Abstract
Biodegradable scaffolds have been extensively used in the field of tissue engineering and regenerative medicine. However, noninvasive monitoring of in vivo scaffold degradation is still lacking. In order to develop a real-time trafficking technique, a series of meso-brominated near-infrared (NIR) fluorophores were synthesized and conjugated to biodegradable gelatin scaffolds. Since the pentamethine cyanine core is highly lipophilic, the side chain of each fluorophore was modified with either quaternary ammonium salts or sulfonate groups. The physicochemical properties such as lipophilicity and net charge of fluorophores played a key role in the fate of NIR-conjugated scaffolds in vivo after biodegradation. The positively charged fluorophore-conjugated scaffold fragments were found in salivary glands, lymph nodes, and most of the hepatobiliary excretion route. However, halogenated fluorophores intensively accumulated into lymph nodes and the liver. Interestingly, balanced-charged gelatin scaffolds were degraded into urine in a short period of time. These results demonstrate that the noninvasive optical imaging using NIR fluorophores can be useful for the translation of biodegradable scaffolds into the clinic.
1. Introduction
Many surgical procedures involve tissue transplantation to repair tissues damaged by trauma or disease [3, 21, 24]. Tissue transplant, however, relies on limited donors with risk of rejection by the patient’s immune system [9]. Many efforts have been focused on regenerating tissues by combining cells from the body or stem cells with biological substitutes [15, 27, 19]. Various materials have been utilized in the attempt to finely tune the structural and chemical characteristics of biodegradable scaffolds, including modified polysaccharides, gelatin-based structures, and collagen nanofibrils [17, 18]. Histological examination is currently the gold standard to analyze the degradation of surgically implanted materials; however, this approach does not provide time-resolved information but only the final end results of the process [25]. Developing a method for continuously and noninvasively monitoring the degradation of implanted scaffolds along with tissue formation is an important undertaking. It is important to monitor the degradation of implanted scaffolds continuously and noninvasively along with tissue formation [20].
Utilizing near-infrared (NIR) fluorescence signals could provide a sensitive technique for the noninvasive continuous monitoring of scaffold degradation [1, 10]. As NIR light is within the window of optical clarity for real-time imaging, it can provide signal with reduced background arising from endogenous fluorophores in the body [2, 5]. In this study, we prepared a series of pentamethine cyanine dyes to establish the molecular characteristics required of the fluorophore for in vivo trafficking. These fluorophores can be synthesized with structural diversity that allows wavelengths to be within the NIR region while exhibiting high water solubility. In addition, a bromine atom was introduced to the central carbon on the polymethine chain for conjugation with the biodegradable scaffolds using the available primary amine of gelatin for substitution. Directly conjugating a NIR fluorescent label to a tissue scaffold could serve as a viable noninvasive method for directly analyzing the rate of degradation and the fate of surgically implanted scaffolds.
2. Experimental details
2.1. Chemicals and reagents
All chemicals and solvents were of American Chemical Society grade or HPLC purity. Sigma-Aldrich (Saint Louis, MO) and TCI America (Waltham, MA) were the commercial sources for the starting materials utilized in the presented synthesis and the reagents were used without purification. The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance (400 MHz) spectrometer using DMSO-d6 (Cambridge Isotope Laboratories, Andover, MA) containing tetramethylsilane (TMS) as an internal calibration standard. UV-Vis/NIR absorption spectra were recorded on a Varian Cary 50 spectrophotometer. High-resolution accurate mass spectra (HRMS) were obtained either at the Georgia State University Mass Spectrometry Facility using a Waters Q-TOF micro (ESI-Q-TOF) mass spectrometer or utilizing a Waters Micromass LCT TOF ES+ Premier Mass Spectrometer. Liquid chromatography with a Waters Delta-Pak 3.9 × 150 mm reversed phase C18 column was coupled with a Waters 2487 single wavelength absorption detector with wavelengths set between 640 and 700 nm depending on the dye’s photophysical properties. Evaporative light scattering detection (ELSD) analyzes trace impurities that cannot be observed by alternate methods; a SEDEX 75 ELSD (Sedere, France) was utilized in tandem with liquid chromatography to observe purity.
