Abstract
Summary The Avon Longitudinal Study of Children and Parents (ALSPAC) was established to understand how genetic and environmental characteristics influence health and development in parents and children. All pregnant women resident in a defined area in the South West of England, with an expected date of delivery between 1st April 1991 and 31st December 1992, were eligible and 13 761 women (contributing 13 867 pregnancies) were recruited. These women have been followed over the last 19–22 years and have completed up to 20 questionnaires, have had detailed data abstracted from their medical records and have information on any cancer diagnoses and deaths through record linkage. A follow-up assessment was completed 17–18 years postnatal at which anthropometry, blood pressure, fat, lean and bone mass and carotid intima media thickness were assessed, and a fasting blood sample taken. The second follow-up clinic, which additionally measures cognitive function, physical capability, physical activity (with accelerometer) and wrist bone architecture, is underway and two further assessments with similar measurements will take place over the next 5 years. There is a detailed biobank that includes DNA, with genome-wide data available on >10 000, stored serum and plasma taken repeatedly since pregnancy and other samples; a wide range of data on completed biospecimen assays are available. Details of how to access these data are provided in this cohort profile.
Why was the cohort set up?
Detailed follow-up of study participants in ALSPAC has focused largely on the offspring, and a previous publication has described the methods of the study,1 with a companion cohort profile updating that previous publication and focusing on the offspring.2 However, the mothers were the participants recruited to ALSPAC and the original investigators were clear that the goal of the study was to determine ways in which genotype and environmental characteristics influence health and development in both children and parents. Substantial information on lifestyle behaviours, including smoking,3,4 diet,5–7 physical activity,8,9 alcohol10–12 and use of illegal drugs,12,13 have been collected repeatedly since pregnancy on mothers and their partners. Information on partners has been collected in one of two key ways: either by responses from the mother about their partner’s behaviour or by responses of the partners themselves when mothers have passed questionnaires on to their partners.
Recently, substantial funding has been obtained to collect additional detailed data on the mothers. This includes funding to complete genome-wide analyses, to complete extraction of obstetric data, to invite all mothers to a follow-up assessment—Focus on Mothers 1 (FoM1)—and to complete a further three follow-up assessments (FoM2–FoM4). These additional funds mean that ALSPAC mothers now provide a unique resource for understanding women's reproductive health and the impact this has on her own future health as well as that of her children.14–16 It also establishes ALSPAC as a truly multi-generational cohort with extensive genetic (genome-wide data on approximately 10 000 mother–offspring pairs) and phenotypic data on mothers and offspring. Recent funding to extend this resource to collect detailed data on fathers, siblings and the next generation—children of the children of the 1990s (COCO90s or ALSPAC-G2)—will further enhance the study's capabilities to understand intergenerational transmission of health and well-being.
Who is in the cohort?
All women resident in a defined geographical area in the South West of England with an expected date of delivery between 1 April 1991 and 31 December 1992 were eligible for inclusion. Full details of how these women were recruited are provided in a previous publication describing ALSPAC and in the accompanying cohort profile focusing on the index children.1,2
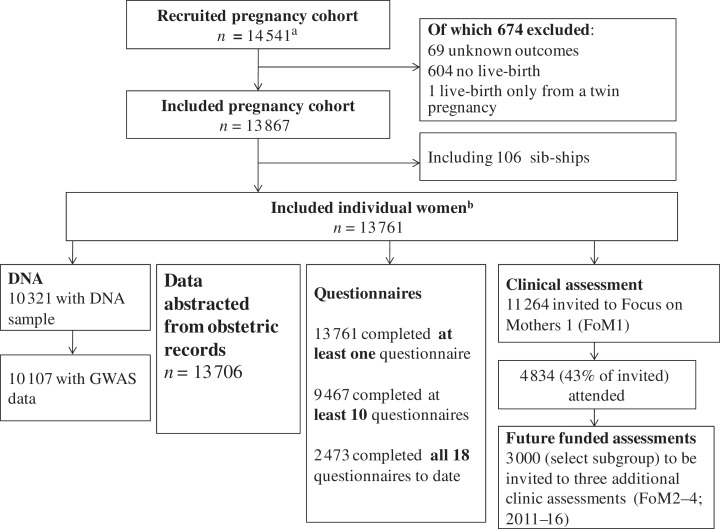
Figure 1 shows the numbers recruited and the flow of these through ALSPAC, focusing on the mothers; an equivalent flow chart focusing on the index children is shown in the companion cohort profile.2 Describing numbers in a pregnancy cohort can be complicated as these differ for ‘pregnancies’, ‘women/mothers’ and ‘children’. For clarity, and consistency with the companion cohort profile of the index children,2 we use the term ‘enrolled pregnancies’ for recruited pregnancies and ‘women’ for unique women in Figure 1. Within the enrolled cohort of pregnancies, there are some women who contribute two pregnancies (i.e. they had a second pregnancy during the recruitment period) and therefore there are a small number of sib-ships within the cohort. The flow diagram in this profile begins with the enrolled pregnancies and that in the accompanying profile2 goes back to ‘eligible pregnancies’, which we have not done here in the interest of simplicity and space. ALSPAC initially enrolled a cohort of 14 541 pregnancies. Of these 14 541 pregnancies, 674 were excluded and the remaining 13 867 pregnancies included 13 761 unique women. As noted in the accompanying profile focusing on the children,2 additional participants were recruited and added to the cohort after the initial recruitment period when children were in school-years. With respect to the mothers, these additional recruitments will not have extracted obstetric data or questionnaire data for questionnaires that were sent prior to their recruitment. They were sent all questionnaires following their recruitment and were invited to the Focus on Mothers assessment (Figure 1).
Figure 1.
