Abstract
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a transgenerational prospective observational study investigating influences on health and development across the life course. It considers multiple genetic, epigenetic, biological, psychological, social and other environmental exposures in relation to a similarly diverse range of health, social and developmental outcomes. Recruitment sought to enrol pregnant women in the Bristol area of the UK during 1990–92; this was extended to include additional children eligible using the original enrolment definition up to the age of 18 years. The children from 14 541 pregnancies were recruited in 1990–92, increasing to 15 247 pregnancies by the age of 18 years. This cohort profile describes the index children of these pregnancies. Follow-up includes 59 questionnaires (4 weeks–18 years of age) and 9 clinical assessment visits (7–17 years of age). The resource comprises a wide range of phenotypic and environmental measures in addition to biological samples, genetic (DNA on 11 343 children, genome-wide data on 8365 children, complete genome sequencing on 2000 children) and epigenetic (methylation sampling on 1000 children) information and linkage to health and administrative records. Data access is described in this article and is currently set up as a supported access resource. To date, over 700 peer-reviewed articles have been published using ALSPAC data.
Why was the cohort set up?
The Avon Longitudinal Study of Parents and Children, known to its participants as ‘Children of the 90s’ (Figure 1), originated in a WHO Europe sponsored meeting held in Moscow in 1985 recommending the establishment of birth cohort studies across Europe to investigate modifiable influences on child health and development. Jean Golding subsequently designed the methodology1 for the European Longitudinal Study of Pregnancy and Childhood (ELSPAC),2 a pan-European series of longitudinal birth cohorts. The Avon Longitudinal Study of Parents and Children, centred on the city of Bristol in the South West of England, was one of these cohorts. The ALSPAC acronym originally denoted the ‘Avon Longitudinal Study of Pregnancy and Childhood’ reflecting the original study focus. As follow-up beyond early childhood was developed, ALSPAC was renamed the ‘Avon Longitudinal Study of “Parents and Children” ’ to reflect the continuing importance of the parents as well as the children.
Figure 1.

The life course of the ALSPAC Study Logo
WHO Europe provided seed funding to develop the common ELSPAC methodology and to undertake pilots of the questionnaires in the UK, Russia and Greece. Subsequently, early funding for ALSPAC was obtained from various sources, including UK Government Departments, the UK Medical Research Council (MRC) and various charities such as the Wellcome Trust, British Heart Foundation, British Lung Foundation and Action Research among many others. North American funding was also obtained, including from several of the National Institutes of Health, and the March of Dimes. The University of Bristol has provided continual support for ALSPAC from soon after the onset of the study; since 2000, this support has been augmented by substantial strategic funding jointly from the Wellcome Trust and the MRC. Additional support for ALSPAC has come through programme and project grant funding from many sources,3 and is listed along with other study information on the ALSPAC website—http://www.bristol.ac.uk/alspac/.
This cohort profile describes recruitment and the first 18 years of follow-up of the ‘Children of the 90s’, i.e. the children of the mothers eligible for recruitment during 1990–92. Recruitment and follow-up of the mothers is described in a companion article.4 Participants are now in early adulthood; however, the intention is for follow-up to be lifelong. The transgenerational and family-based nature of ALSPAC is being consolidated through the ALSPAC ‘Focus on Mothers’ mothers study, the ‘Focus on Fathers’ fathers study and follow-up of the next ALSPAC generation (ALSPAC-G2 study) through ‘COCO90s’ (Children of Children of the 90s). These initiatives are not described further here.
Who is in the cohort?
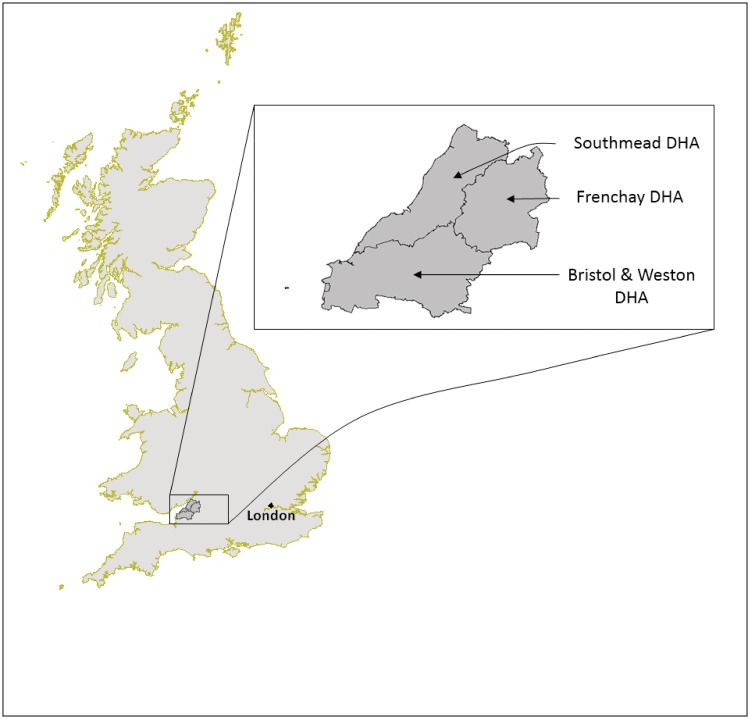
All pregnant women residents in that part of the old administrative county of Avon comprising the three Health Districts shown in Figure 2 were eligible to participate in ALSPAC if their estimated delivery date (EDD) fell between 1 April 1991 and 31 December 1992 inclusive. Any resulting child from these pregnancies is considered eligible. The catchment area covered the three health administration districts within the South-West Regional Health Authority that became the ‘Bristol & District Health Authority’. This area (1991 total population ∼0.9 million) includes the City of Bristol (1991 population ∼0.5 million) and surrounding urban and rural areas, including towns, villages and farming communities; but excludes the area of Avon around the City of Bath. Pregnant women migrating into the catchment area were eligible up to the point of delivery; pregnant women originally resident in Avon but migrating out of the catchment area prior to delivery were excluded unless they had completed the questionnaire scheduled for the third trimester of pregnancy. A small number of women and their children falling outside the above strict definition of eligibility contributed data and have been included in ALSPAC. This is discussed below.
Figure 2.
The ALSPAC Eligible Study Area; the study area within the UK and details illustrating the three eligible NHS District Health Authorities (DHAs). © Crown Copyright/database right 2011. An Ordnance Survey/EDINA supplied service
ALSPAC recruitment campaign
Recruitment to ALSPAC was opportunistic and aimed to recruit women as early in pregnancy as possible. ALSPAC attempted to make contact with eligible women through media information encouraging study contact and recruitment staff visiting community locations. In parallel, routine antenatal and maternity health services were used to promote the study and distribute an ‘expression of interest’ card. Through returning this card, women were able to request further information on the study or to decline participation. Completed cards contained sufficient detail (address and EDD) to allow ALSPAC staff to determine eligibility. Women requesting further information were sent a study information booklet followed by an initial questionnaire ∼1 week later. Invitation cards indicated that study consent was ‘opt out’, i.e. women not actively declining participation would be included in future data collection follow-up.