2.2. Synthesis of highly charged cyanine fluorophores
A suspension of 4-iodophenylhydrazine hydrochloride (1) in glacial acetic acid (75 mL) was vigorously stirred and heated to reflux. After complete dissolution, 3-methyl-2-butanone (3 mol eq) was added to the reaction mixture. The refluxing solution was allowed to react for 24 h and, after consumption of the starting material was confirmed by TLC, the liquid was removed under reduced pressure. The resulting oil was dissolved in dichloromethane (30 mL) and washed with saturated sodium bicarbonate (3 × 50 mL). The organic layer was extracted and dried over sodium sulfate. After concentration via rotary evaporation, the product was obtained as a highly viscous reddish-brown oil and was used in further reactions without purification. 5-Iodo-2,3,3-trimethylindolenine (3): Yield 66%; oil; 1HNMR (400MHz, DMSO-d6): δ 1.29 (s, 6H), 2.28 (s, 3H), 7.24 (d, J = 8.2 Hz, 1H), 7.65 (dd, J = 8.2 Hz, J = 1.6 Hz, 1H), 7.75 (d, J = 1.6 Hz, 1H); 13CNMR (100 MHz, DMSO-d6): δ 14.8, 22.4, 54.1, 119.5, 122.9, 124.2, 131.4, 148.4, 152.5, 186.6.
Indolenine derivative 3 was transferred to a round bottom flask that was oven dried and purged with nitrogen. Anhydrous acetonitrile (50 mL) was added and the mixture was heated under nitrogen. Alkylating agent (3-bromopropyl)trimethylammonium bromide was added to the flask. The mixture was heated to reflux for 72 h. The dicationic salt was obtained after precipitation using diethyl ether and washing with acetone, ethyl acetate and hexanes. 5-Iodo-2,3,3-trimethyl-1-(3-(trimethylammonio)propyl)-3H-indol-1-ium bromide (5): Yield 81%; MP 147–149 °C; 1HNMR (400 MHz, DMSO-d6): δ 1.61 (s, 6H), 2.52 (t, J = 7.6 Hz, 2H), 3.22 (s, 9H), 3.70 (tt, J = 7.6 Hz, J = 8.0 Hz, 2H), 4.60 (t, J = 8.0 Hz, 2H), 4.80 (s, 3H), 7.65 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.4 Hz, 1H), 8.20 (s, 1H); 13CNMR (100 MHz, DMSO-d6): δ 13.4, 21.0, 21.5, 44.3, 53.9, 54.6, 62.3, 95.7, 116.6, 133.0, 138.5, 140.7, 143.6, 198.3; Composition in theory: C(37.39%), H(4.98%), Br(29.26%), N(5.13%); Composition found: C(37.66%), H(4.59%), Br(29.21%), N(5.44%); HRMS (ESI) calculated for [C17H27IN2]2+ m/z 193.1613, found m/z 193.2221.
Substituted 2,3,3-trimethyl indolenine salts bearing a N-heterocyclic quaternary ammonium moiety (2 mol eq) were individually reacted with the brominated methine reagent 7 (1 mol eq) in the presence of acetic anhydride (8 mL) and sodium acetate (4 mol eq) at 70 °C. The reaction was monitored using UV-Vis-NIR absorption spectroscopy in methanol and LC-MS. After 2–4 h, the reactions were complete. The solvent was removed in vacuo. The crude material was purified using Fluka Silica gel 90 Å C18-reversed phase column chromatography using a gradient elution of methanol:water to obtain the final compounds in high purity for further conjugation. 2-((1E,3Z,5E)-3-Bromo-5-(5-iodo-3,3-dimethyl-1-(3-(trimethylammonio)propyl)indolin-2-ylidene)penta-1,3-dien-1-yl)-5-iodo-3,3-dimethyl-1-(3-(trimethylammonio)propyl)-3H-indol-1-ium bromide (11): 91% yield; M.P. 240–242 °C; 1HNMR (400 MHz, DMSO-d6): δ 1.74 (s, 12H), 2.17 (t, J = 7.0 Hz, 4H), 3.11 (s, 18H), 3.56–3.66 (m, 4H), 4.23 (t, J = 7.0 Hz), 6.33 (d, J = 12.0 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 8.0 Hz, 2H), 8.17 (s, 2H), 8.58 (d, J = 12.0 Hz); 13CNMR (400 MHz, DMSO-d6), δ: 20.6, 26.7, 41.8, 49.4, 52.4, 62.6, 90.6, 102.9, 114.3, 116.7, 131.9, 137.2, 141.3, 143.8, 150.2, 173.9. Composition in theory: (x3H2O): C (37.65%), H (4.95%), N (4.75%); Composition found: C (37.61%), H (4.78%), N (5.02%); HRMS (ESI) calculated for [C37H52BrI2N4]3+ m/z 295.5222, found m/z 295.4423.