ALSPAC mother participant flow. aThis number of recruited pregnancies does not include 717 women who completed one early pregnancy questionnaire and then experienced a pregnancy loss prior to 23 weeks. A description of these pregnancies (the miscarriage substudy) is provided in the supplementary web-material available as Supplementary Data at IJE online. bFor some subsequent data collection, additional women who were recruited ∼5–8 years postnatally were included. The description of these additional recruitments is provided in the accompanying cohort profile of index children.2 For obstetric data abstractions and for the earlier questionnaires only the 13 761 women were eligible. Later questionnaires and invites to the Focus on Mothers clinic assessment were sent to some additional women who were recruited 5–8 years postnatally and so the potential denominator for these is somewhat greater than 13 761 and varies for different questionnaires
The 1991 census was used to compare the population of mothers with infants <1 year of age resident in Avon with those in the whole of Britain, and to further compare participants in ALSPAC (using data collected ∼8 months postnatal) with mothers in Avon. The 8-month postnatal questionnaire was completed by ∼80% of the enrolled cohort of pregnancies, meaning that this comparison illustrates socio-demographic differences resulting from both incomplete enrolment and lack of response to the 8-month questionnaire. Mothers of infants in Avon were slightly more likely than those in Britain to live in owner-occupied accommodation and to have a car available to the household and less likely to have one or more persons per room and be non-White; the proportion of women who were married was similar in Avon and Britain (Table 1). ALSPAC participants at 8 months post childbirth were more likely than mothers in Britain, and also those in Avon, to live in owner-occupied accommodation and have a car in their household and less likely to be non-White. ALSPAC mothers were more likely to be married than either the equivalent population of Avon or Britain. Interestingly, despite generally having higher socio-economic position indicators on average than equivalent women in both Avon and Britain, ALSPAC mothers were somewhat more likely to be living in overcrowded conditions (a higher proportion with on average more than one person per room) than either Avon or British women.
Table 1.
Socio-demographic characteristics of mothers in Great Britain, Avon and those who participated in ALSPAC
| Characteristic | Whole of Great Britain (%) | Avon (%) | ALSPAC participants (%) |
|---|---|---|---|
| Owner occupier | 63.4 | 68.7 | 79.1 |
| 1 + person/room | 30.8 | 26.0 | 33.5 |
| Car in household | 75.6 | 83.7 | 90.8 |
| Married couple | 71.8 | 71.7 | 79.4 |
| Non-White mother | 7.6 | 4.1 | 2.2 |
Data for mothers in Great Britain and Avon from the 1991 census and based on women with an infant <1 year of age. Data from ALSPAC based on questionnaire responses at ∼8 months postnatal collected between 1992 and 1993; ∼80% of the enrolled pregnancy cohort from ALSPAC completed this questionnaire.
To date, two key ‘sub-studies of ALSPAC mothers’ have been completed in those who (i) experienced a pregnancy loss prior to 23 weeks gestation17 and (ii) are lesbian mothers.18–20 An additional two substudies have recently started: (i) a qualitative study with repeat in-depth interviews being conducted over the next 5 years to examine women's attitudes towards ageing and health as they go through midlife and the peri-menopause and (ii) a photographic project used to illustrate how women see themselves in midlife. All four of these substudies are described in more detail in the supplementary web-material available as Supplementary Data at IJE online.
How often have they been followed up?
During the index pregnancy, most women were sent three questionnaires; some who were recruited late in pregnancy will only have been sent one or two and a small number completed four. Since the index pregnancy, over a period of some 20 years, women have been sent 16 additional questionnaires that refer to their own health and well-being (questionnaires that they completed that asked about their study child are not discussed here).
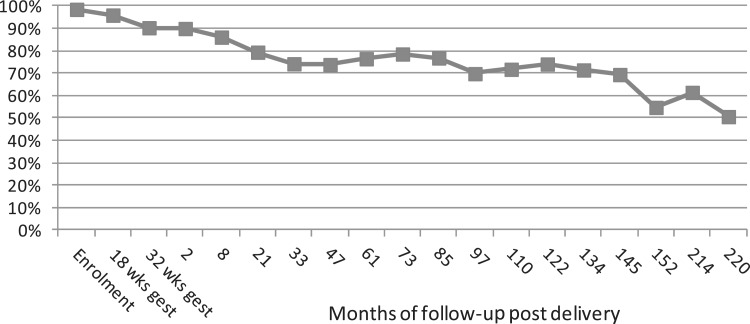
Figure 2 shows the response to the first 18 (3 antenatal and 15 postnatal) questionnaires to mothers about themselves; the 16th postnatal questionnaire, which focuses specifically on symptoms of polycystic ovarian syndrome and incontinence, has recently been mailed to the mothers. Response declined slightly over pregnancy and the first 33 months postnatal, but then remained constant at ∼70% until 152 months (∼12–13 years) postnatal, when there was a decline, with recent responses being between 50% and 60%. A similar pattern has been seen in recent responses from the index children,2 and this recent decline may reflect ‘study fatigue’ or be related to the size of the questionnaires, which have been larger in recent years as the number of collaborators in ALSPAC has increased. As a response to this, we are limiting the size of subsequent questionnaires and exploring the acceptability and response to web-based questionnaires.
Figure 2.
ALSPAC mother questionnaire response. The denominator is the number of women who were sent the questionnaire at each time period. This varies from 13 761 at the start of the study to 8512 for more recent questionnaires, as women who have died or requested to be withdrawn from further questionnaires or have a study break are taken into account. A total of 11 264 women remain in contact with the study
The decline in questionnaire responses over time somewhat exaggerates participation attrition as 11 264 (82%) of mothers still remain engaged with the study and they exhibit different patterns of questionnaire response. For example, some will complete every other questionnaire or not complete one for several occasions and then respond. This means that we can often include a large proportion of participants in analyses using repeat (change) in questionnaire characteristics and can also combine data from questionnaires close together to increase numbers with a specific outcome.
In 2008, funding was secured to invite all mothers still engaged with the study to a follow-up clinic (FoM1). Of the 11 264 eligible women, 4834 (43%) attended and provided valid data for the FoM1 follow-up clinic. Table 2 shows differences in baseline characteristics between the 6430 women who were invited but did not attend FoM1 and the 4834 who did attend. Women who did not attend were younger, from lower social class backgrounds and less likely to have a university degree and were more likely to have had two or more children prior to the index pregnancy, compared with those who did attend. Those who did not attend also had higher pre-pregnancy body mass index (BMI) and were more likely to experience hypertensive disorder of pregnancy, though mean gestational weight gain and the occurrence of gestational diabetes were similar in those attending and those not.
Table 2.