ALSPAC eligible sample
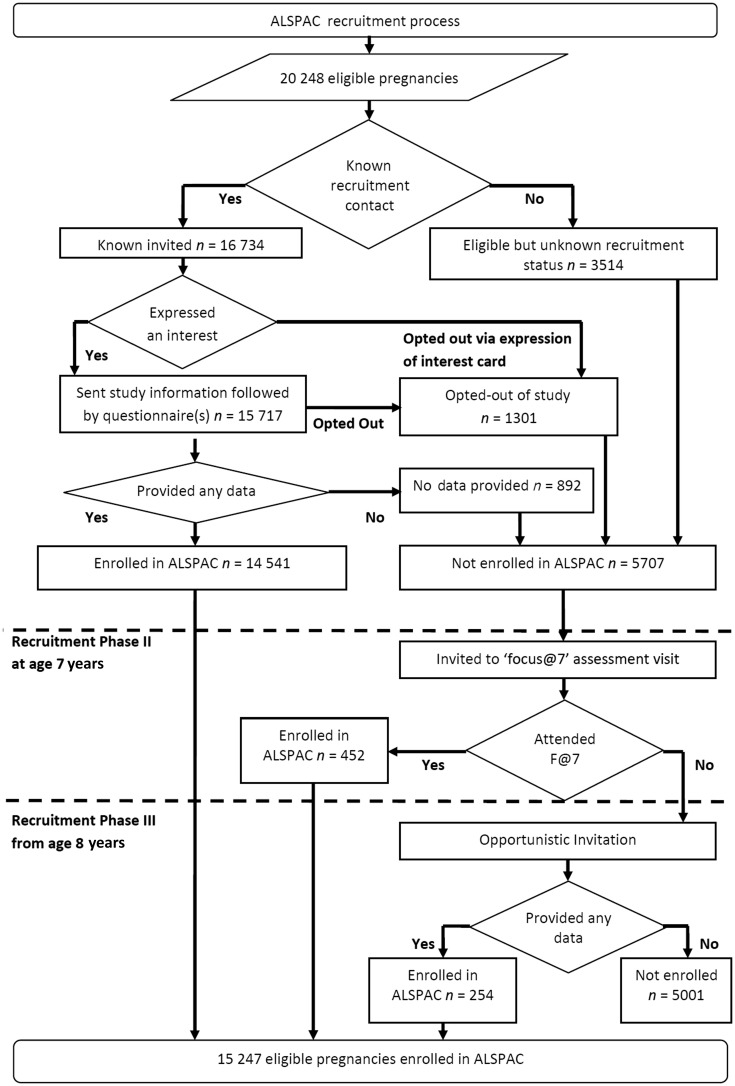
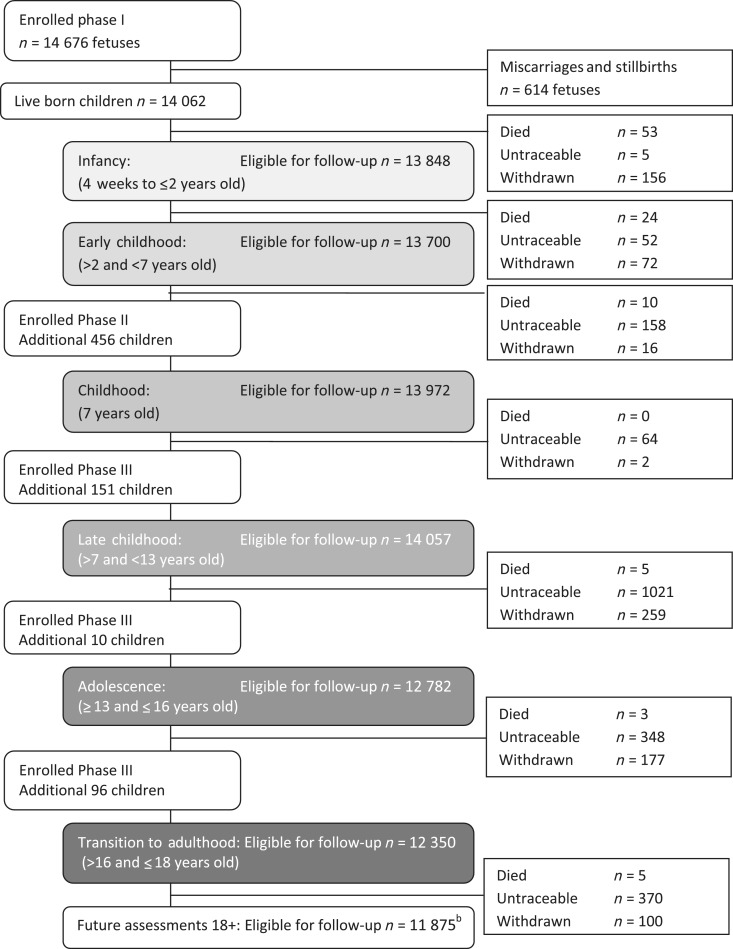
During the 1990–92 recruitment campaign, the ALSPAC target sample was a dynamic population for whom no convenient sampling frame to support systematic invitation of all eligible individuals was available. The eligible study sample has been defined retrospectively, based on ALSPAC recruitment records and maternity, birth and child health records.5 Comparing these health records with ALSPAC study records, we are able to describe study recruitment (Figure 3). Recruitment was not complete, which is unsurprising given its opportunistic nature. It is impossible to fully ascertain retrospectively where non-recruitment represents failure to invite as opposed to active non-response. Records of the returned expression of interest cards and the proportion of these women declining participation are known (Figure 3); however, the number of invitation cards distributed is unknown. The methods used in retrospectively defining the cohort allowed the use of more accurate geocoding resources, unavailable to recruiters in the early 1990s. These resources identified 229 pregnancies (233 children), living on the geographical periphery of the study area, that were incorrectly assumed to be eligible in 1990–92. As these individuals enrolled and contributed data, we have continued to include them in both the ‘eligible sample’ and the ‘enrolled sample’; as such they are eligible for continuing follow-up and are included in all figures provided in this article.
Figure 3.
The ALSPAC enrolment campaign flow diagram
Recruitment outwith the period 1990–92
The substantial majority (82.6%) of women are known to have been invited to enrol during the 1990–92 recruitment campaign. Subsequent work to define the ‘eligible sample’ identified additional pregnancies where the offspring were eligible for recruitment, but no replies to recruitment in 1990–92 had been received (either positive or negative). With funding to complete a ‘Focus@7’ follow-up assessment of all of the participants at 7 years of age, the opportunity was taken to attempt to recruit all known eligible children who would have fitted the original eligibility criteria, excluding those who had previously refused enrolment. Invitations describing the study were sent to this group, inviting the mothers to enrol (recruitment Phase II in Figure 3). In addition to this systematic recruitment, ALSPAC made subsequent opportunistic contact with additional families who were ‘eligible’ but not ‘enrolled’ (recruitment Phase III in Figure 3). Contact was initiated by eligible families seeking enrolment or during ALSPAC community outreach and promotion activities. Due to the nature of these recruitment methods, it is not possible to determine how many individuals were ‘invited’ during recruitment Phases II and III. Where recruitment occurred in Phases II and III, ALSPAC have not been able to collect the pregnancy, infancy and early childhood data that were collected from parental self-completed questionnaires from the sample recruited during pregnancy (Phase I).