2-((1E,3Z,5E)-3-Bromo-5-(3,3-dimethyl-5-sulfonato-1-(3-(trimethylammonio)propyl) indolin-2-ylidene)penta-1, 3-dien-1-yl)-3,3-dimethyl-1-(3-(trimethylammonio)propyl)-3H-indol-1-ium-5-sulfonate bromide (12): 77% yield. M.P. 252–254 °C; 1HNMR (400 MHz, DMSO-d6): δ 2.05 (s, 12H), 2.71 (t, J = 7.6 Hz, 4H), 3.47 (s, 18H), 3.86 (tt, J = 7.6 Hz, 7.2 Hz, 4H), 4.64 (t, J = 7.2 Hz, 4H), 6.78 (d, J = 13.2 Hz, 2H), 7.73 (d, J = 8.0 Hz, 2H), 8.21 (d, J = 8 Hz, 2H), 8.25 (s, 2H), 8.56 (d, J = 13.2 Hz, 2H); 13CNMR (100 MHz, DMSO-d6), δ: 20.3, 26.3, 40.6, 49.7, 52.3, 63.6, 103.0, 110.2, 116.4, 119.1, 126.6, 140.3, 141.3, 142.5, 150.6, 176.7. Composition in theory: (x3H2O): C (47.95%), H (6.31%), N (6.05%); Composition found: C (47.46%), H (6.48%), N (6.05%); HRMS (ESI) calculated for [C37H52BrN4O6S2]+ m/z 792.8822, found m/z 792.8854.
2.3. LC-MS spectroscopy
The purity of all compounds was measured using liquid chromatography-mass spectrometry (LC-MS) on a Waters system consisting of a 1525 binary HPLC pump with a manual 7725i Rheodyne injector, a 996 Photodiode Array (PDA) detector, and a 2475 multiwavelength fluorescence detector. The column eluate was divided in two using a flow splitter (Upchurch Scientific). A portion of the eluate flowed into an ELSD (Richards Scientific) while the rest flowed into a Micromass LCT ESI-TOF spectrometer (Waters) equipped with a Symmetry (R) C18 (4.6 × 150 mm, 5 μm) reverse-phase HPLC column. For mass spectrometry mobile phase was solvent A = 0.1% formic acid (FA) in water and solvent B = CH3CN with 95% A for 5 min and a linear gradient from 5% to 40% CH3CN (from A to B for 30 min) at a flow rate of 1 mL min−1, capillary voltage was −3317 V, and sample cone voltage was −50 V.
2.4. Optical property measurements
All optical measurements were performed at 37 °C in 100% fetal bovine serum (FBS) buffered with 50 mM HEPES, pH 7.4. Absorbance and fluorescence emission spectra of the series of NIR fluorophores were measured using fiberoptic HR2000 absorbance (200–1100 nm) and USB2000FL fluorescence (350–1000 nm) spectrometers (Ocean Optics, Dunedin, FL). NIR excitation was provided by a 655 nm red laser pointer (Opcom Inc., Xiamen, China) set to 5 mW and coupled through a 300 μm core diameter, NA 0.22 fiber (Fiberguide Industries, Stirling, NJ). For fluorescence quantum yield (QY) measurements, oxazine 725 in ethylene glycol (QY = 19%) was used as a calibration standard, under conditions of matched absorbance at 655 nm. In silico calculations of physicochemical properties such as the partition coefficient (logD at pH 7.4), pKa, total polar surface area (TPSA), H-bond donors/acceptors, and rotatable bonds were calculated using Marvin and JChem calculator plugins (ChemAxon, Budapest, Hungary).
2.5. Implantation of NIR scaffolds
Animals were housed in an AAALAC-certified facility, and all animal studies were performed under the supervision of Beth Israel Deaconess Medical Center’s Institutional Animal Care and Use Committee (IACUC) in accordance with approved institutional protocol #101-2011. NCRNU nude mice (20–30 g, 6–8 weeks, spontaneous mutant T-cell deficient mice) were purchased from Taconic Farms (Germantown, NY). Prior to transplant scaffolds, mice were anesthetized with an intraperitoneal injection of ketamine (100 mg kg−1) and xylazine (10 mg kg−1). Gelatin-based biodegradable scaffold Gelfoam was purchased from Pfizer Inc. (New York, NY) and used after washing with ethanol (EtOH). The series of charged cyanine fluorophores were conjugated on the biodegradable gelatin scaffold through the nucleophilic substitution on the meso bromine in a mixture of water and EtOH (50/50 v/v%). After rinsing the unconjugated dyes 10 times, the NIR scaffolds were pre-wetted with Matrigel (BD Bioscience, Bedford, MA) and implanted into the subcutaneous pocket of flank of nude mice. A control unconjugated scaffold was also transplanted into each mouse.
