Abstract
Previous work has shown that muscle contraction elevates interstitial adenosine triphosphate concentration ([ATP]i), which is likely due to the release of ATP from active skeletal muscle. ATP activation of purinergic receptors P2X on thin muscle afferent fibers further enhances cardiovascular responses to contraction. Thus, the purposes of this study were: (1) to examine the mechanisms by which ATP is released from muscle in response to mechanical stimulation; and (2) to study the effects of interstitial ATP concentrations on modulating pressor response to muscle contraction. Static contraction of the triceps surae muscle was evoked by electrical stimulation (at 5 Hz and 2.5 times motor threshold) of the tibial nerve in 9 anesthetized cats. Muscle interstitial ATP samples were collected from microdialysis probes inserted into the muscles. Dialysate ATP concentrations were determined using the luciferin–luciferase assay. In a control experiment, contraction was induced after 0.5 ml of saline was injected into the arterial blood supply of the hindlimb muscles. This increased [ATP]i by 220% (P < 0.05 vs. baseline). After gadolinium (1 mM), a blocker of mechanically sensitive channels, was injected into the muscles, contraction increased [ATP]i by 112% (P < 0.05 vs. control). In contrast, glibenclamide (an inhibitor of the ATP-binding cassette protein), monensin, and brefeldin A, which interfere with vesicular formation (or trafficking) and inhibit exocytosis, did not significantly affect ATP release by muscle contraction. In addition, a regression analysis showed that [ATP]i was linearly related to the pressor response to muscle contraction. The data suggest that ATP release from skeletal muscle is mediated via involvement of mechanosensitive channels. These findings further support a physiological role for release of ATP in modulating cardiovascular responses during static muscle contraction.
Keywords: autonomic reflex, muscle contraction, purinergic signaling
During muscle contraction, adenosine triphosphate (ATP) and its metabolites are released from skeletal muscle, thus raising the interstitial concentration of this important nucleotide.9,11,16 Once released, it leads to activation of P2X receptors on muscle-fiber afferents and reflexively increases arterial blood pressure and heart rate.3–7,13 Thus, ATP released from muscle during contraction can act as a stimulus or modulator for transduction of the reflex.
A previous study suggested that muscle contraction is the necessary and sufficient stimulus for the rise in ATP concentrations in the interstitium.11 First, the increase in ATP is linearly related to muscle tension. Second, the rise in interstitial ATP (ATPi) during stimulation is not blocked by removal of reflex afferent inputs. This suggests that a large portion of the ATP increase is unlikely to have come from sympathetic nerves, a part of the muscle reflex. Third, the rise in ATPi is eliminated by muscle paralysis, which supports the concept that ATPi comes from skeletal muscle and not from motor nerves. However, the mechanisms by which ATP is released from muscle in response to muscle contraction are unknown.
Similar to muscle contraction, passive muscle stretch that predominantly activates mechanosensitive receptors also increases the concentrations of ATPi.12 It is known that activation of muscle mechanoreceptors is a component of the muscle pressor reflex. Recently, it has been shown that blocking muscle mechanoreceptors with gadolinium attenuates the pressor response to muscle stretch.15,17 It was also noted that stretch-evoked reflex cardiovascular responses are suppressed in the conscious condition, but they are exaggerated by anesthesia.14 Thus, it is necessary to determine if activation of muscle mechanoreceptors can alter the release of ATP in muscle when using anesthetized animals.
In ureter epithelium, distension-evoked ATP release is inhibited after blocking vesicular exocytosis with brefeldin A or monensin.10 It is also believed that ATP transport across the plasma membrane is associated with activity of ATP-binding cassette protein (ABC protein).19 Glibenclamide is known to inhibit members of the ABC protein family.20 Studies further suggest that ATP leaves red blood cells via one or more ion channels in the ABC protein or directly via the proteins.2 In rat hepatocytes and HTC hepatoma cells, increases in cell volume stimulate ATP release.18 Gadolinium (Gd3+), a blocker of mechanosensitive channels, attenuates volume-sensitive ATP release by more than 90%.18 Moreover, Gd3+ also prevents cell-swelling–induced ATP release.1
The first purpose of this study was to determine the potential mechanism by which ATP is released from skeletal muscle during contraction. We examined whether elevation of interstitial ATP concentrations ([ATP]i) evoked by muscle contraction would be attenuated by Gd3+, glibenclamide, and brefeldin A or monensin after administration into the arterial blood supply of hindlimb muscles, respectively. The second purpose was to further ascertain a relationship between [ATP]i and the reflex pressor response to muscle contraction. We hypothesized that ATP would be released from contracting muscle via mechanosensitive channels, and altered ATP concentrations in the interstitium would affect the muscle pressor reflex.