ALSPAC recruitment rates
The ‘eligible sample’ comprises 20 248 pregnancies. The mothers of 14 541 (71.8%) pregnancies were recruited antenatally during 1990–92 (recruitment Phase I). Of these 14 541 pregnancies, 68 have no known birth outcome; of the remaining 14 472 pregnancies, 195 were twin, 3 were triplet and 1 was quadruplet, meaning that there are 14 676 known foetuses. These pregnancies resulted in 14 062 live-born children of whom 13 988 were alive at 1 year of age. Post-natal recruitment to the ‘Focus@7’ clinical assessment at the age of ∼7 years recruited a further 456 children from 452 (2.2% of eligible) pregnancies (Phase II). Recruitment during Phase III (ages 8–18 years) added a further 257 children from 254 (1.2% of eligible) pregnancies, giving an overall total of 15 247 (75.3% of eligible) enrolled pregnancies; from these pregnancies there were 14 775 live-born children of which 14 701 were alive at one year of age (Table 1).
Table 1.
ALSPAC eligible sample pregnancy size and birth outcomes
| ALSPAC enrolled pregnancies n = 15 247 | Non-enrolled pregnancies n = 5001 | |
|---|---|---|
| ALSPAC eligible sample n = 20 248 pregnancies | ||
| Pregnancy size n (%) | ||
| Singletons | 14 971 (98.2)a | 4909 (98.2)b |
| Sets of Twins | 204 (1.3)a | 43 (0.9)b |
| Sets of Triplets | 3 (<0.1)a | 1 (<0.1)b |
| Sets of Quads | 1 (<0.1)a | 0 (<0.1)b |
| No known birth outcome | 68 (0.4)a | 48 (1.0)b |
| ALSPAC eligible sample n = 20 390 children/foetuses | ||
| Outcomes (numbers of foetuses/offspring) n (%) | ||
| Foetal loss <20 weeks | 547 (3.6)c | 138 (2.8)d |
| Foetal death/sb 20+ weeks | 68 (0.4)c | 37 (0.7)d |
| Neonatal death <7 days | 45 (0.3)c | 11 (0.2)d |
| Neonatal death 7–27 days | 8 (<0.1)c | 2 (<0.1)d |
| Post-neonatal death 28 days–1 year | 21 (0.1)c | 15 (0.3)d |
| Alive > 1 year | 14 701 (95.5)c | 4797 (96.0)d |
| Total | 15 390e (100)c | 5000e (100)d |
aPercentage of 15 247 enrolled pregnancies.
bPercentage of 5001 non-enrolled pregnancies.
cPercentage of 15 390 enrolled children (foetal).
dPercentage of 5000 non-enrolled children (foetal).
eThe sample contains two twin pregnancies where only one child enrolled (recruitment phases II and III). These are reported as an enrolled twin pregnancy with birth outcomes of a single enrolled and a single non-enrolled child.
The birth outcomes of the 20 248 eligible pregnancies are detailed in Table 1; this information is based on ALSPAC recruitment records and maternity, birth and child health records. The full extent of miscarriage (foetal loss prior to 20 weeks of gestation) is likely to be under-represented in both the 1990–92 ALSPAC recruitment records and the records used to identify the eligible sample. Of the 20 390 known foetuses, the birth outcomes include 19 600 (96.1%) live births, 685 (3.4%) miscarriages and 105 (0.5%) stillbirths (defined as foetal loss after 20 weeks of gestation and stillborn deliveries). There are an additional 116 pregnancies with no known birth outcome. The ‘Children of the 90s’ ‘enrolled sample’ consists of 14 775 (75.7% of the 19 600 eligible live births) live-born children from the 15 247 pregnancies (15 458 foetuses) where an individual, either the mother/primary caregiver or the child, has provided data. Of these enrolled participants, ALSPAC has collected data relating directly to 14 009 (71.5% of 19 600 eligible live births) children, through self-reported data from the child or data provided about the child by the biological mother/primary caregiver. The ‘eligible sample’ remains eligible regardless of their participation history or movement away from the study area (questionnaires and invitation to clinical assessments are sent worldwide); if participants withdraw from the study, they remain eligible and are free to re-enrol into the study if they wish (32 families re-joined the study in this way by the age of 18 years).
External validity and possible participation biases
The regional basis of ALSPAC has brought advantages both in logistics and in the creation and maintenance of a study identity; however, these advantages may have been offset by limitations in external validity when generalizing ALSPAC findings to the national population. Moreover, as recruitment of eligible mothers to ALSPAC was incomplete, it is possible that systematic differences between those recruited and those not recruited may introduce bias to subsequent estimates based on participants.
In the companion article based on ALSPAC mothers, comparisons of socio-economic characteristics between the original recruited ALSPAC mothers, mothers of a child of similar age living in Avon at the time of ALSPAC initiation and mothers living anywhere in the UK are shown.4 Here we present comparisons of demographic and standard school assessment data, typically taken at 16 years of age, between a national sample, the ALSPAC ‘enrolled sample’ and individuals with different enrolment, participation and study attrition histories (Tables 2–5). The data are taken from the National Pupil Database (NPD) ‘Key Stage 4’ (KS4) dataset, recording pupil census and assessment data for all pupils in English schools. Of these, 14 878 ‘eligible’ children have been linked to a subset of the NPD KS4 records that relate to government-maintained establishments (GMEs). As such, this offers the best available, yet not complete, comparison group (see footnotes in Table 2).
Table 2.
A comparison of academic attainment between the national NPDa sample and ALSPAC children who have enrolled, have recently participated or are lost to follow-up
| ALSPAC enrolment and current participation status |
||||||
|---|---|---|---|---|---|---|
| Comparative indicator | National NPD KS4 GMEa sampleb | Enrolled in ALSPACc | No recent participation | Recently participatedd | Eligible for future follow-up | Lost to attrition |
| Academic attainmentat the age of 16 yearse | ||||||
| n | 1 759 174 | 11 008 | 5473 | 5535 | 9452 | 1556 |
| Mean score | 308 | 317 | 287 | 347 | 324 | 278 |
| IQR (25–75) | 266–374 | 280–380 | 242–350 | 314–398 | 290–380 | 224–350 |
aNPD KS4 GME: The National Pupil Database (NPD) ‘Key Stage 4’ (KS4) dataset records, pupil census and assessment data for all pupils in English schools at the mean age of 16 years. ALSPAC has linked 14 878 ‘eligible’ children to a subset of the NPD KS4 records that relate to government-maintained establishments (GME). This NPD KS4 GME sample has a 89.5% coverage of all English pupils nationally and 84.3% regionally (the area including and surrounding the cities of Bristol and Bath). The NPD GME excludes privately funded and specialist care establishments and as such offers the best available, yet not complete, comparison group. The majority of the remaining ALSPAC ‘eligible sample’ whom we have not linked to the NPD KS4 GME are thought to live outside England or relate to individuals where there is insufficient information to establish accurate linkage.
bAll pupils, excluding those in ALSPAC, from English GMEs who sat their KS4 assessments during the same academic years as the ALSPAC cohort (academic years 2007–09).
cAll pupils, from English GMEs, who are from families that have enrolled in ALSPAC by completing an ALSPAC questionnaire or clinical assessment.
dAll pupils, from English GMEs, who completed an ALSPAC assessment in the ‘transition to adulthood’ phase (completed a ‘CCS’ or ‘CCXC’ questionnaire or attended the TF17 Focus clinical assessment).
eAcademic attainment at the age of 16 years (total points score for the pupils eight highest scoring assessments).