Baseline characteristics in women who attended the first follow-up clinic assessment of mothers (∼17–18 years postnatal) and those who were invited but did not attend
| Mean (SD) for continuously measured variables and prevalence
(%) for categorical variables |
|||
|---|---|---|---|
| Characteristic | Did not attend follow-up clinic N = 6430 | Did attend follow-up clinic N = 4834 | P-value* |
| Age at birth of child (years) | 27.2 (5.0) | 29.6 (4.5) | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 23.2 (4.1) | 22.5 (3.4) | <0.001 |
| Weight gain in pregnancy (kg) | 12.4 (4.9) | 12.6 (4.3) | 0.07 |
| Head of household manual occupational social class (%) | 23.8 | 11.8 | <0.001 |
| Had a university degree at time of index pregnancy (%) | 9.7 | 18.7 | <0.001 |
| Already had 2 or more children at time of index pregnancy (%) | 7.1 | 4.9 | <0.001 |
| Experienced HDP in index pregnancy (%) | 17.1 | 14.8 | 0.001 |
| Experienced gestational diabetes in index pregnancy (%) | 0.4 | 0.6 | 0.25 |
*Assessing the null hypothesis that there is no difference in distributions between those who attended and those who did not attend the follow-up clinic; unpaired t-test for continuous variables and chi-square test for categorical variables.
HDP, hypertensive disorder of pregnancy.
Further funding was secured in 2011 to complete three further clinics (FoM2–FoM4) on the subgroup of 3000 women who were pre- or peri-menopausal at the time of FoM1 and likely to make a transition through one or more stages of the menopausal transition during the next 5 years. These assessments will take place between now and 2016.
What has been measured?
The ALSPAC resource has a scale and richness that is unprecedented in epidemiological studies and it is beyond the scope of a single paper to list all data, at each time point, which have been collected. Instead, here, and in the companion children's cohort profile,2 we summarize the key areas of data collection. A comprehensive guide to all measurements in all participants can be found on our website (http://www.bris.ac.uk/alspac/). Key measurements that have been completed or are planned on the ALSPAC mothers are summarized in Table 3 and the biobank resource for the mothers is summarized in Table 4.
Table 3.
ALSPAC mother's summary of measurements
| Self-reported questionnaire measures |
From pregnancy to 20 years postnatal; numbers with data vary from ∼5000 to
13 700; more than 9467 have completed at least 10 of the 18 questionnaires
to date.a
|
| Obstetric data abstracted from medical records |
Available on 13 706 womena
|
| Opportunistic clinic assessments |
Completed on mothers when they attended Focus assessments with their index child
and when staff and resources allowed these assessments; numbers and time points
vary and are detailed by each measure
|
| FoM1 follow-up clinic assessment |
Completed 2009–11, N = 4834a
|
| Funded and ongoing future FoM2–4 clinics |
These data will be collected on 3000 women who were pre- or peri-menopausal at
the time of FoM1 and likely to change through one or more of the stages of the
menopausal transition over the next 5 years; FoM2 began in August 2011 and to date
(February 2012) approximately 700 women have attended and completed the
assessments
|
| Record linkage |
All recruited women who provided permission have been linked to the National
Health Service (NHS) Central Register, which provides data on the following.
|
aNumbers given for a particular questionnaire or focus follow-up visit assessment refer to the number who completed at least some of the questions/measurements. For individual measurements, the N will vary but only by small amounts. Where it is markedly different (e.g. for placental weights in the obstetric data abstraction), this is noted.
Environmental toxin data have also been collected from households and samples in ALSPAC; these are detailed in the companion cohort profile focusing on the offspring.2
Table 4.
Biological samples on ALSPAC mothersa
| Time point | Sample type (and additivesb) | Maximum number of aliquots | Number of participants with at least one aliquot remainingc |
|---|---|---|---|
| Pregnancy (1990 to early 1993) | Serum 1 ml | 3 | 6120 |
| Serum (AFP residue) 500 µl | 3 | 3449 | |
| Serum (rubella residue) 500 µl | 1 | 2598 | |
| Plasma (hep) 1 ml | 6 | 6167 | |
| Plasma (hep) 500 µl | 5 | 467 | |
| Whole blood (EDTA) 2 ml | 1 | 7501 | |
| Red cells (EDTA) | 1 | 1507 | |
| Blood spot (heparin) | 10 spots | 5731 | |
| Red cells (heparin) | 1 | 1651 | |
| White cells (heparin) | 1 | 2316 | |
| Urine 1 ml | 4 | 8575 | |
| Urine 5 ml | 5 | 7723 | |
| Delivery (1991 to early 1993) | Placenta (formalin) | 1 (whole placenta) | 8933 |
| Early postnatal (1993) | Hair | 1 | 4928 |
| Toe nails | 1 | 4959 | |
| 11–15 years postnatal (2004–08) | Plasma (hep) 500 µl | 4 | 5211 |
| Plasma (hep) 200 µl | 9 | 5221 | |
| 16–18 years postnatal (2009–11) | Plasma (hep) 500 µl | 4 | 4467 |
| Plasma (hep)200 µl | 9 | 4424 | |
| Plasma (EDTA) 500 µl | 3 | 4449 | |
| Plasma (EDTA) 200µl | 5 | 4381 | |
| Plasma (fluoride) 300 µl | 5 | 4298 | |
| White cells (EDTA) | 1 | 3967 | |
| Continuous since recruitment until now | DNA | NA | 10 321 |
| Cell lines | NA | 5140 |
NA, not applicable.
aData compiled and accurate on 29 February 2012.
bKey to sample additives: Hep: heparin, EDTA: ethylenediamineteraacetic acid.
cThe last aliquot of most samples is not available for collaborative research studies unless special permission is gained from the executive (i.e. at the moment we keep at least one aliquot of any sample for a potential major research project rather than allow exhaustion) and since compiling this table some aliquots may have been reserved for analyses and therefore fewer may be available.
Obstetric medical record data
The information sheets given to the women in pregnancy stated that data from medical records would be abstracted, unless she specifically indicated that she did not want this to occur. Initially, very detailed prenatal, labour and early postnatal data were abstracted on specific subgroups; for example, all women whose child was delivered by caesarean section or had an instrumental delivery, based on funded nested studies; and data were also abstracted on a random sample of 2500 healthy controls. In total, these early abstractions were completed on 8369 pregnancies. Recent funding has supported abstraction on remaining records, but with a focus on the antenatal period primarily. Thus, detailed antenatal data and some pregnancy and postnatal data have now been abstracted on all 13 706 pregnancies where the medical records could be retrieved. Additional information on other complications of pregnancy, results of ultrasound examinations, details of hospital admissions, investigations and treatments up to 14 days postpartum are available on 8369 pregnancies (Table 3).