METHODS
Animal Surgical Preparation
All experimental procedures were approved by the animal care committee of this institute and complied with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. Experiments were performed on 9 adult male cats (4.0 –5.0 kg).
The cats were anesthetized initially with ketamine (25 mg/kg intramuscularly). An endotracheal tube was inserted into the trachea and attached to a ventilator (Model 683; Harvard Apparatus, South Natick, Massachusetts). The cats were then anesthetized by inhalation of an isoflurane– oxygen mixture. Polyethylene catheters (PE-90) were inserted into an external jugular vein and a common carotid artery for drug administration and measurements of arterial blood pressure, respectively. The gaseous anesthetic was discontinued after α-chloralose (80 mg/kg) and urethane (200 mg/kg) were injected intravenously. Throughout the experiment, supplemental injections of α-chloralose (15 mg/kg) and urethane (40 mg/kg) were given if the cats exhibited a corneal reflex or withdrew a limb in response to a noxious stimulus. The femoral artery and arterial collaterals of one hindlimb were carefully isolated. An arterial branch of the femoral artery was cannulated with a PE-10 catheter. This allowed arterial injection of drugs while the arterial blood supply to hindlimb muscles was maintained. The triceps surae muscles of both limbs were isolated, and the Achilles (calcaneal) tendons were cut. A tie was placed around the tendon and attached to a tension transducer. The legs were stabilized with ties around the ankles at the top of the knees. The pelvis was stabilized in a spinal unit, and the knee joints were secured by attaching the patellar tendon to a steel post. The tibial nerves on one leg were carefully isolated and placed on platinum bipolar stimulating electrodes. A pool was formed around the exposed neural and muscular tissue using skin flaps sutured to brass bars, and the exposed region was immersed in a pool of warm (37°C) mineral oil.
The ventilator was set to a tidal volume of 20 ml/stroke and a rate of 20 –30 strokes/min. Arterial blood gases and pH were periodically checked by a pH blood gas analyzer (ABL 510; Radiometer, Copenhagen, Denmark). pH was maintained at ~7.35–7.45, PCO2 at ~30 – 40 mm Hg, and HCO3 at ~20 –25 mmol/L by adjusting the ventilator or by intravenous injection of 1 M of sodium bicarbonate solution. Body temperature was continuously monitored with a rectal thermometer (Series 400; Yellow Springs Instruments, Yellow Springs, Ohio) and maintained between 37.5° and 38.5°C by a water-perfused heating pad and external heat lamps.
Insertion of Microdialysis Probes
Semipermeable fibers with a molecular weight cutoff of 30,000 (0.20 mm i.d., 0.22 mm o.d.; Spectrum Laboratories, Laguna, California) were used to construct the microdialysis probes. Each end of a single fiber was inserted ~1 cm into a hollow polyamide tube (0.25 mm i.d., 0.36 mm o.d.) and glued. The length of the probe semipermeable fiber was 4 cm (between two polyamide tubes). The skin directly over the triceps surae muscle on both hindlimbs was dissected away, and four microdialysis probes were inserted into the gastrocnemius muscle of each hindlimb. Samples that were collected from the probes in the leg contralateral to the contracting hindlimb served as control. The probes were inserted into the muscle parallel to fiber orientation via a cannula. The microdialysis probes were attached to a perfusion pump (Model 102; CMA) and perfused at a rate of 5 μl/min with Ringer’s solution. The dialysate was collected in 250-μl microcentrifuge tubes, immediately sealed (to prevent evaporation), and stored at −80°C in a freezer until analyzed. Dialysate ATP was measured by luminometry using a luciferin–luciferase assay.