Table 3.
A comparison of socio-demographic characteristics between the National NPD sample and children who have completed any ALSPAC questionnaire or clinical assessment
| National NPD KS4 GME samplea | Enrolled in ALSPACb | Odds ratio |
|||
|---|---|---|---|---|---|
| Characteristic | Category | n/n (%) | n/n (%) | OR (95% CI) | P > chi2‡ |
| Child sex | Female | 871 375/1 770 654 (49.21) | 5470/11 008 (49.69) | 1.02 (0.98–1.06) | 0.313 |
| Child ethnicity | White | 1 508 926/1 744 429 (86.50) | 10 505/10 933 (96.09) | 3.85 (3.50–4.24) | <0.001 |
| Low household income | FSMc | 218 033/1 745 353 (12.49) | 682/10 959 (6.22) | 0.46 (0.43–0.50) | <0.001 |
aAll pupils, excluding those in ALSPAC, from English GMEs who sat their KS4 assessments during the same academic years as the ALSPAC cohort (academic years 2007–09).
bAll pupils, from English GMEs, who are from families that have enrolled in ALSPAC by completing an ALSPAC questionnaire or clinical assessment.
cEligible for ‘free school meals’ (FSM), indicating a joint parental income of ≤£16 000.7 ‡Chi-squared test for homogeneity (equal odds).
Table 4.
A comparison of socio-demographic characteristics between those children who did and did not participate in the 16–18-year data collection assessments
| Enrolled in ALSPACa | Recently participated | Odds ratio |
|||
|---|---|---|---|---|---|
| Characteristic | Category | n/n (%) | n/n (%) | OR (95% CI) | P > chi2‡ |
| Child sex | Female | 5470/11 008 (49.69) | 3181/5535 (57.47) | 1.88 (1.74–2.03) | <0.001 |
| Child ethnicity | White | 10 505/10 933 (96.09) | 5322/5508 (96.62) | 1.34 (1.10–1.62) | 0.004 |
| Low household income | FSMb | 682/10 959 (6.22) | 240/5519 (4.35) | 0.51 (0.44–0.60) | <0.001 |
aAll pupils, from English GMEs, who are from families that have enrolled in ALSPAC by completing an ALSPAC questionnaire or clinical assessment.
bEligible for ‘free school meals’ (FSM), indicating a joint parental income of ≤£16 000.7 ‡Chi-squared test for homogeneity (equal odds).
Table 5.
A comparison of socio-demographic characteristics between those children lost to attrition and those who remain eligible for future follow-up
| Enrolled in ALSPACa | Ineligible for future follow-up due to study attrition | Odds ratio |
|||
|---|---|---|---|---|---|
| Characteristic | Category | n/n (%) | n/n (%) | OR (95% CI) | P > chi2‡ |
| Child sex | Female | 5 470/11 008 (49.69) | 726/1556 (46.66) | 0.86 (0.77–0.96) | 0.010 |
| Child ethnicity | White | 10 505/10 933 (96.09) | 1473/1545 (95.34) | 0.81 (0.62–1.05) | 0.103 |
| Low household income | FSMb | 682/10 959 (6.22) | 177/1544 (11.46) | 2.28 (1.91–2.74) | <0.001 |
aAll pupils, from English GMEs, who are from families that have enrolled in ALSPAC by completing an ALSPAC questionnaire or clinical assessment.
bEligible for ‘free school meals’ (FSM), indicating a joint parental income of ≤£16 000.7 ‡Chi-squared test for homogeneity (equal odds).
Comparisons (Table 2) show that those children in the ALSPAC ‘enrolled sample’ have a higher educational attainment at the age of 16 years than the NPD KS4 GME national sample, a difference that is not present when comparing the ALSPAC ‘eligible sample’ (mean attainment score of 309, data not shown) with the NPD KS4 GME national sample. This difference in mean attainment increases with increasing completeness of participation in ALSPAC; conversely children who have not recently participated or are lost to follow-up through study attrition have a lower educational attainment than the national average. The ALSPAC ‘enrolled sample’ (Table 3) are more likely to be White and less likely to be eligible for free school meals than the NPD KS4 GME national sample. Recent responders (Table 4) are more likely to be female, White and less likely to be eligible for free school meals, whereas those lost to attrition (Table 5) are more likely to be male and eligible for free school meals.
To an extent the differences in ethnic composition identified in the comparison between the ALSPAC ‘enrolled sample’ and the national NPD KS4 GME sample can be attributed to regional differences and demographic changes within the UK since the birth of the cohort. The 1991 census data report that 4.1% of mothers with infants <1 year of age resident in Avon, described themselves as ‘non-White’, compared with 7.6% of mothers across the whole of Britain. Nationally, 14.0% of pupils in the NPD KS4 GME data are described as non-White compared with 8.0% of pupils regionally. In economic terms, the South West of England, which incorporates the ALSPAC catchment area, has a similar gross disposable household income per head (£13 300) to the English average (£13 500). This, however, masks regional differences; using the same measure, the South West ranks fourth highest of the 12 UK regions.6
Although these data are accurate at the time of writing ALSPACs, initiatives to address incomplete participation, described later in the article, are intended to address these biases and may result in changes in these results.
How often have they been followed up?
Assessments have been administered frequently, with 68 data collection time points between birth and 18 years of age (Supplementary Table S1, available as Supplementary data at IJE online). These include 34 child-completed questionnaires (CCQs, 25 including multiple domains and 9 focusing on pubertal development), 9 ‘focus’ clinical assessments and 25 questionnaires about the child completed by the mother or other main caregiver (MCQs). To aid the clarity of our reporting, we have retrospectively allocated each data collection into six phases (Supplementary Table S1a–f, available as Supplementary data at IJE online); ‘infancy’ (≥4 weeks and ≤2 years of age), ‘early childhood’ (>2 years and <7 years), ‘childhood’ (7 years of age), ‘late childhood’ (>7 years and <13 years), ‘adolescence’ (≥13 years and <16 years) and ‘transition to adulthood’ (>16 years and ≤18 years). The ‘infancy’ phase included four MCQs and a subsample, selected from the past 6 months of ALSPAC births, who were invited to the first four ‘Children in Focus’ (CiF) study assessment clinics. ‘Early childhood’ included 11 MCQs, 6 CCQs and the CiF subsample who were invited to a further 6 clinical assessments. ‘Childhood’ included the first focus (‘Focus@7’) study assessment clinic and one CCQ. ‘Late childhood’ included six MCQs, nine CCQs and four ‘Focus’ clinics. ‘Adolescence’ included three MCQs, seven CCQs and two ‘Focus’ clinics and finally ‘transition to adulthood’ included one MCQ, two CCQs and the latest clinic assessment visit (‘Focus@17’). The enrolled sample was increased in ‘childhood’ through recruitment Phase II, and recruitment Phase III ran from ‘late childhood’ through ‘transition to adulthood’ inclusive.