Trained research midwives/nurses abstracted data from medical records. For the 5437 abstracts that were recently completed, data were abstracted directly onto an electronic database with inbuilt quality control. Early abstractions were onto a paper pro-forma and were later transferred to an electronic database. There is no between type of data abstraction, between abstractor or variation in mean values of abstracted data. For the 5437 records abstracted onto electronic databases, repeated data entry checks demonstrated error rates consistently <1%.
The detailed repeat measurements of weight, blood pressure, proteinuria and glycosuria have been used to derive trajectories and/or latent classes representing different patterns of change in these, and also to derive medical conditions using different criteria, for example the International Society for the Study of Hypertension in Pregnancy (ISSHP) criteria for gestational hypertension and pre-eclampsia.21 Details of the derived obstetric data, including the statistical methods used to derive them, are provided in the supplementary web-material available as Supplementary Data at IJE online.
Opportunistic clinic assessment
Opportunistic measures were obtained from mothers accompanying their children to follow-up Focus visits held when the children were 12–13, >13–14 and 15–16 years of age. These measurements were conducted if clinic staff and time were available. Table 3 details the complete list of measurements and numbers at each time point.
Completed FoM1 clinic assessment
Between November 2008 and March 2011, all ALSPAC mothers who were alive and had not withdrawn (N = 11 264) were invited to a clinic assessment—FoM1—and 4834 women completed the assessment. This clinic was funded to examine the association of pregnancy characteristics with later cardiometabolic health in the mother, and the measurements conducted reflect this focus on cardiometabolic health (Table 3).
Planned future clinic assessments
We have secured funding to complete a further three clinics on the mothers (FoM2–4). The focus of these clinics (together with data collected at FoM1) is to examine changes in hormonal, cardiometabolic function, musculoskeletal health, cognitive function and physical capability between mid-1940s and mid-1950s and to explore the extent to which menopausal/hormonal, life circumstances (e.g. changes in occupation, income and household/family structure), behaviour (e.g. change in smoking and physical activity) and genetic and epigenetic characteristics might explain any changes. In view of this focus, FoM2–4 will be conducted on a subgroup of the ALSPAC mothers (N = 3000) who at the FoM1 clinic assessment were aged ≥47 years, still menstruating regularly and who had not had a hysterectomy or oophorectomy—these characteristics aim to identify women who were pre- or peri-menopausal and likely to go through at least one stage of the menopausal transition over the period of the assessments. FoM2 began in July 2011 and by the middle of February 2012, 968 women had completed this assessment, with a further 369 booked into appointments to the end of April 2012.
Record linkage
ALSPAC mothers are flagged for mortality and cancer with the Office of National Statistics registries. To date, several hundred cancer events have been registered among this cohort, with 454 registrations of cervical cancer, 288 of breast cancer, 107 of any type of skin cancer, 15 of colorectal cancer and 10 of lung cancer, to October 2011.
Biological samples and measurements
Table 4 summarizes the biobank samples that have been collected on ALSPAC mothers; this table also provides information on the availability of these resources to potential collaborators.
Repeated blood samples on each woman collected as part of routine antenatal care were retrieved and these, together with further collections during the follow-up clinics of the children and mothers, have been used to establish a maternal DNA bank and immortalized lymphoblastoid cell lines. DNA was extracted using any of a phenol–chloroform, salting-out or guanidine hydrocholoride extraction methods, and concentration has been determined using PicoGreen assessment; 95% of the samples have at least 20 µl of DNA at a concentration of 50 ng/µl. A recently awarded Wellcome Trust grant provides funds for genome-wide assays (Illumina 660 W-quad BeadChip) on all 10 321 women who provided consent for genetic testing and on whom high-quality genomic DNA at sufficient concentration and volume (minimum 20 µl of DNA at a concentration of 50 ng/µl) is available. A large number of candidate genetic variants have been typed on the mothers. Recently, completion of the genome-wide assays means that there are now approximately 10 000 mother–offspring pairs in ALSPAC with genome-wide data; this reduces to approximately 8000 when within each group of mothers and offspring all relatives (to level of cousin) are removed.
DNA methylation has been assessed on subgroups of the mothers; for example, on their pregnancy DNA samples in those with different levels of pre-pregnancy BMI or of weight gain. Recent funding will allow global DNA methylation using the Illumina Human Methylation 450 K DNA Bead Chip to be completed on all women with relevant samples during pregnancy and then on samples collected at each of FoM1–FoM4.
Routine blood test results from the obstetric records, including rubella immunity status, blood group and haemoglobin levels are available. To date, total cholesterol, vitamin D [25(OH)D2 and D3], calcium, phosphate, fatty acid profile, trace metals and albumin have been assessed on the stored pregnancy samples. In addition, funding is available, and assays have begun to complete measurements of thyroid function and thyroid antibodies during pregnancy. A number of nested case–control studies have also had more expensive assays completed on case–control subgroups, generally of a few hundred participants each. For example, IGF1, IGF2, IGF-binding protein 3, sex hormone binding globulin and testosterone were assayed in 151 cases of cervical cancer, 69 cases of breast cancer and 443 healthy controls.22
Assays of fasting glucose, insulin, pro-insulin, total and high-density lipoprotein cholesterol (low-density lipoprotein cholesterol will be estimated from these), triglyceride levels and C-reactive protein and sex hormones have been (or are being) completed on the FoM1 samples, with similar measurements funded for the samples that will be collected at FoM2–4 (Table 3).
In addition to blood samples, random urine samples were collected during pregnancy, the placentas from the index pregnancy have been retained and stored and hair and toenail samples were collected in 1993 when the offspring were infants. Two studies analysing anatomical and some histopathological features of the placentas are currently funded. The first is to determine if the size and shape of the placental surface predicts hypertension in children. The second is a case–control study examining vascular branching structures and histopathological characteristics to investigate the intrauterine origins of autism risk. Once these studies are completed, the placental data will be incorporated into the database and available for collaborators to use. To date, creatinine has been measured on the pregnancy urine samples and iodine and cotinine are currently being assayed.