2.6. Optical imaging system and fluorescence microscopy
The real-time intraoperative Fluorescence-Assisted Resection and Exploration (FLARE™) imaging system has been described in detail previously [26]. Noninvasive optical imaging was performed from the same animal every 2–3 days. In this study, 670 nm excitation was used at a fluence rate of 4 mW cm−2, with white light (400–650 nm) at 40 000 lx. Color and NIR fluorescence images were acquired simultaneously with custom software at rates up to 15 Hz over a 15 cm diameter field of view. A pseudo-colored lime green was used for NIR fluorescence in the color-NIR merged images. The imaging system was positioned at a distance of 18 inches from the surgical field. For microscopic imaging, lymph nodes were extracted from in vivo study, fixed in 10% formalin for 10 min, molded with Tissue-Tek OCT compound (Fisher Scientific, Pittsburgh, PA), and frozen in liquid nitrogen. Frozen sections were cut to a 20 μm thickness, and their structure and degradation pattern were examined by a four-channel fluorescence microscope. The wavelength used for excitation and emission filters was 650 ± 22 nm and 710 ± 25 nm, respectively.
3. Results and discussion
3.1. Synthesis of highly charged cyanine fluorophores
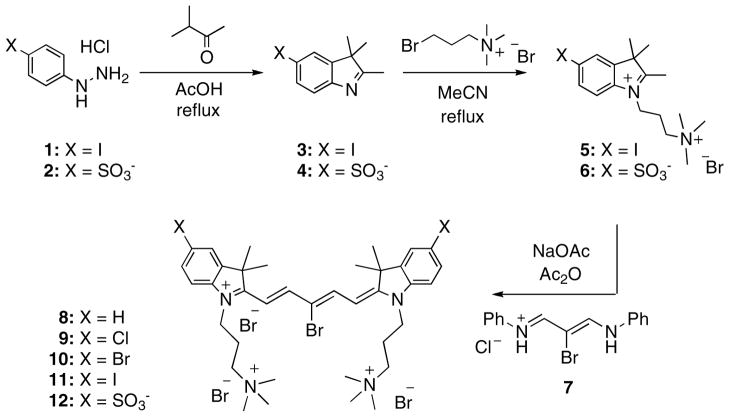
The synthetic route for the preparation of substituted cyanine dyes begins with the acid catalyzed formation of indolenine derivatives 3 and 4 starting with the phenylhydrazine analogs 1 and 2. The indolenine derivatives were then alkylated in boiling acetonitrile using 3-bromopropyltrimethylammonium bromide which afforded the highly charged heterocyclic salts 5 and 6. The individual salt was then used to prepare the pentamethine cyanine dyes 11 and 12 by treatment with sodium acetate and reagent 7 in warm acetic anhydride. Reagent 7 was prepared from the reaction of mucobromic acid with dilute aniline in ethanol, which generated the mono-halogenated compound. The design of this reagent allowed the further nucleophillic replacement of this bromine atom at the meso position for a direct covalent conjugation to the gelatin scaffold. Additional cyanine dyes 8–10, depicted in figure 1, were also synthesized and tested but the detailed methodology and characterization will be published elsewhere (Henary et al).
Figure 1.
Chemical schemes for highly charged cyanine fluorophores.