Experimental Protocols
After the microdialysis probes were inserted, a minimum 90-min equilibration period was allowed to obtain stabilized resting ATP concentrations. The samples for baseline data were collected for 20 min before contraction. Contractions induced by electrical stimulation of the tibial nerves were then performed in 9 cats. Rhythmic contractions were conducted at frequencies of 5 Hz (2.5 times motor threshold and 0.1-ms duration), on the basis of our previous report.11 The stimulation was sustained for 10 min. The samples for recovery data were collected for 20 min after the end of electrical stimulation. Prior to each contraction, an injection was made into the muscle, via the catheter inserted into the femoral arterial branch, of 0.5 ml of saline, 1 mM of either Gd3+, glibenclamide, monensin, or brefeldin A. It is noted that the half-life (t1/2) of these chemicals is more than 2 hours. The chemicals were selected for injection in a random fashion. All chemicals were dissolved in saline. The injected volume was 0.5 ml. The same volume of saline was injected to flush the arterial catheter. The duration of injection was 1 min. There was a 60-min rest period between each bout of contraction.
Experimental Data Acquisition and Analysis
Arterial blood pressure was measured by connecting the carotid arterial catheter to a pressure transducer (Model P23ID; Statham). Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. Heart rate (HR) was derived from the arterial pressure pulse. Ties were placed around the tendons that were attached to a force transducer (Model F10; Grass Instruments, West Warwick, Rhode Island) for measurement of developed tension. All measured variables were continuously recorded on an eight-channel chart recorder (Model TA 4000; Gould, Valley View, Ohio) and an iMac computer with PowerLab system software (ADInstruments, Castle Hill, Australia).
Control values were determined by analyzing at least 30 s of data immediately before electrical stimulation. The peak response of each variable was determined by the peak change from the control value. Experimental data (MAP, heart rate, muscle time–tension index, and ATP concentrations) were analyzed by one-way analysis of variance (ANOVA) with repeated measures. As appropriate, Tukey’s post hoc analyses were utilized. All values are expressed as mean ± SE. For all analyses, differences were considered statistically significant at P < 0.05. All statistical analyses were performed using SPSS for Windows, version 11.5 (SPSS Sci).
RESULTS
ATPi Response to Muscle Contraction
The percent recovery rate of microdialysis probes for ATP was examined in vitro in our previous study.11 The recovery rates are ~30%, as ATP concentrations are less than 10 μM. The relationship between standard and dialysate concentrations is linear. This indicates that dialysate ATP concentration is directly proportional to its concentration in the dish. Dialysate ATP would be linearly related to interstitial ATP obtained from the cat hindlimb. Thus, in this report we present dialysate concentrations. The concentrations of interstitial ATP are ~2– 4 μM as the recovery rate is considered. In addition, percentage change of dialysate ATP from the baseline value is reported in order to minimize the effect of resting ATP concentrations, likely due to methods of ATP measurements and anesthesia.
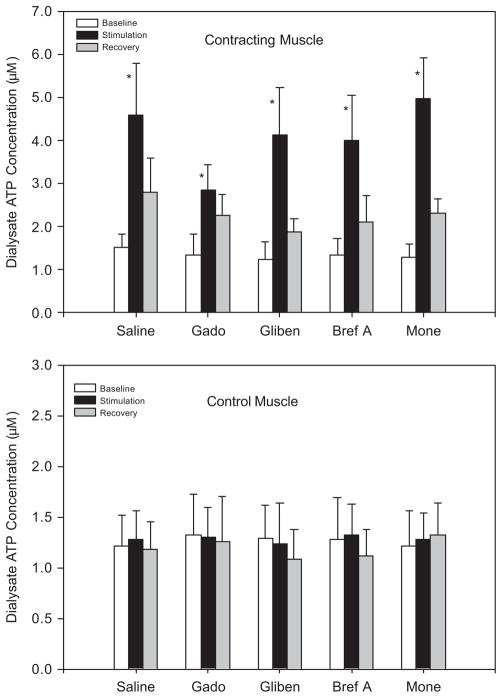
Resting ATP concentrations were not different in either experimental or control muscle in the five study groups (Fig. 1). The dialysate ATP concentrations were significantly increased in contracting muscle during electrical stimulation–induced contraction (Fig. 1, upper panel). After the end of stimulation, dialysate ATP recovered. In addition, dialysate ATP concentrations in the contralateral control leg were not significantly altered by muscle contraction (Figure 1, bottom panel). This suggests that muscle reflex activation and the resultant engagement of sympathetic efferent nerves were not likely responsible for the increase in dialysate ATP in the experimental leg.