What has it found? Key findings and publications
Since data collected in ALSPAC spans at least two generations, the study has facilitated analyses that have made major contribution to a considerable number of areas, with full details on the study website (http://www.bris.ac.uk/alspac/). Examples of some of the scientific areas that data from the ALSPAC mothers study have contributed to are summarized below.
Understanding of relationships between maternal characteristics and offspring outcomes
There are several hundred publications that have examined a wide range of maternal exposures with later offspring outcomes. By way of examples, we have shown that greater maternal gestational weight gain is associated with a more adverse cardiometabolic risk profile in offspring,23 that hypertensive disorders of pregnancy are associated with offspring blood pressure,15 but not other cardiovascular outcomes,16 and that maternal glycaemia in pregnancy is associated with offspring obesity and fasting insulin and glucose.24,25 Using ALSPAC data, associations of prenatal anxiety with offspring diurnal salivary cortisol patterns,26 childhood behaviour27–29 as well as childhood asthma30 have been demonstrated. Greater maternal prenatal fish consumption has been shown to be positively associated with verbal IQ and other outcomes in the offspring.31 Data from ALSPAC and others studies show that maternal insulin glucose kinase genotype is associated with offspring birth weight.32 Of relevance to parenting programmes aimed at improving child development and maternal outcomes, we have shown that targeting at-risk groups based on young maternal age, as current UK policy recommends, will miss the majority of those with adverse outcomes, whereas using other maternal characteristics (education, financial difficulties, partner status, smoking in pregnancy and antenatal depression) would markedly increase the proportion of those at risk who are correctly identified.33,34
Identifying causal developmental risk factors
The availability of questionnaire data on the mother’s partners (sometimes reported by the mother and sometimes by the partner) as well as mothers, has been used to assess whether there is a direct biological effect of intrauterine exposures on offspring health status.35,36 For example, comparing associations of prenatal maternal and paternal smoking suggest that foetal exposure to maternal smoking in utero predisposes offspring to shorter birth length and lower weight and ponderal index at birth, as well as faster increases in length and adiposity in infancy,37,38 but does not appear to be causally related to adiposity in later childhood.38 Similar parental comparisons suggest that exposure to maternal smoking in utero is not causally related to childhood IQ, blood pressure or bone density.4,11,39 We have also compared maternal and paternal pre-pregnancy BMI, assessed by questionnaire at the time of recruitment, in their associations with offspring BMI4 and bone density,40 with the similarity of maternal- and paternal-offspring associations suggesting that maternal pre-pregnancy greater BMI does not cause, via intrauterine mechanisms, greater BMI or lower bone density in offspring in later life. In contrast, maternal pre-pregnancy BMI was found to be more strongly associated with offspring dual-energy X-ray absorptiomtry determined fat mass at mean age 10 years compared with paternal pre-pregnancy BMI, though the difference between the two was small.41
The strong collaborative links between ALSPAC and a large number of other birth cohorts across the world have also facilitated cross-cohort comparisons that contribute to understanding causality. For example, the confounding structures related to whether mothers breastfeed or not are markedly different between populations in high income countries such as the ALSPAC mothers (breastfeeding more likely in those from higher socio-economic backgrounds) than in those from low- or middle-income countries (breastfeeding not related to socio-economic position or more likely in those from lower socio-economic backgrounds). We have used this difference to demonstrate that breastfeeding is likely causally related to greater IQ in offspring in later life, but is not causally related to their later BMI or blood pressure.42
Women's reproductive health and its relationship to other health outcomes
A key programme of work that has utilized data collected around the time of recruitment has been associated with pre- and postnatal depression. This has characterized different features of these conditions and identified risk factors for depression both during and following pregnancy.43–47 A key finding in this programme of work was that depressive symptoms had a higher prevalence during pregnancy than postnatally.48
Using the repeat measurements of blood pressure in pregnancy, we have demonstrated different trajectories of blood pressure change between women who are normotensive, have existing hypertension or experience gestational hypertension or pre-eclampsia, which raise the possibility of being able to identify women earlier in pregnancy who may be destined for pre-eclampsia.49 We have also shown that established risk factors for pre-eclampsia and gestational hypertension are also associated with baseline blood pressure and change in blood pressure and proteinuria during pregnancy suggesting a continuum of risk that extends to women who do not meet conventional diagnostic thresholds.50,51 With data from the largest sample size and with most repeat measurements to date, we have found that in normal pregnancy the physiological increase in blood pressure starts at 18 weeks of gestation (95% CI 17–19 weeks), rather than the 20-week point, which is used in definitions of hypertensive disorder of pregnancy.50
We have found that hypertensive disorders of pregnancy and gestational diabetes are associated with different cardiovascular risk factors in women 18 years post-pregnancy.14 Using the opportunistic clinic measurements as outcomes, we have shown that greater gestational weight gain is associated with greater adiposity and higher blood pressure in the mothers 15–16 years post-pregnancy.52 We have attempted to understand more about different patterns of gestational weight gain and what might explain these differences, and have observed that genetic variants associated with greater adiposity in general in European populations are not associated with gestational weight gain,53 but age, education, parity and smoking are associated with different patterns of weight gain in pregnancy.54
Genetic influences on health and using genetic variants to understand causal effects of modifiable risk factors
Even prior to the recent collection of genome-wide data in ALSPAC mothers, it contributed (as a replication sample) to several genome-wide association studies that have identified variants that are robustly associated with body composition.55–57 With the recently completed genome-wide data on ALSPAC mothers, we are currently leading, and contributing to, a large number of genome-wide association studies concerned with maternal genetic contributions to their pregnancy characteristics and to perinatal outcomes. The ALSPAC mothers have also contributed to important candidate gene associations. For example, variants in the Factor V Leiden gene were found to be associated with pre-eclampsia but not with intrauterine growth restriction.58 In other candidate gene approaches, we have shown that in women who smoke at the start of pregnancy a common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5–CHRNA3–CHRNB4) is associated with being less likely to quit smoking during pregnancy,59 whereas a variant in the catechol O-methyltransferase (COMT) gene, that is believed to affect brain dopamine levels, is weakly related to how heavily women smoke, but not to other characteristics of smoking in pregnancy.60 A non-synonymous variant in the alcohol dehydrogenase 1B was found to be strongly associated with self-reported prenatal alcohol use in the ALSPAC mothers,10 and genetic variants in the fatty acid desaturase gene cluster predict the amount of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women.61 We have also shown that the Val66Met polymorphism in the brain-derived neurotrophic factor is associated with BMI,62 but does not appear to be clearly associated with depression.63 The mothers have also contributed to a number of studies where genetic variants are used as instrumental variables to examine the causal effect of non-genetic modifiable risk factors (Mendelian randomization studies).64,65 For example, they have contributed to studies using this approach, which suggest that folate intake by women is not related to their BMI66 or that of their offspring in later life.67
What are the main strengths and weaknesses?