3.2. Physicochemical and optical properties
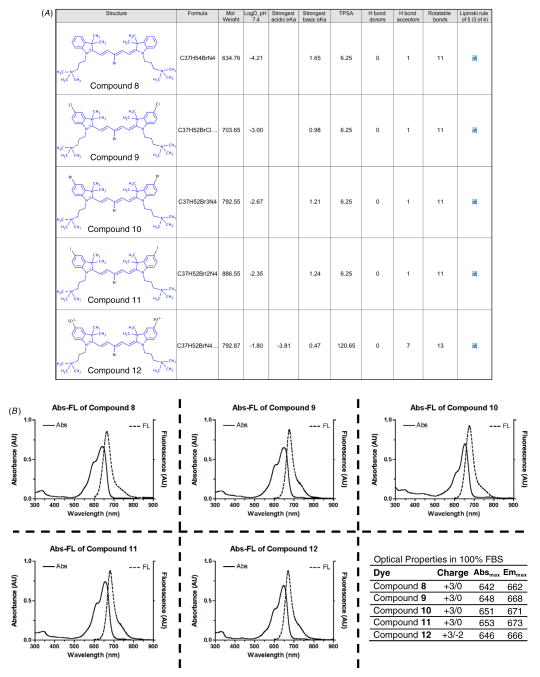
As shown in figure 2(a), physicochemical properties of highly charged NIR fluorophores were calculated by Marvin and JChem software (ChemAxon, Budapest, Hungary) to evaluate a certain pharmacological or biological activity based on Lipinski’s rule of five [22]. The rule describes molecular properties important to its bioavailability in the human body, including their absorption, distribution, metabolism and excretion. Identifying calculable parameters such as logD at pH 7.4, pKa, total polar surface area (TPSA), and H-bond donors/acceptors can be used for estimation of lipophilicity and cell permeability of new NIR fluorophores. The logD values of halogenated compounds 9–11 are in the range of −3 to −2.35, higher than H-compound 8 (−4.21), and lower than sulfonated compound 12 (−1.80). The increased logD values in terms of lipophilicity may promote intracellular accumulations as results of in vitro live cell binding test (data not shown). Although sulfonated compound 12 also has acceptable logD value in accordance with Lipinski’s rule, the TPSA value is close to the 140 Å2, which tend to be poor at permeating cell membranes. In addition, the lower net charge (+1) of sulfonated compound 12 resulted in distinguished molecular properties compared with other halogenated compounds (net charge = +3). Pentamethine cyanine fluorophores have wavelengths of absorption and fluorescence that are a function of the length of a polymethine bridge between two terminal heterocyclic nitrogen atoms [23], and all of the final compounds show maximum absorption and fluorescence emission spectra in 650–700 nm in FBS, and their NIR range optical properties would substantially reduce interfering autofluorescence from the body and increase accuracy in noninvasive monitoring in animals (Figure 2(b). All fluorophores were highly water-soluble and less cytotoxic up to 10 μM concentrations [13], and showed high physicochemical stability (>90%) in 100% FBS in dark conditions (data not shown).
Figure 2.
Physicochemical (A) and optical properties (B) of highly charged NIR fluorophores in FBS, pH 7.4. In silico calculations of physicochemical properties were calculated using Marvin and JChem calculator plugins (ChemAxon, Budapest, Hungary). Each compound after purification was diluted to a concentration of 1–5 μM and subjected to absorbance spectrometry (solid line) and fluorescence spectrometry (dotted line).
3.3. Engineering NIR scaffolds
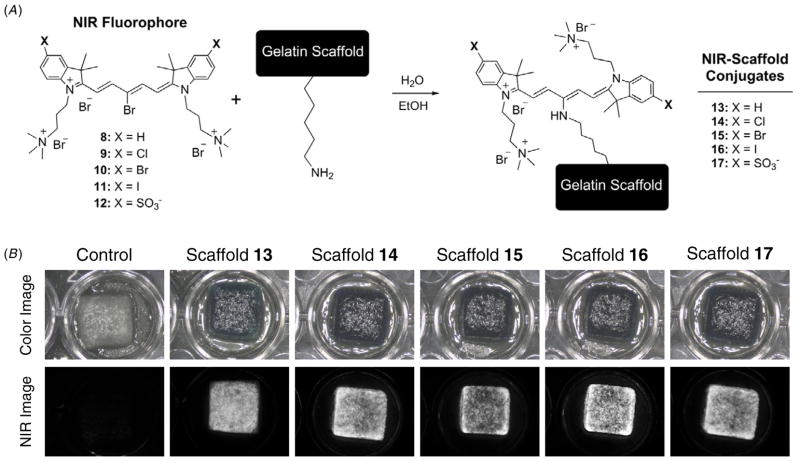
The gelatin-based biodegradable NIR scaffolds were prepared by nucleophilic substitution reactions between the bromine group on the meso-position of the NIR fluorophores and the primary amine groups of gelatin units in water and EtOH (figure 3(a)). To increase the observable fluorescent signal without inducing quenching in each NIR scaffold, the conjugation ratios were tested in various dye concentrations. Fluorescence quenching was observed when the treated dye amount was greater than 50 nmol (theoretical conjugation ratio = 1, dye/gelatin unit), which was calculated by contrast-to-background ratio by measuring NIR fluorescence signals (data not shown). The physical integrity of the NIR scaffolds such as shape and gross morphology was preserved after NIR fluorophore conjugation (figure 3(b)).