FIGURE 1.
Dialysate ATP concentrations before (baseline), during (stimulation), and after (recovery) 10-min muscle contraction. Either saline, gadolinium (Gado), glibenclamide (Gliben), brefeldin A (Bref A), or monensin (Mone) was injected into the femoral artery 10 min before the start of contraction. Electrical stimulations significantly elevated dialysate ATP concentrations in contracting muscle (upper panel), but not in control muscle (bottom panel). The number of animals = 9. Values are expressed as mean ± SE. *P < 0.05 vs. baseline and recovery.
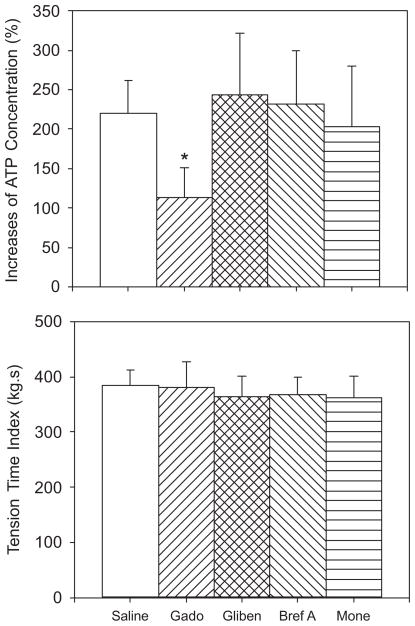
Arterial injection of glibenclamide, monensin, and brefeldin A did not affect ATP increases in contracting muscle (Fig. 2, upper panel). However, the percentage increase of dialysate ATP concentrations was significantly attenuated after Gd3+ was injected into the arterial blood supply of the hindlimb muscles as compared with control saline injection (Fig. 2, upper panel). The effect of gadolinium was not due to an effect of this agent on developed muscle tension because there were no differences in muscle tension among the different groups (Fig. 2, bottom panel).
FIGURE 2.
Percentage increase of dialysate ATP concentrations (upper panel) and muscle time–tension index (bottom panel) during 10-min contraction in 9 cats. Saline, gadolinium (Gado), glibenclamide (Gliben), brefeldin A (Bref A), and monensin (Mone) were administered into the femoral artery before electrical stimulation–induced contraction, respectively. Gadolinium significantly attenuated an increase in ATP concentrations at the similar developed muscle tension. The number of animals = 9. Values are expressed as mean ± SE. *P < 0.05 vs. saline control. Glibenclamide, brefeldin A, and monensin did not significantly affect the concentrations of interstitial ATP.