Additional follow-up of the ‘eligible sample’ has been made through school-administered questionnaires and assessments completed by the child’s teacher. There have been numerous subpopulation studies using quantitative and qualitative methodologies that are described later in this article. The ‘eligible sample’ have been linked to National Health Service (NHS) death and cancer registries (99% linkage match rate) and education attainment and school census data (82% linkage match rate, reflecting that only English GME schools are available; this excludes private education that has a 15.7% coverage locally). Both the participant's home and school addresses have been geocoded to a national reference table,8 which allows onward linkage to neighbourhood socio-demographic and environmental exposure indicators.
What is attrition like?
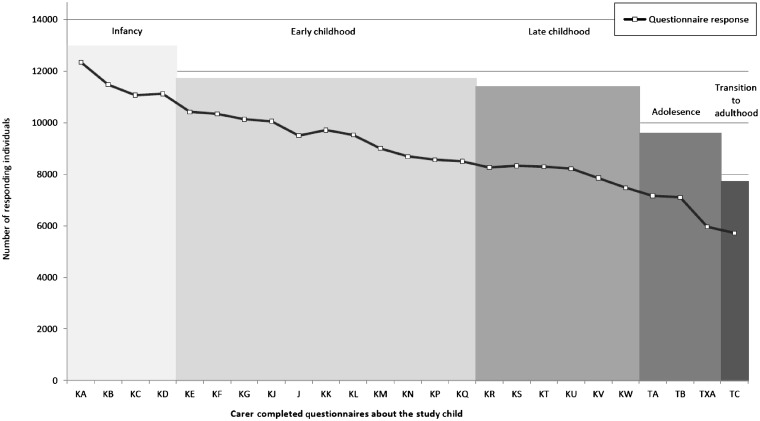
Response rates to each data collection are summarized in Supplementary Table S1 (available as Supplementary data at IJE online) and Figures 4 and 5. Attrition rates were at their greatest when the child was in infancy and are increasing again as the children enter adulthood. The response rates suggest selective participation among respondents with a larger group of ‘active’ participants than the response to any single data collection would suggest; e.g. the average response rate to the 12 measures during the ‘adolescence’ phase is 6155 (48.2% of the 12 776 eligible for follow-up during this phase); however, 9600 (75% of 12 776) individuals responded at least once during this phase (Supplementary Table S1, available as Supplementary data at IJE online).
Figure 4.
Response rates to mother/primary carer completed questionnaires about the child (assessments from 4 weeks old to 18 years of age)
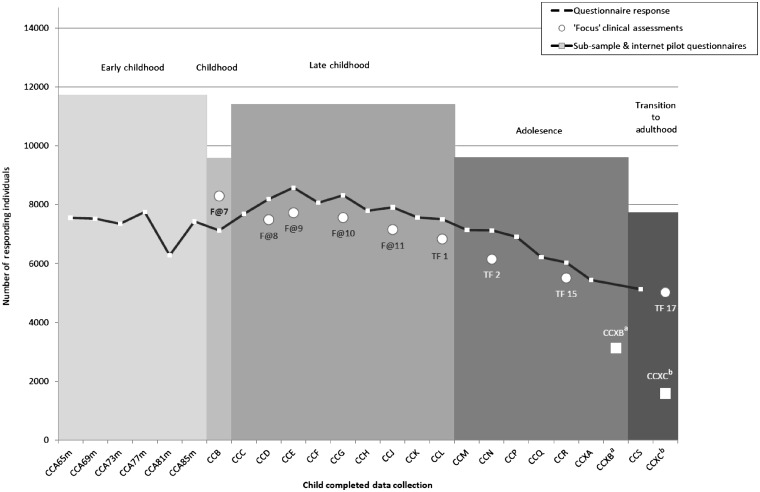
Figure 5.
Response rates to child study clinical assessments (excluding CiF sub-sample clinics) and self-completed questionnaires (assessments from 65 months to 18 years). aCCXB questionnaire was administered to a subsample of the cohort. bCCXC questionnaire piloted a new internet-based data collection and did not receive the normal reminder follow-up procedure
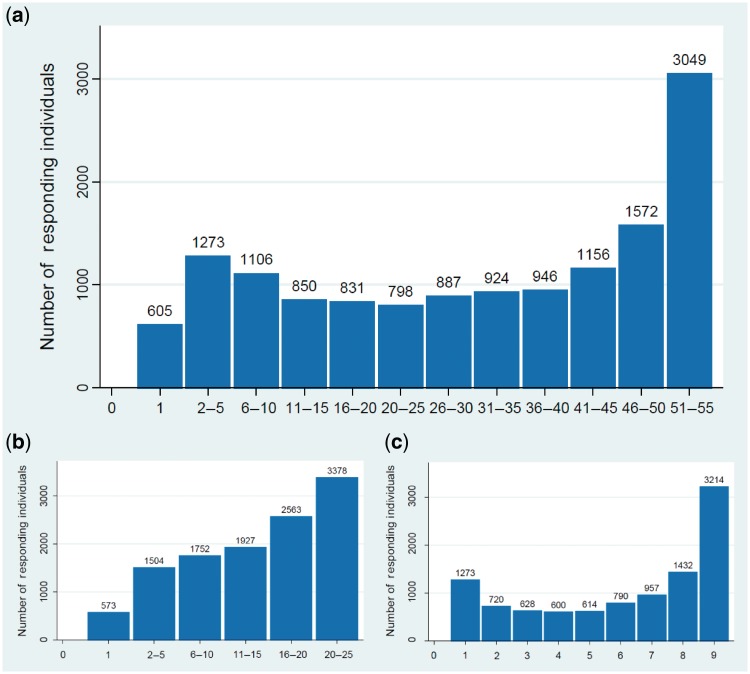
A defining feature of the ALSPAC data set is the breadth of repeat measures taken at frequent intervals across the life course. Loss to follow-up caused by permanent attrition to the study (Figure 6) reduces the proportion of the sample who are eligible for follow-up at each time point. In addition, the fluctuations in response described previously decreases the participating sample that consistently responds (Figure 7). While these factors impact on the availability of repeat measures across all time points, a core sub-sample of over 3000 families have responded to all the 55 assessments open to the ‘full’ sample (defined in the footnotes to Figure 7) and 5777 have responded to 75% or more of these assessments (≥41 individual assessments).
Figure 6.
ALSPAC Study attritiona flow diagram. aIndividuals excluded from follow-up due to a ‘permanent’ status change that remained in place up to the age of 18 years (e.g. individuals became untraceable and were not found again before the age of 18 years). Individuals for whom a change in status meant they were temporarily excluded from follow-up are not included and, therefore, this diagram under-represents attrition at individual data collection time points. bPost-18-year age tracing and recruitment initiatives in ALSPAC may result in some individuals being included in future follow-up
Figure 7.