Figure 3.
Schematic drawing for engineering NIR scaffolds (A) and gross morphology of NIR scaffolds after extensive washing in water and ethanol (B). All NIR fluorescence images have identical exposure and normalizations.
3.4. Intraoperative optical imaging
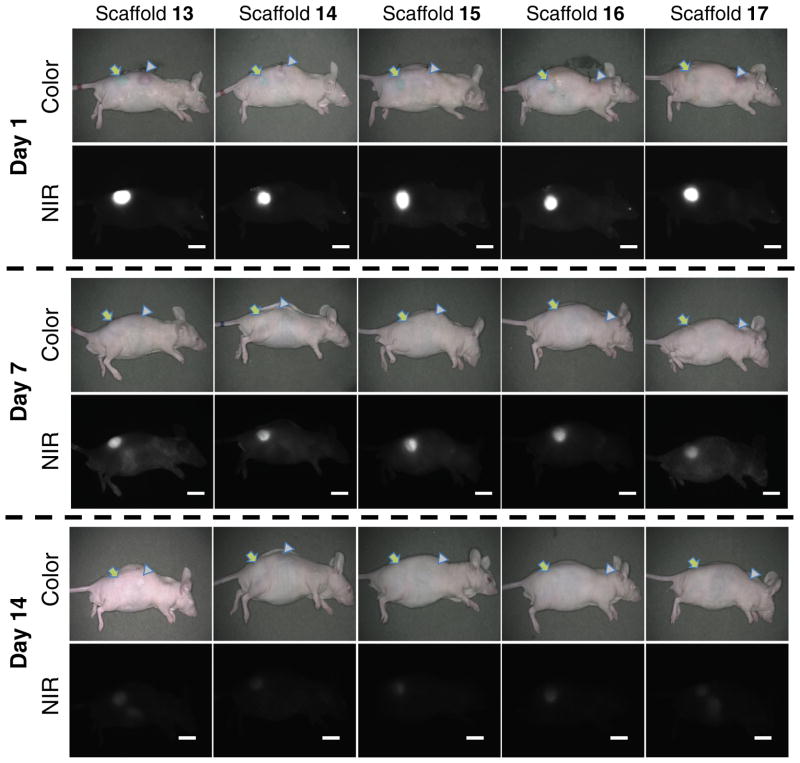
To investigate the effect of local enzyme-mediated biodegradation in the body, NIR scaffolds and non-treated scaffolds as a control group were implanted into the subcutaneous pocket of flank region separately. It is known that mechanical stress around the implanted matrix is a major determining factor for the degree of the foreign body reaction, which stimulates collagenase production and release from fibroblasts, macrophages, and neutrophils [14, 12]. As shown in figure 4, a significant decrease in the NIR fluorescence signal of all scaffolds was observed over the implantation time using the FLARE™ imaging system [16, 6, 7]. The scaffolds began shrinking after implantation, indicating their biodegradation by the subsequent enzymatic cleavage in the body. This result clearly indicates that the NIR fluorescence traces the biodegradability of scaffolds through noninvasive real-time imaging, as the control scaffold monitoring could not be achieved without surgical resection.
Figure 4.
NIR scaffold-implanted nude mice. Images were obtained at day 1, day 7, and day 14 post-implantation. Arrows = NIR scaffolds; arrowheads = control scaffolds; scale bars = 1 cm. All NIR fluorescence images have identical exposure and normalizations.