Cardiovascular Responses to Muscle Contraction
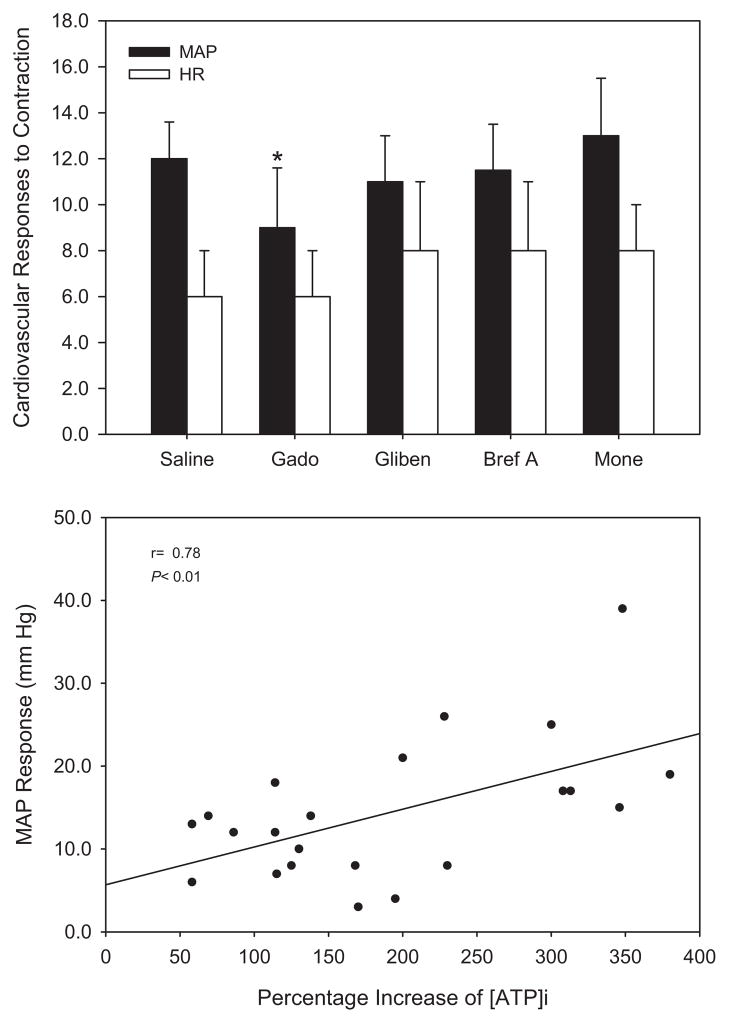
Baseline values for MAP and heart rate (HR) were 126 ± 12 mm Hg and 165 ± 15 beats/min, respectively. Rhythmic muscle contraction evoked by electrical stimulation of the tibial nerves significantly increased MAP in all experimental groups. Note that the pressor response was significantly attenuated with the prior injection of Gd3+. Peak MAP and HR responses to contraction after arterial injection of saline, Gd3+, glibenclamide, monensin, and brefeldin A were not significantly altered. The results are presented in Figure 3 (upper panel).
FIGURE 3.
(Top) Peak mean arterial pressure (MAP) and heart rate (HR) responses during 10-min electrical stimulation of the tibial nerves in 9 cats. Ten minutes before stimulation, either saline, gadolinium (Gado), glibenclamide (Gliben), brefeldin A (Bref A), or monensin (Mone) was administered into the femoral artery. Muscle contraction significantly increased blood pressure in all groups. However, the prior injection of gadolinium significantly attenuated the pressor response. The number of animals = 9. Values are expressed as mean ± SE. *P < 0.05 vs. saline control. (Bottom) Effects of [ATP]i on modulating pressor response to contraction. Regression analysis suggests that [ATP]i is linearly related to the pressor response to muscle contraction.
To determine whether the increases in MAP response were linked to [ATP]i during contraction, a linear regression analysis was performed, and a significant relationship is demonstrated (r = 0.78, P < 0.01) in Figure 3 (bottom panel).
DISCUSSION
The purposes of this study were to determine the mechanisms by which ATP is released from contracting muscle, and the role played by [ATP]i in modulating the pressor response to muscle contraction. The results demonstrate that blockade of mechanosensitive channels with Gd3+ significantly attenuates contraction-increased ATP concentrations in the interstitium. Because increased interstitial ATP is due to the release of ATP from contracting muscle,11 our data suggest that mechanosensitive channels, at least in part, mediate ATP release from muscle into the interstitium during muscle contraction. The magnitude of the rise in interstitial ATP induced by contraction is linked to the level of tension generated.11 Because Gd3+ did not reduce the level of muscle tension generated, we must conclude that the effect of this agent was evoked by directly blocking mechanoreceptor channels on skeletal muscle. The findings from this report further support this concept and, for the first time, offer data suggesting that ATP is released from contracting muscle via mechanosensitive channels. Furthermore, regression analysis has shown that [ATP]i is linearly related to the pressor response to muscle contraction, suggesting that elevated ATP concentrations in the interstitium are very likely to affect the pressor response to activation of muscle afferent fibers. Evidence has supported the role of ATP in modulating the muscle pressor reflex via activation of P2X receptors on small afferent fiber nerves.3–7,13 Thus, our findings further support a physiological role for release of ATP in the processing of the muscle pressor reflex.