The main strengths of the ALSPAC mothers’ cohort are: its sample size; the duration of follow-up and availability of repeat measures; genome-wide data and extensive phenotypic data; and funds to collect repeat measurements of DNA methylation. The ability to link these data to those of other family members—currently, the index children with very detailed genetic and phenotypic data,2 but with funds to also collect genetic and phenotypic data on the partners of these women and on their grandchildren—is also a major strength.
Limitations include the majority of participants of White ethnicity, which limits the generalizability of findings to other ethnic groups and ethnic comparisons, though has some advantages where a homogeneous ethnic group is required (e.g. in some genetic association studies). Whereas it is logical and convenient to recruit women into a birth cohort study when they are already pregnant, it is not necessarily the best eligibility criterion for questions related to women's health in general. This is because the index pregnancy is a somewhat arbitrary baseline in the context of the participating women's life course. An additional, and no less important, reason is that although a majority of women do experience pregnancy and have children, some do not and this group is not represented in a cohort of women recruited in pregnancy. That said, current Office of National Statistics data show that for UK women in the age range of the ALSPAC mothers, 85–88% will have at least one child (http://www.statistics.gov.uk/statbase/Product.asp?vlnk=5768). Therefore, our findings would be relevant to the large majority of UK women and for many associations to women from high-income countries in general. A related limitation is that women who had been recruited to ALSPAC but then experienced an early pregnancy loss (before 23 weeks) were not followed up (see supplementary web-material available as Supplementary Data at IJE online for details of the miscarriage substudy).
A further limitation is that it is only recently that a detailed follow-up examination of the mothers has been funded. This means that understanding the effects of early postnatal exposures (that require clinical assessment) on later maternal health or of changes in exposures (e.g. changes in measured body composition, blood pressure or blood glucose levels) through pregnancy and the early postnatal period cannot be assessed with this resource. This ‘delay’ between initial recruitment and a dedicated clinic follow-up of the mothers may also have influenced the response to the FoM1. It has at times been difficult for clinic staff, as mothers increasingly questioned why they were not allowed some assessments (notably dual-energy X-ray absorptiometry scans of bone density) that were performed on their index children whom they diligently brought repeatedly to assessments.
As with all prospective cohort studies, the number of women being followed up declines over time. Attrition in ALSPAC has been greater for those who experienced greater adversity during the index pregnancy (including factors such as early pregnancy complications, inadequate housing and lack of social support),68 and women who attended the recent FoM1 clinic assessment were older, less socio-economically deprived and more healthy at recruitment than those who were invited but did not attend. We have recently explored likely selection bias from attrition in ALSPAC and this suggests that, with respect to health inequalities, attrition results in underestimation of perinatal effects and later offspring education effects, but may not have a marked impact on maternal behaviours such as smoking.68 We have also used multiple imputation to examine bias related to attrition and to appropriately correct for this in several publications, although this can be a complex procedure, depending on the analysis model under consideration.69
Can I get hold of the data? Where can I find out more?
The ALSPAC executive is very keen to support collaboration and easy access to all aspects of the data by any researchers. Details of how to access the data can be found on the study website (http://www.bristol.ac.uk/alspac/), in the supplementary web-material available as Supplementary Data at IJE online accompanying this cohort profile, and in the companion cohort profile focusing on the index children.2
Supplementary Data
Supplementary Data are available at IJE online.
Funding
The UK Medical Research Council (MRC); the Wellcome Trust and the University of Bristol currently provide core funding support for ALSPAC. Specific funds for recent detailed data collection on the mothers has been obtained from the US National Institute of Health (R01 DK077659) and Wellcome Trust (WT087997MA) for completion of selected items of obstetric data extraction; Wellcome Trust (WT088806) for completion of genome wide data; British Heart Foundation (SP/07 1008/24066) for completion of FoM1; Wellcome Trust (WT092830/Z/10/Z) for completion of FoM2 and FoM3; Joint UK Research Councils (Lifelong health and well-being initiative, G1001357) for completion of FoM4, additional assays on bloods collected at FoM1–FoM3 and qualitative and photographic data collection. A.F. is funded by an MRC Research Fellowship (G0701594), C.M.W. by a Wellcome Trust grant (WT087997MA) and A.B. by the Wellcome Trust/MRC strategic award. A.F., C.M.W., G.D.S. and D.A.L. work in a Centre that receives funding from the MRC (G0600705).
Supplementary Material
Acknowledgements
The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Conflict of interest: None declared.
KEY MESSAGES.
The ALSPAC mothers cohort has contributed to understanding the associations of maternal pregnancy-related changes with the mother's and her offspring's future health.
Through the use of genetic variants as instrumental variables, comparisons of maternal and paternal exposures with offspring outcomes, and cross-cohort comparisons with studies from low- and middle-income countries, ALSPAC has provided evidence on whether different intrauterine exposures are causally associated with later offspring outcomes.
It has contributed to understanding genetic determinants of a number of health-related phenotypes.
It has contributed to understanding the relationship of women's reproductive health to healthy ageing and well-being.
