to directly regulate HER2 expression (39)driving CSCs in MMTV-HER2 mouse breast cancer (40). In addition, progesterone induced RANK-L drives mammary stem cell self-renewal through NF-κB activation (41, 42). Interestingly HER2 also activates NF-κB signaling through Akt mediated I-κB phosphorylation (43) generating a positive feedback loop. These results suggest that the RANK-L inhibitor denosumab, already approved for the treatment of bone metastasis, may also target CSCs by blocking RANK-L induced HER2 expression. It has been reported that approximately 30% of women with early stage breast cancer harbor occult micrometastasis in their bone marrow at the time of diagnosis, a state associated with a worse prognosis (44). These micrometases have been found to be highly enriched in cells expressing CSC markers (45). The ability of trastuzumab to target occult bone marrow metastasis in both HER2-amplified and non-amplified breast cancer may also play a role in reducing tumor recurrence in the adjuvant setting (Illustrated in Figure 7B).
These studies have important implications for the development of clinical trials utilizing HER2 targeting agents by suggesting that a much larger group of women with breast cancer may benefit from HER2 blockade in the adjuvant setting than currently receive these treatments. Our data also supports a CSC model in which a subpopulation of cells with stem cell properties mediates tumor recurrence. If this is the case then effective adjuvant therapies will need to successfully target this CSC population.
Supplementary Material
Acknowledgments
We would like to thank Dr. Stephen Ethier for supplying SUM159 cells, Nancy McAnsh, Alan Burgess and Anosike Nwokoye for their technical help in pathologic examinations, Denise Poirier for her tireless effort and UMMCC Core facilities. This work was supported by NIH grants, CA129765 and CA101860, the Breast Cancer Research Foundation, Komen for the Cure, the Taubman Institute and Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes-On-Sale. M.S. Wicha is supported by a Stand Up To Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR DT0409).
Footnotes
Disclosure of potential conflicts of interest
M. S. Wicha has financial holdings in OncoMed Pharmaceuticals, receives research support from Dompe and MedImmune, serves on the scientific advisory board of VERISTEM.
H. Korkaya receives research support from MedImmune.
D. F. Hayes has received research support from Pfizer, Novartis and Veridex and holds stock option for his role on the scientific advisory board for OncImmune.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. First-line Herceptin monotherapy in metastatic breast cancer. Oncology. 2001;61 (Suppl 2):37–42. doi: 10.1159/000055400. [DOI] [PubMed] [Google Scholar]
- 5.Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–6. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 6.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 7.Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17:2235–49. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 9.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 10.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 11.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 12.Spielmann M, Roche H, Delozier T, Canon JL, Romieu G, Bourgeois H, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–34. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 13.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–11. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 14.Houssami N, Macaskill P, Balleine RL, Bilous M, Pegram MD. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res Treat. 2011;129:659–74. doi: 10.1007/s10549-011-1632-x. [DOI] [PubMed] [Google Scholar]
- 15.Perez EA, Reinholz MM, Hillman DW, Tenner KS, Schroeder MJ, Davidson NE, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28:4307–15. doi: 10.1200/JCO.2009.26.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–12. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korkaya H, Wicha MS. HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res. 2009;15:1845–7. doi: 10.1158/1078-0432.CCR-08-3087. [DOI] [PubMed] [Google Scholar]
- 19.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauraniemi P, Hautaniemi S, Autio R, Astola J, Monni O, Elkahloun A, et al. Effects of Herceptin treatment on global gene expression patterns in HER2-amplified and nonamplified breast cancer cell lines. Oncogene. 2004;23:1010–3. doi: 10.1038/sj.onc.1207200. [DOI] [PubMed] [Google Scholar]
- 23.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 24.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe A, Dolled-Filhart M, Camp RL, Rimm DL. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97:1808–15. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 26.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 27.Franceschi RT, Ge C, Xiao G, Roca H, Jiang D. Transcriptional regulation of osteoblasts. Ann N Y Acad Sci. 2007;1116:196–207. doi: 10.1196/annals.1402.081. [DOI] [PubMed] [Google Scholar]
- 28.Thomas GP, Baker SU, Eisman JA, Gardiner EM. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol. 2001;170:451–60. doi: 10.1677/joe.0.1700451. [DOI] [PubMed] [Google Scholar]
- 29.Georgoulias V, Bozionelou V, Agelaki S, Perraki M, Apostolaki S, Kallergi G, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK19 mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012 doi: 10.1093/annonc/mds020. [DOI] [PubMed] [Google Scholar]
- 30.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. discussion 95-6. [DOI] [PubMed] [Google Scholar]
- 31.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–50. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454–6. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 35.Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–21. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 36.Nakanishi T, Chumsri S, Khakpour N, Brodie AH, Leyland-Jones B, Hamburger AW, et al. Side-population cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signalling. Br J Cancer. 2010;102:815–26. doi: 10.1038/sj.bjc.6605553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–8. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011;37:422–30. doi: 10.1016/j.ctrv.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Cao N, Li S, Wang Z, Ahmed KM, Degnan ME, Fan M, et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res. 2009;171:9–21. doi: 10.1667/RR1472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y, Luo JL, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci U S A. 2007;104:15852–7. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–7. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 42.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 43.Merkhofer EC, Cogswell P, Baldwin AS. Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene. 2010;29:1238–48. doi: 10.1038/onc.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cote RJ, Morrissey DM, Houghton AN, Beattie EJ, Jr, Oettgen HF, Old LJ. Generation of human monoclonal antibodies reactive with cellular antigens. Proc Natl Acad Sci U S A. 1983;80:2026–30. doi: 10.1073/pnas.80.7.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuben JM, Lee BN, Gao H, Cohen EN, Mego M, Giordano A, et al. Primary breast cancer patients with high risk clinicopathologic features have high percentages of bone marrow epithelial cells with ALDH activity and CD44CD24lo cancer stem cell phenotype. Eur J Cancer. 2011;47:1527–36. doi: 10.1016/j.ejca.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.