In guinea-pig ureter epithelium, increases in distension pressure evoke ATP release from epithelium in a pressure-dependent manner.10 After 10 μM of brefeldin A or 100 μM of monensin were directly applied to perfusion medium to block vesicular exocytosis, ATP release was inhibited.10 In addition, ATP leaves red blood cells via one or more ion channels in the ABC protein or directly via the proteins.2 ATP transport across the plasma membrane is also associated with activity of ABC protein.19 Glibenclamide is an inhibitor of members of the ABC protein family [sulfonylurea receptor and cystic fibrosis transmembrane conductance regulator (CFTR) protein].20 In an in vitro study, 100 μM glibenclamide CFTR inhibited channel activity by >90%.10 In the present study, 1 mM of brefeldin A, monensin, and glibenclamide were injected into the arterial blood supply of the hindlimb muscles. We believe that sufficient amounts of these chemicals were delivered into the injected muscle tissues. If they had interfered with vesicular exocytosis and inhibited ABC protein family to reduce the release of ATP from muscle, an increase in ATP concentrations by muscle contraction would have been attenuated.
It was reported that 200 μM of Gd3+ added to cultured cell buffer inhibits ATP permeability enhanced by increasing cell volume in rat hepatocytes and HTC hepatoma cells.18 Moreover, Gd3+ can also prevent cell-swelling–induced ATP release in eukaryotic cells.1 Inhibition of ATP release requires ~100 –500 μM of Gd3+.1 Given that Gd3+ injected into the femoral artery crosses the blood–vascular barrier, gadolinium, in a concentration of 1 mM, was applied in this study. This significantly attenuated the release of ATP from contracting muscle by ~50%.
It is unclear where activity of mechanosensitive channels was blocked after arterial injection of gadolinium. Previous studies have suggested that gadolinium, at a concentration of 10 mM, injected into contracting muscle blocks the pressor response to muscle contraction.8,21 Recent reports further indicate that passive stretch–induced reflex pressor response is attenuated by intravenous injection of 20 mM gadolinium.15,17 Because elevated ATP has been reported to stimulate small-fiber muscle afferents and increase cardiovascular responses to muscle stimulation,3–7,13 it is reasoned that activities of mechanosensitive channels in skeletal muscle as well as sensory afferent fibers were likely to be antagonized with 10 mM of gadolinium in the processing of the muscle pressor reflex. Therefore, we injected gadolinium into the arterial blood supply of the hindlimb muscle to attenuate mechanosensitive channels in muscle tissues, where muscle sensory nerves are located, because systemic administration of gadolinium could cause confounding effects. Moreover, a lower dose of gadolinium (1 mM) was administered to establish a relationship between [ATP]i and the reflex muscle response. Notably, the data show that blocking mechanosensitive channels significantly decreased the release of ATP from contracting muscle after 1 mM of gadolinium was injected the arterial supply of the hindlimb muscles, but the MAP response to muscle contraction was attenuated to a lesser degree. Thus, attenuation of the muscle pressor reflex by a lower dose of gadolinium was most likely due to a decrease in ATP concentration in the interstitium. In addition, the increase in MAP response was shown to be linearly related to [ATP]i during contraction. This further suggests that elevated ATP concentrations in the interstitium per se can modulate the pressor response to activation of muscle afferent fibers.
In conclusion, we have demonstrated that muscle contraction increases the release of ATP from active muscle. A mechanism of ATP release is associated with activation of mechanosensitive channels. ATP is released into the interstitium by muscle contraction and, in turn, stimulates or modulates small-fiber muscle afferent nerves in the interstitial space in the processing of the muscle pressor reflex.