3.5. Biodistribution and clearance of NIR scaffolds
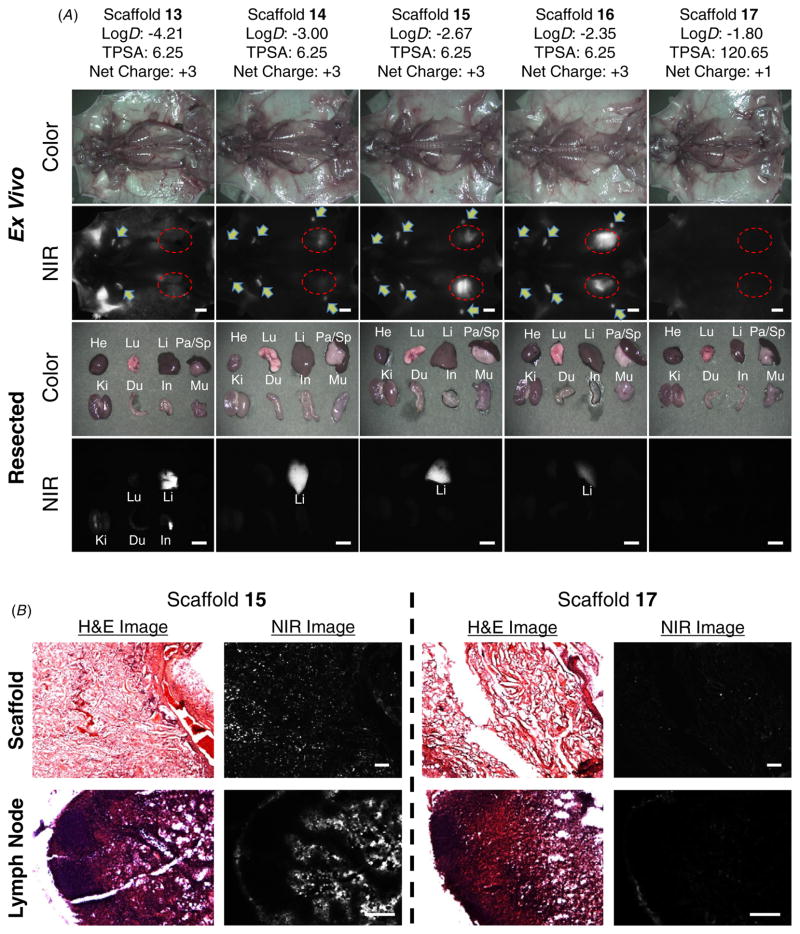
To determine the biodistribution and clearance of degraded gelatin units in the body, whole-body optical imaging was performed using the FLARE™ intraoperative imaging system [4, 11]. As shown in figure 5(a), the biodegraded fragments of halogenated gelatin scaffolds 14–16 were found mainly in the liver and lymph nodes, whereas the fractions of scaffold 17 were excreted in the kidneys and bladder. Non-halogenated gelatin scaffold 13 was found in the background tissues such as muscle, skin and adipose, and most of the hepatobiliary excretion route. The NIR fluorescence signal decreased over the period of implantation time, and only a small amount of signal was observed in the animal sacrificed 2 weeks post-implantation. This result demonstrates that the NIR scaffold could be removed completely from the body after it has performed its mechanical supporter function during tissue ingrowth. The resected organs and scaffolds after imaging were observed through a NIR fluorescence microscope after staining with hematoxylin and eosin (H&E). Figure 5(b) shows tissue cross-sections of the scaffold area extracted 14 days post-implantation, and there were proliferating fibroblasts in granulation tissue. This result proves that the host tissue had penetrated and deposited into the scaffold and consequentially induced scaffold degradation. Additionally, the lymph nodes region resected from the scaffold 15 implanted mouse showed the NIR fluorescence signal around lymph nodes, while the lymph node from the scaffold 17 implanted mouse did not show any NIR fluorescence signal over the entire resected area [8].
Figure 5.
(A) Biodistribution and clearance of NIR fluorophore-conjugated scaffolds at day 14 post-implantation. Abbreviations used are: Du, duodenum; He, heart; In, intestine; Ki, kidneys; Li, liver; Lu, lungs; Mu, muscle; Pa, pancreas; and Sp, spleen. Arrows = lymph nodes; red dotted circles = scaffolds; scale bars = 1 cm. (B) H&E and NIR imaging of resected scaffolds and lymph nodes from (A). Scale bars = 200 μm. All NIR fluorescence images have identical exposure times and normalization.
4. Conclusions
In this study, we designed several meso-brominated NIR-fluoresce pentamethine cyanine fluorophores and conjugated them to biodegradable scaffolds. We successfully tracked the biodegradation pattern and rate of implanted NIR-scaffolds using real-time noninvasive imaging. Physicochemical properties of the fluorophore such as net charge, lipophilicity and polar surface area are the key factors in determining the fate of degraded scaffold fragments in the body. These bioconjugatable NIR fluorophores would be useful not only for tracking scaffold degradation but also for targeting regional lymph nodes and specific organs in long-term survival.
Acknowledgments
We thank Dr John V Frangioni (BIDMC) for many helpful discussions and Alex Allardyce (ChemAxon, Budapest, Hungary) for technical support. We also thank David Burrington, Jr, for editing, and Eugenia Trabucchi for administrative assistance. This study was supported by the Georgia Cancer Coalition grant and the National Institutes of Health (NIBIB) grants R01-EB-010022 (HSC) and R01-EB-011523 (HSC), and the Dana Foundation Program in Brain and Immuno-Imaging (HSC). EAO was supported through internal Funds from RIG and Mentor Grants at GSU and SHK was from the WCU Program (R31-20029) from the Korea Ministry of Education, Science and Technology (KMEST). MH appreciates the Georgia State University Center for Diagnostics and Therapeutics (CDT) for their support.