ALSPAC assessment response across multiple data collection time points. (a) Response to all 55* child-based questionnaires and ‘Focus’ clinical assessments across multiple time points. Number of questionnaires about the child (completed by the child or by the child’s mother/carer) or Focus clinical assessments completed by the child (excludes CiF sub-sample clinics and the puberty, CCXB and CCXC subsample questionnaire assessments). (b) Response to 25* CCQs across multiple time points. Number of questionnaires completed by the child (excludes the puberty, CCXB and CCXC subsample questionnaires). (c) Attendance at 9* child ‘Focus’ clinical assessment visits. Number of ‘Focus’ clinical assessments attended by the child (excludes the CiF sub-sample clinics)
We are addressing incomplete participation in several ways. We are conducting a postal campaign seeking consent to collect data from health and administrative sources (described later) and inviting re-enrolment in ALSPAC by participants who have now reached the age of 18 years. We plan to extend this campaign to eligible individuals who have never previously participated. We are currently seeking support under Section 251 of the NHS Act (2006) to collect routine data on non-responders to this campaign. Through data linkage, we can increase the completeness of the study data set in participants and better characterize non-participants in order to investigate possible participation biases. Linkage offers additional benefits through providing data to inform ALSPAC’s efforts to fully trace all enrolled participants. We continue to engage the sample through new media, including a study children’s Facebook page, and through targeted community engagement and data collection. These initiatives and key ALSPAC developments are developed in consultation with cohort participants through an advisory committee (ALSPAC Teenage Advisory Panel, TAP), participant representation on the ALSPAC Ethics and Law Committee and participant focus groups.
What has been measured?
The ALSPAC resource has a scale and richness that is unprecedented in epidemiological studies. The resource contains key phenotypes, genetic and biological samples collected at multiple time points allowing the assessment of developmental trajectories and critical periods of development. Current data collection focuses on the children’s transition into adulthood, obtaining measures as the children reach their physical maxima and observing emerging patterns in risk taking and anti-social behaviour. Measures have been selected to record the antecedent exposures of future health and psychosocial outcomes. A comprehensive guide can be found on our website.9 Key measures are summarized in Tables 6 and 7.
Table 6.
ALSPAC phenotype measures
|
aWPPSI: Wechsler Preschool and Primary Scale of Intelligence, DXA: Dual-emission X-ray Absorptiometry bone mineral scan, pQCT: Peripheral Quantitative Computed Tomography bone mineral scan, WISC: Wechsler Intelligence Scale for Children, CIS-R: Clinical Interview Schedule Revised symptom score, ESYTC: Edinburgh Study of Youth Transitions and Crime questionnaire assessment
Table 7.
ALSPAC environmental measures
|
The ALSPAC phenotype resource
The phenotype resource (Table 6) and environmental data (Table 7) comprise self-completed child, mother and teacher questionnaires as well as clinical assessments.
The ALSPAC biobank
Since early pregnancy, mothers and children have provided biological samples including blood, urine, hair, toenails, teeth, saliva and placenta. To ensure maximum efficiency of sample use, each sample has been divided into small aliquots and stored to ensure long-term preservation. The biobank is summarized in Supplementary Table S2 (available as Supplementary data at IJE online); full details of the ALSPAC biobank including a list of child samples and the assayed results are available on the study website.9
The ALSPAC genetic resource
DNA samples for 11 343 of the children have been collected, and 6902 of these have had a lymphoblastoid cell line transformed to date. The intention is to collect buccal saliva samples from children with no existing DNA data. Genome-wide data have been obtained for 8365 of the children. A list of ∼700 genotypes assayed on the available >10 000 participants with DNA samples are on the ALSPAC website.10 Most of these were obtained before genome-wide data became available, but some represent variants not included or imputable from genome-wide data.
A subsample of 2000 of the most heavily phenotyped participants will have complete genome sequencing to 6-fold depth (UK10K and Sanger Institute). For 1000 participants, ALSPAC has obtained gene expression data (Illumina 48 k chip) and copy number variation data (custom 105 k Agilent CGH chip from the WTCCC). DNA methylation data sampling (Illumina HumanMethylation450 BeadChip) is being run on 1000 mother and child pairs (where mother and child DNA is available from all of the following: child DNA at birth, 7 or 9 mean years of age and 15 or 17 mean years of age and mother DNA from pregnancy and child aged 15–17 years); and also whole-methylome sequencing is currently being piloted.
ALSPAC linkage to routine health and administrative records
Information on individuals eligible to participate in ALSPAC is currently available through linkage to the ONS (deaths and cancer registrations, 99% coverage of the ‘eligible sample’), the National Pupil Database (∼82% coverage of the ‘eligible sample’ depending on time point) and the General Practice Research Database11 (∼4% coverage of the ‘eligible sample’). The Project to Enhance ALSPAC through Record Linkage (PEARL) is extending the use of linkage to administrative data to enhance the ALSPAC data resource and address issues of possible participation bias. Existing or intended administrative data sources used by ALSPAC are listed in Table 8.
Table 8.
ALSPAC linkage to health and administrative records
|
aEither linkage, data owner approval and/or data collection mechanisms in development. bLinkage and data owner approvals in place and data collected
The use of ALSPAC as a sampling frame
The ALSPAC cohort has frequently been used to study subpopulations and controls identified in a variety of ways, including by genotype (e.g. smoking-related genotypes used for recall of sample for detailed assessment of smoking style), environment and/or phenotype. These have included qualitative projects (young people’s views on record linkage, drug use in teenagers) and quantitative projects (e.g. bone fractures,12 physical exercise13 and diet, children and siblings in families with parental separation14). The ALSPAC sample has also been used to select cases for inclusion in randomized control trials including the value of thermometers in a child’s nursery and comparisons between two designs of orthoptic screening for visual defects (as part of the CiF 4–37-month clinics).15
What has it found? Key findings and publications
There have been >700 articles published by February 2012; details of these can be found on the ALSPAC study website.16 A summary of some of the key ALSPAC findings has recently been published elsewhere.17 Notable examples of how ALSPAC findings have informed health and social policy include the following: (i) providing evidence to help persuade policy makers to support the ‘back to sleep’ policy change. This campaign started in the UK prior to ALSPAC's initiation, and initially advised parents not to place their babies to sleep in the prone position to reduce the risk of cot death. ALSPAC reassured sceptical health professionals and policy makers to proceed to recommending the supine position by demonstrating that this position was not associated with factors detrimental to child health.18,19 This finding also helped to persuade the US National Institutes of Health to carry out their own ‘Back to Sleep’ campaign that started in 2004. (ii) The finding that the application of skin creams containing peanut oil as a base ingredient to broken skin20,21 sensitized children to peanut allergy has led to some manufacturers altering the composition of the creams and the Committee on Safety in Medicines recommending that warning labels be included on all medicinal products containing peanut oil. (iii) ALSPAC has contributed to the debate on the consumption of fish during pregnancy. Established advice in the UK and USA was that on balance the risks of consuming toxins while eating more than two portions of fish per week outweighed the known benefits of eating fish. ALSPAC findings have influenced UK and US guidelines concerning fish consumption in pregnancy by demonstrating that benefits to child behaviour and verbal IQ,22 early development23 and visual stereoacuity24 outweigh potential harm of neurotoxicity from mercury and other sources of contamination. (iv) Evidence from ALSPAC including the influence of socio-economic position on life chances and aspirations were used to support the Independent Review on Poverty and Life Chances by Frank Field MP ‘The Foundation Years: Preventing Poor Children becoming Poor Adults’25 and the Marmot Review Fair Society, Healthy Lives.26
Genetic investigations using the candidate approach to studying gene variation and phenotypic outcome have described the influence of the filaggrin gene (FLG)27 on child susceptibility to atopic eczema and asthma. ALSPAC was the largest follow-up sample in the discovery that variation at the FTO28,29 gene is associated with increased adiposity and predisposition to obesity; using DXA assessment measures ALSPAC showed that the association was only present for fat mass and not lean mass. ALSPAC holds DNA collected at multiple time points, allowing researchers to explore DNA methylation and epigenetics.30,31 To realize the full potential of the resource ALSPAC is involved in genetic consortium studies including UK10K and EGG (early growth genetics).