Acknowledgments
This work was supported by NIH grants R01 HL075533 (to J.L.), R01 HL078866 (to J.L.), and R01 HL60800 (to L.S.). The authors thank Jennie Stoner for her outstanding secretarial skills. We also thank Gretchen Monahan and Kris Rice for measuring the ATP samples.
Abbreviations
- ABC
ATP-binding cassette
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- ATPi
interstitial ATP
- [ATP]i
interstitial ATP concentration
- CFTR
cystic fibrosis transmembrane conductance regulator
- Gd3+
gadolinium
- HR
heart rate
- MAP
mean arterial pressure
- P2X
purinergic receptors subtype 2X
References
- 1.Boudreault F, Grygorczyk R. Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol Cell Physiol. 2002;282:C219–C226. doi: 10.1152/ajpcell.00317.2001. [DOI] [PubMed] [Google Scholar]
- 2.Demolombe S, Escande D. ATP-binding cassette proteins as targets for drug discovery. Trends Pharmacol Sci. 1996;17:273–275. doi: 10.1016/0165-6147(96)10037-7. [DOI] [PubMed] [Google Scholar]
- 3.Gao Z, Kehoe V, Xing J, Sinoway L, Li J. Temperature modulates P2X receptor–mediated cardiovascular responses to muscle afferent activation. Am J Physiol Heart Circ Physiol. 2006;291:H1255–H1261. doi: 10.1152/ajpheart.01303.2005. [DOI] [PubMed] [Google Scholar]
- 4.Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H939–H945. doi: 10.1152/ajpheart.00911.2006. [DOI] [PubMed] [Google Scholar]
- 5.Hanna RL, Hayes SG, Kaufman MP. alpha, beta-methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol. 2002;93:834– 841. doi: 10.1152/japplphysiol.00237.2002. [DOI] [PubMed] [Google Scholar]
- 6.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94:1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- 7.Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol. 2004;96:1166–1169. doi: 10.1152/japplphysiol.01020.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Cell Physiol. 2001;280:H2153–H2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- 9.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6– 8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- 10.Knight GE, Bodin P, de Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Heart Renal Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- 11.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- 12.Li J, King NC, Sinoway LI. Interstitial ATP and NE concentrations in active muscle. Circulation. 2005;111:2748–2751. doi: 10.1161/CIRCULATIONAHA.104.510669. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–2643. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- 14.Matsukawa K, Nakamoto T. Muscle mechanosensitive reflex is suppressed in the conscious condition: effect of anesthesia. J Appl Physiol. 2008;104:82– 87. doi: 10.1152/japplphysiol.00938.2007. [DOI] [PubMed] [Google Scholar]
- 15.Matsukawa K, Nakamoto T, Inomoto A. Gadolinium does not blunt the cardiovascular responses at the onset of voluntary static exercise in cats: a predominant role of central command. Am J Physiol Cell Physiol. 2007;292:H121–H129. doi: 10.1152/ajpheart.00028.2006. [DOI] [PubMed] [Google Scholar]
- 16.Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol. 2001;536:593– 603. doi: 10.1111/j.1469-7793.2001.0593c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamoto T, Matsukawa K. Muscle mechanosensitive receptors close to the myotendinous junction of the Achilles tendon elicit a pressor reflex. J Appl Physiol. 2007;102:2112–2120. doi: 10.1152/japplphysiol.01344.2006. [DOI] [PubMed] [Google Scholar]
- 18.Roman RM, Feranchak AP, Davison AK, Schwiebert EM, Fitz JG. Evidence for Gd3+ inhibition of membrane ATP permeability and purinergic signaling. Am J Physiol Gastrointest Liver Physiol. 1999;277:G1222–G1230. doi: 10.1152/ajpgi.1999.277.6.G1222. [DOI] [PubMed] [Google Scholar]
- 19.Schwiebert EM. ABC transporter-facilitated ATP conductive transport. Am J Physiol Cell Physiol. 1999;276:C1–C8. doi: 10.1152/ajpcell.1999.276.1.C1. [DOI] [PubMed] [Google Scholar]
- 20.Sheppard DN, Welsh MJ. Effect of ATP-sensitive K channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]