Contributor Information
Gilson Khang, Email: gskhang@jbnu.ac.kr.
Maged Henary, Email: mhenary1@gsu.edu.
Hak Soo Choi, Email: hchoi@bidmc.harvard.edu.
References
- 1.Artzi N, Oliva N, Puron C, Shitreet S, Artzi S, Bon Ramos A, Groothuis A, Sahagian G, Edelman ER. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nature Mater. 2011;10:704–9. doi: 10.1038/nmat3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashitate Y, Kim SH, Tanaka E, Henary M, Choi HS, Frangioni JV, Flaumenhaft R. Two-wavelength near-infrared fluorescence for the quantitation of drug antiplatelet effects in large animal model systems. J Vasc Surg. 2012;56:171–80. doi: 10.1016/j.jvs.2011.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chachques JC. Cellular cardiac regenerative therapy in which patients? Expert Rev Cardiovasc Ther. 2009;7:911–9. doi: 10.1586/erc.09.84. [DOI] [PubMed] [Google Scholar]
- 4.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imaging. 2010;9:291–310. [PMC free article] [PubMed] [Google Scholar]
- 5.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009;9:2354–9. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Design considerations for tumour-targeted nanoparticles. Nature Nanotechnol. 2010;5:42–7. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nature Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HS, et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem, Int Ed Engl. 2011;50:6258–63. doi: 10.1002/anie.201102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. New Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–55. [PMC free article] [PubMed] [Google Scholar]
- 12.Gross J, Harper E, Harris ED, McCroskery PA, Highberger JH, Corbett C, Kang AH. Animal collagenases: specificity of action, and structures of the substrate cleavage site. Biochem Biophys Res Commun. 1974;61:605–12. doi: 10.1016/0006-291x(74)91000-6. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Burgess K. Fluorescent indicators for intracellular pH. Chem Rev. 2010;110:2709–28. doi: 10.1021/cr900249z. [DOI] [PubMed] [Google Scholar]
- 14.Hilborn J, Bjursten LM. A new and evolving paradigm for biocompatibility. J Tissue Eng Regen Med. 2007;1:110–9. doi: 10.1002/term.4. [DOI] [PubMed] [Google Scholar]
- 15.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 16.Hyun H, et al. cGMP-Compatible preparative scale synthesis of near-infrared fluorophores. Contrast Media Mol Imaging. 2012;7:516–24. doi: 10.1002/cmmi.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki N, et al. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: a evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules. 2004;5:828–33. doi: 10.1021/bm0400067. [DOI] [PubMed] [Google Scholar]
- 18.Kempen DH, Yaszemski MJ, Heijink A, Hefferan TE, Creemers LB, Britson J, Maran A, Classic KL, Dhert WJ, Lu L. Non-invasive monitoring of BMP-2 retention and bone formation in composites for bone tissue engineering using SPECT/CT and scintillation probes. J Control Release. 2009;134:169–76. doi: 10.1016/j.jconrel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011;32:8771–82. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Jeong CG, Hollister SJ. Non-invasive monitoring of tissue scaffold degradation using ultrasound elasticity imaging. Acta Biomater. 2008;4:783–90. doi: 10.1016/j.actbio.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko IK, Song HT, Cho EJ, Lee ES, Huh YM, Suh JS. In vivo MR imaging of tissue-engineered human mesenchymal stem cells transplanted to mouse: a preliminary study. Ann Biomed Eng. 2007;35:101–8. doi: 10.1007/s10439-006-9204-7. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 23.Peng X, et al. Fluorescence ratiometry and fluorescence lifetime imaging: using a single molecular sensor for dual mode imaging of cellular viscosity. J Am Chem Soc. 2011;133:6626–35. doi: 10.1021/ja1104014. [DOI] [PubMed] [Google Scholar]
- 24.Stroncek D, et al. Developments in clinical cell therapy. Cytotherapy. 2010;12:425–8. doi: 10.3109/14653240903511952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung H-J, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25:5735–42. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 26.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, Ngo L, Khamene A, Azar F, Frangioni JV. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–52. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25:5821–30. doi: 10.1016/j.biomaterials.2004.01.038. [DOI] [PubMed] [Google Scholar]