Environmental studies have explored the antecedents of asthma where environmental exposures including prenatal maternal anxiety and paracetamol use,32–34 exposure to a range of cleaning products35,36 and excessive hygiene regimens37 have been found to influence the development of asthma in the child. Analysis of early life influences including maternal age, diet and smoking do not appear to influence blood pressure in the child38–41 although maternal weight gain in pregnancy is associated with increased risk of child adiposity and adverse cardiovascular risk factors.42 ALSPAC has also shown that fat mass contributes to higher bone mineral density.43,44
What are the main strengths and weaknesses?
The main strengths of ALSPAC are its general population base and sample size, the breadth and frequency of data collection and the availability of repeat measures, the extensive biobank, the breadth of genetic sampling and the ongoing support and commitment from the study families.
ALSPAC also has some weaknesses. Despite its size, it lacks power to study rarer exposures and outcomes. Although ALSPAC has collected repeat measures across frequent time points, the early (up to the age of 5 years) collection of data at study assessment clinics was limited to a 10% subsample. Nevertheless, the data collected have been very informative in regards to early growth and development. Collecting data from young adults is notoriously difficult and it is no surprise that ALSPACs response rates have decreased over time. To address this, ALSPAC is investing resources into collecting data from health and administrative records as well as participation initiatives aimed at increasing response. Incomplete recruitment and subsequent attrition have further reduced power and the availability of repeat measures across multiple time points and may have introduced bias in relation to estimation of some effects. Moreover, the demographic profile of the catchment area population and the effects of subsequent differential attrition have led to an over-representation of more affluent groups and an under-representation of non-White minority ethnic groups compared with the national population. This may influence external validity of some study findings based on prevalence, although it should not influence adversely the longitudinal results provided the features affecting bias are included. An advantage of current analyses is the relatively new statistical techniques for taking account of missing data, and these are being used increasingly.45
Can I get hold of the data? Where can I find out more?
Further details can be found on the ALSPAC website: http://www.bristol.ac.uk/alspac/, including the full ALSPAC collaboration policy (http://www.bristol.ac.uk/alspac/documents/alspac-policy.pdf). This resource also details all ALSPAC data (on mothers, index children and other relatives) that are currently available to external collaborators. At the present time, the ALSPAC resource is set up as a supported access resource rather than as an open access resource. In brief, any researcher wanting to use ALSPAC data must complete an ALSPAC Research Proposal Form describing the proposed collaboration and send it to the ALSPAC Executive (alspac-exec@bris.ac.uk). The ALSPAC executive will reply, usually within 2 weeks, to inform the applicant of the outcome and provide advice on the next stages. The vast majority of requests are approved as the system only checks availability of data and whether a cost will need to be charged (in most cases <£1000) to cover the staff resources required to put together a data set for collaborators. We do not police overlaps between requests, but since July 2011 all approved projects are detailed on the website (address as above), so potential collaborators can see what is already being done with the resource. We also do not police the scientific quality of projects, which is the responsibility of the investigators, but we do ensure that the study is correctly described in articles/other reports going for publication. Once a project has been agreed, the researchers are assigned a data buddy to support collaboration. The data buddy will provide the data set and advice on analysis of the data set. Any newly derived variables (including new assays on stored bloods) by the collaborator will be put into the main ALSPAC database and made available to other researchers once the collaborator has completed their project. As part of the collaborative agreement, we expect collaborators to produce appropriately detailed information about such new variables.
The ALSPAC executive plan to move towards more open access collaboration in the future and details of this will appear on the website. It is likely that access to genetic (particularly GWAS) and molecular data will have some supported access rather than being completely open access. The access requirements and use of external data collected through record linkage will be determined by the contractual agreements ALSPAC has with the data owners. Access to these linkage data will be supported by ALSPAC.
Supplementary Data
Supplementary Data are available at IJE online.
Funding
The UK Medical Research Council (MRC), the Wellcome Trust and the University of Bristol currently provide core funding support for ALSPAC (WT092731). Data collection is funded from a wide range of sources, which are detailed on the ALSPAC website: http://www.bristol.ac.uk/alspac/participants/ethics/funding/. AB is funded by the Wellcome Trust (WT086118/Z/08/Z) and the MRC/Wellcome Trust strategic support. AF is funded by an MRC Research Fellowship (G0701594). DAL, AF and GDS work in a Centre that receives funding from the MRC (G0600705).
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Conflict of interest: None declared.
KEY MESSAGES.