References
- 1.Golding J, Pembrey M, Jones R. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyd A, Macleod J, Fraser A, et al. Cohort Profile: The ‘Children of the 90s’; the index offspring of The Avon Longitudinal Study of Parents and Children (ALSPAC) Intl J Epidemiol. 2012;42:111–27. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey Smith G, Steer C, Leary S, Ness A. Is there an intrauterine influence on obesity? Evidence from parent–child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC) Arch Dis Child. 2007;92:876–80. doi: 10.1136/adc.2006.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald-Wallis C, Tobias JH, Davey Smith G, Lawlor DA. Parental smoking during pregnancy and offspring bone mass at age 10 years: findings from a prospective birth cohort. Osteoporos Int. 2011;22:1809–19. doi: 10.1007/s00198-010-1415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers I, Emmett P ALSPAC Study Team. Diet during pregnancy in a population of pregnant women in South West England. Eur J Clin Nutr. 1998;52:246–50. doi: 10.1038/sj.ejcn.1600543. [DOI] [PubMed] [Google Scholar]
- 6.Northstone K. Emmett PM. Dietary patterns of men in ALSPAC: associations with sociodemographic and lifestyle characteristics, nutrient intake and comparison with women's dietary patterns. Eur J Clin Nutr. 2010;64:978–86. doi: 10.1038/ejcn.2010.102. [DOI] [PubMed] [Google Scholar]
- 7.Brion MJ, Ness AR, Rogers I, et al. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: exploring parental comparisons and prenatal effects. Am J Clin Nutr. 2010;91:748–56. doi: 10.3945/ajcn.2009.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattocks C, Ness A, Deere K, et al. Early life determinants of physical activity in 11 to 12 year olds: cohort study. BMJ. 2008;336:26–29. doi: 10.1136/bmj.39385.443565.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Blair SN, Teng Y, Ness AR, Lawlor DA, Riddoch C. Physical activity during pregnancy in a prospective cohort of pregnant women: results from the Avon Longitudinal Study of Parents and Children. Eur J Epidemiol. 2011;26:237–47. doi: 10.1007/s10654-010-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuccolo L, Fitz-Simon N, Gray R, et al. A non-synonymous variant in ADH1B is strongly associated with prenatal alcohol use in a European sample of pregnant women. Hum Mol Genet. 2009;18:4457–66. doi: 10.1093/hmg/ddp388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alati R, Macleod J, Hickman M, et al. Intrauterine exposure to alcohol and tobacco use and childhood IQ: findings from a parental–offspring comparison within the Avon Longitudinal Study of Parents and Children. Pediatr Res. 2008;64:659–66. doi: 10.1203/PDR.0b013e318187cc31. [DOI] [PubMed] [Google Scholar]
- 12.Zammit S, Thomas K, Thompson A, et al. Maternal tobacco, cannabis and alcohol use during pregnancy and risk of adolescent psychotic symptoms in offspring. Br J Psychiatry. 2009;195:294–300. doi: 10.1192/bjp.bp.108.062471. [DOI] [PubMed] [Google Scholar]
- 13.Macleod J, Hickman M, Bowen E, Alati R, Tilling K, Davey Smith G. Parental drug use, early adversities, later childhood problems and children's use of tobacco and alcohol at age 10: birth cohort study. Addiction. 2008;103:1731–43. doi: 10.1111/j.1360-0443.2008.02301.x. [DOI] [PubMed] [Google Scholar]
- 14.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated CVD risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geelhoed JJM, Fraser A, Tilling K, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure, independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–99. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawlor DA, Macdonald-Wallis C, Fraser A, et al. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eu Heart J. 2012;33:335–45. doi: 10.1093/eurheartj/ehr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrow A, Farrow SC, Little R, Golding J. The repeatability of self-reported exposure after miscarriage. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Int J Epidemiol. 1996;25:797–806. doi: 10.1093/ije/25.4.797. [DOI] [PubMed] [Google Scholar]
- 18.Golombok S, Perry B, Burston A, et al. Children with lesbian parents: a community study. Dev Psychol. 2003;39:20–33. doi: 10.1037//0012-1649.39.1.20. [DOI] [PubMed] [Google Scholar]
- 19.Perry B, Burston A, Stevens M, Steele H, Golding J, Golombok S. Children's play narratives: what they tell us about lesbian-mother families. Am J Orthopsychiatry. 2004;74:467–79. doi: 10.1037/0002-9432.74.4.467. [DOI] [PubMed] [Google Scholar]
- 20.Stevens M, Perry B, Burston A, Golombok S, Golding J. Openness in lesbian-mother families regarding mother's sexual orientation and child's conception by donor insemination. J Reprod Infant Psychol. 2003;21:347–62. [Google Scholar]
- 21.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Preg. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 22.Jeffreys M, Northstone K, Holly J, Emmett P, Gunnell D. Levels of insulin-like growth factor during pregnancy and maternal cancer risk: a nested case–control study. Cancer Cause Control. 2011;22:945–53. doi: 10.1007/s10552-011-9767-y. [DOI] [PubMed] [Google Scholar]
- 23.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Nelson SM, Lawlor DA. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–64. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawlor DA, Fraser A, Lindsay RS, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia. 2010;53:89–97. doi: 10.1007/s00125-009-1560-z. [DOI] [PubMed] [Google Scholar]
- 25.Patel S, Fraser A, Davey Smith G, et al. Associations of gestational diabetes, existing diabetes and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes Care. 2012;35:163–71. doi: 10.2337/dc11-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–17. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor TG, Heron J, Glover V. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry. 2002;41:1470–77. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–36. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- 30.Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009;123:847–53. doi: 10.1016/j.jaci.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–85. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 32.Weedon MN, Frayling TM, Shields B, et al. Genetic regulation of birth weight and fasting glucose by a common polymorphism in the islet cell promoter of the glucokinase gene. Diabetes. 2005;54:576–81. doi: 10.2337/diabetes.54.2.576. [DOI] [PubMed] [Google Scholar]
- 33.Chittleborough CR, Lawlor DA, Lynch J. Prenatal prediction of poor maternal and offspring outcomes: implications for selection into intensive parent support programs. Matern Child Health J. 2012;16:909–20. doi: 10.1007/s10995-011-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chittleborough CR, Lawlor DA, Lynch JW. Young maternal age and poor child development: predictive validity from a birth cohort. Pediatrics. 2011;127:e1436–44. doi: 10.1542/peds.2010-3222. [DOI] [PubMed] [Google Scholar]