ALSPAC evidence provided reassurance that the supine sleeping position in babies, recommended in the ‘back to sleep’ campaign, did not appear to be detrimental to child health.18,19
ALSPAC evidence on the influence of socio-economic position on life chances and aspirations has contributed to reviews of UK national policy.25,26
ALSPAC data combining a wide range of phenotypic and environmental data with genetic information collected across multiple time points have allowed important contributions to genetic27–29 and epigenetic epidemiology.30,31
ALSPAC has provided important evidence on the influence of environmental exposures, such as prenatal maternal paracetamol use and household cleaning products and hygiene regimens, on the risk of asthma in the child.32–37
References
- 1.Golding J. Research protocol. European Longitudinal Study of Pregnancy and Childhood (ELSPAC) Paediatr Perinat Epidemiol. 1989;3:460–69. doi: 10.1111/j.1365-3016.1989.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 2.The ELSPAC website. http://ivdmr.fss.muni.cz/english/view.php?cisloclanku=2005050401 (29 February 2012, date last accessed)
- 3.ALSPAC Funders. http://www.bristol.ac.uk/alspac/participants/ethics/funding/ (29 February 2012, date last accessed)
- 4.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers. Int J Epidemiol. 2012;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd A, Golding J, Macleod JAA. Scottish Health Informatics Programme (SHIP) Bi-annual Conference. UK: St. Andrews; 2011. Using linkage to health and social administrative records to quantify the response rate to initial recruitment in the Avon Longitudinal Study of Parents and Children (ALSPAC) and assess possible bias associated with non-response (Abstract) [Google Scholar]
- 6.Marais J, Schuster S. Regional Household Income. London: Office for National Statistics; 2007. http://www.ons.gov.uk/ons/rel/regional-accounts/regional-household-income/march-2007/regional-household-income.pdf (29 February 2012, date last accessed) [Google Scholar]
- 7.Hobbs G, Vignoles A. Is free school meal status a valid proxy for socio-economic status (in schools research)? CEE Discussion Papers, Centre for the Economics of Education, LSE, 2007. [Google Scholar]
- 8.The Office of National Statistics (ONS) National Statistics Postcode Directory. http://www.census.ac.uk/guides/Lookup_tables.aspx (29 February 2012, date last accessed)
- 9.The ALSPAC Resource. http://www.bristol.ac.uk/alspac/sci-com/resource/ (29 February 2012, date last accessed)
- 10.ALSPAC Genotype Data. http://www.bristol.ac.uk/alspac/documents/genotypes-feb-2010.pdf (29 February 2012, date last accessed)
- 11.The General Practice Research Database (GPRD) http://www.gprd.com/home/ (29 February 2012, date last accessed)
- 12.Clark EC, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23:173–79. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattocks C, Ness A, Leary S, et al. Use of accelerometers in a large field based study of children: protocols, design issues and effects on precision. J Phys Act Health. 2008;5(Suppl 1):S98–111. doi: 10.1123/jpah.5.s1.s98. [DOI] [PubMed] [Google Scholar]
- 14.Gass K, Jenkins J, Dunn J. Are sibling relationships protective? A longitudinal study. J Child Psychol Psychiatry. 2007;48:167–75. doi: 10.1111/j.1469-7610.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- 15.Williams C, Harrad RA, Harvey I, Frankel S, Golding J. Methodology for a randomized controlled trial of preschool vision screening: a new approach with the ‘ALSPAC’ project. Opthalmic Epidemiol. 1996;3:63–76. doi: 10.3109/09286589609080111. [DOI] [PubMed] [Google Scholar]
- 16.ALSPAC Peer-Reviewed Publications. http://www.bristol.ac.uk/alspac/sci-com/pubs/ (29 February 2012, date last accessed)
- 17.Golding J. Determinants of child health and development: the contribution of ALSPAC—a personal view of the birth cohort study. Arch Dis Child. 2010;95:319–22. doi: 10.1136/adc.2009.178954. [DOI] [PubMed] [Google Scholar]
- 18.Hunt L, Fleming P, Golding J. Does the supine sleeping position have any adverse effects on the child? I: health in the first 6 months. The ALSPAC Study Team. Pediatrics. 1997;100:E11. doi: 10.1542/peds.100.1.e11. [DOI] [PubMed] [Google Scholar]
- 19.Dewey C, Fleming P, Golding J. Does the supine sleeping position have any adverse effects on the child? II: development in the first 18 months. ALSPAC Study Team. Pediatrics. 1998;101:E5. doi: 10.1542/peds.101.1.e5. [DOI] [PubMed] [Google Scholar]
- 20.Lack G, Fox D, Northstone K, et al. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 21.Khakoo A, Lack G. Preventing food allergy. Curr Allergy Asthma Rep. 2004;4:36–42. doi: 10.1007/s11882-004-0041-1. [DOI] [PubMed] [Google Scholar]
- 22.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–85. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 23.Daniels JL, Longnecker MP, Rowland AS, et al. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402. doi: 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- 24.Williams C, Birch EE, Emmett PM, et al. Stereoacuity at age 3.5 y in children born full-term is associated with prenatal and postnatal dietary factors: a report from a population-based cohort study. Am J Clin Nutr. 2001;73:316–22. doi: 10.1093/ajcn/73.2.316. [DOI] [PubMed] [Google Scholar]
- 25.Field F. The Report of the Independent Review on Poverty and Life Chances. London: Cabinet Office; 2010. The Foundation Years: Preventing Poor Children Becoming Poor Adults. [Google Scholar]
- 26.Patel C, North F, Head J, et al. Fair Society, Healthy Lives: Strategic Review of Health Inequalities in England Post 2010 (The Marmot Review) London: University College London; 2010. [Google Scholar]
- 27.Henderson J, Northstone K, Lee SP, et al. The burden of disease associated with filaggrin mutations: a population-based longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872–77. doi: 10.1016/j.jaci.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timpson NJ, Emmett PM, Frayling TM, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88:971–78. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Relton CL, Groom A, Elliot HR, et al. Epigenetic epidemiology: evidence for the role of epigenetic variation in complex disease. Ann Nutr Metab. 2009;55(Suppl 1):165. [Google Scholar]
- 31.Relton CL, Davey Smith G. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med. 2010;7:e1000356. doi: 10.1371/journal.pmed.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaheen SO, Newson RB, Sherriff A, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57:958–63. doi: 10.1136/thorax.57.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaheen SO, Newson RB, Henderson AJ, et al. Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood. Clin Exp Allergy. 2005;35:18–25. doi: 10.1111/j.1365-2222.2005.02151.x. [DOI] [PubMed] [Google Scholar]
- 34.Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers’ anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009;123:847–53. doi: 10.1016/j.jaci.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherriff A, Farrow A, Golding J, et al. Frequent use of chemical household products is associated with persistent wheezing in pre-school age children. Thorax. 2005;60:45–49. doi: 10.1136/thx.2004.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson J, Sherriff A, Farrow A, et al. Household chemicals, persistent wheezing and lung function: effect modification by atopy? Eur Respir J. 2008;31:547–54. doi: 10.1183/09031936.00086807. [DOI] [PubMed] [Google Scholar]
- 37.Sherriff A, Golding J. Hygiene levels in a contemporary population cohort are associated with wheezing and atopic eczema in preschool infants. Arch Dis Child. 2002;87:26–29. doi: 10.1136/adc.87.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leary SD, Ness AR, Emmett PM, Davey Smith G, Headley JE. Maternal diet in pregnancy and offspring blood pressure. Arch Dis Child. 2005;90:492–93. doi: 10.1136/adc.2004.070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts RJ, Leary SD, Davey Smith G, Ness AR, the ALSPAC Study Team Maternal age in pregnancy and offspring blood pressure in childhood in the Avon Longitudinal Study of Parents and Children (ALSPAC) J Hum Hypertens. 2005;19:893–900. doi: 10.1038/sj.jhh.1001915. [DOI] [PubMed] [Google Scholar]
- 40.Brion MJ, Leary SD, Davey Smith G, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49:1422–28. doi: 10.1161/HYPERTENSIONAHA.106.085316. [DOI] [PubMed] [Google Scholar]
- 41.Brion MJ, Leary SD, Lawlor DA, Davey Smith G, Ness AR. Modifiable maternal exposures and offspring blood pressure: a review of epidemiological studies of maternal age, diet and smoking. Pediatr Res. 2008;63:593–98. doi: 10.1203/PDR.0b013e31816fdbd3. [DOI] [PubMed] [Google Scholar]
- 42.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–64. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timpson NJ, Sayers A, Davey Smith G, Tobias JH. How does body fat influence bone mass in childhood? A Mendelian randomization approach. J Bone Miner Res. 2009;24:522–33. doi: 10.1359/jbmr.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab. 2010;95:699–706. doi: 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steer CD, Golding J, Bolton PF. Traits contributing to the autistic spectrum. PLoS One. 2010;5:e12633. doi: 10.1371/journal.pone.0012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.