- 35.Lawlor DA, Leary S, Davey Smith G. Theoretical underpinning for the use of intergenerational studies in life course epidemiology. In: Lawlor DA, Mishra G, editors. Family Matters: Using Family Based Studies to Determine the Mechanisms Underlying Early Life Determinants of Adult Chronic Diseases. Oxford: Oxford University Press; 2009. pp. 1–12. [Google Scholar]
- 36.Davey Smith G. Assessing intrauterine influences on offspring health outcomes: can epidemiological findings yield robust results? Basic Clin Pharmacol Toxicol. 2008;102:245–56. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 37.Leary S, Davey Smith G, Ness A. Smoking during pregnancy and components of stature in offspring. Am J Hum Biol. 2006;18:502–12. doi: 10.1002/ajhb.20518. [DOI] [PubMed] [Google Scholar]
- 38.Howe LD, Matijasevich A, Tilling K, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. Int J Epidemiol. 2012;41:722–32. doi: 10.1093/ije/dys025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brion MJ, Leary SD, Davey Smith G, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49:1422–28. doi: 10.1161/HYPERTENSIONAHA.106.085316. [DOI] [PubMed] [Google Scholar]
- 40.Macdonald-Wallis C, Tobias JH, Davey Smith G, Lawlor DA. The relationship of maternal pre-pregnancy body mass index with offspring bone mass in childhood: is there evidence for an intrauterine effect? Am J Clin Nutr. 2010;4:872–80. doi: 10.3945/ajcn.2010.29501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawlor DA, Timpson N, Harbord R, Leary S, et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and the FTO gene as an instrumental variable for maternal adiposity: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) PLoS Med. 2008;5:e33. doi: 10.1371/journal.pmed.0050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brion M-J A, Lawlor DA, Matijasevich A, et al. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int J Epidemiol. 2011;40:670–80. doi: 10.1093/ije/dyr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fergusson DM, Horwood LJ, Thorpe K. Changes in depression during and following pregnancy. ALSPAC Study Team. Study of Pregnancy and Children. Paediatr Perinat Epidemiol. 1996;10:279–93. doi: 10.1111/j.1365-3016.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 44.Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115:1043–51. doi: 10.1111/j.1471-0528.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 45.Micali N, Simonoff E, Treasure J. Pregnancy and post-partum depression and anxiety in a longitudinal general population cohort: The effect of eating disorders and past depression. J Affect Disord. 2011;131:150–57. doi: 10.1016/j.jad.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 46.Munafo MR, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine Tob Res. 2008;10:1609–20. doi: 10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- 47.Golding J, Steer C, Emmett P, Davis JM, Hibbeln JR. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009;20:598–603. doi: 10.1097/EDE.0b013e31819d6a57. [DOI] [PubMed] [Google Scholar]
- 48.Evans J, Heron J, Francomb H, Oke S, Golding J The ALSPAC Study Team. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323:257–60. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macdonald-Wallis C, Lawlor DA, Fraser A, May M, Nelson SM, Tilling K. Blood pressure change in normotensive, gestational hypertensive, preeclamptic and essential hypertensive pregnancies. Hypertension. 2012;59:1241–48. doi: 10.1161/HYPERTENSIONAHA.111.187039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Established preeclampsia risk factors are related to patterns of blood pressure change in normal term pregnancy: findings from the Avon Longitudinal Study of Parents and Children. J Hypertens. 2011;29:1703–11. doi: 10.1097/HJH.0b013e328349eec6. [DOI] [PubMed] [Google Scholar]
- 51.Macdonald-Wallis C, Lawlor DA, Heron J, Fraser A, Nelson SM, Tilling K. Relationships of risk factors for pre-eclampsia with patterns of occurrence of isolated gestational proteinuria during normal term pregnancy. PLoS One. 2011;6:e22115. doi: 10.1371/journal.pone.0022115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraser A, Tilling K, MacDonald-Wallis C, Sattar N, Nelson SM, Lawlor DA. Associations of gestational weight gain with mothers BMI, waist circumference and blood pressure measured 16 years post-pregnancy: the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2011;93:1285–92. doi: 10.3945/ajcn.110.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawlor DA, Fraser A, MacDonald-Wallis C, Palmer T, Davey Smith G, Tilling K. Maternal and offspring adiposity related genetic variants and gestational weight gain. Am J Clin Nutr. 2011;94:149–55. doi: 10.3945/ajcn.110.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tilling K, Fraser A, Macdonald-Wallis C, et al. Patterns of weight gain in pregancy and their determinants: findings from the Avon Longitudinal Study of Parents and Children. Longitud Life Course Studies. 2010;1:99. [Google Scholar]
- 55.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loos RJ, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–75. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudding T, Heron J, Thakkinstian A, et al. Factor V Leiden is associated with pre-eclampsia but not with fetal growth restriction: a genetic association study and meta-analysis. J Thromb Haemost. 2008;6:1869–75. doi: 10.1111/j.1538-7836.2008.03134.x. [DOI] [PubMed] [Google Scholar]
- 59.Freathy RM, Ring SM, Shields B, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–27. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munafo MR, Freathy RM, Ring SM, St Pourcain B, Davey Smith G. Association of COMT Val(108/158)Met genotype and cigarette smoking in pregnant women. Nicotine Tob Res. 2011;13:55–63. doi: 10.1093/ntr/ntq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2011;93:211–19. doi: 10.3945/ajcn.110.006189. [DOI] [PubMed] [Google Scholar]
- 62.Shugart YY, Chen L, Day IN, et al. Two British women studies replicated the association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) and BMI. Eur J Hum Genet. 2009;17:1050–55. doi: 10.1038/ejhg.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Lawlor DA, Lewis SJ, et al. Genetic association study of BDNF in depression: finding from two cohort studies and a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:814–21. doi: 10.1002/ajmg.b.30686. [DOI] [PubMed] [Google Scholar]
- 64.Davey Smith G, Ebrahim S. “Mendelian randomisation”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 65.Lawlor DA, Harbord RM, Sterne JAC, Timpson NJ, Davey Smith G. Mendelian randomization and instrumental variables. Stat Med. 2008;27:1133–63. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 66.Lewis SJ, Lawlor DA, Nordestgaard BG, et al. The methylenetetrahydrofolate reductase C677T genotype and the risk of obesity in three large population-based cohorts. Eur J Endocrinol. 2008;159:35–40. doi: 10.1530/EJE-08-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis SJ, Leary S, Davey Smith G, Ness A. Body composition at age 9 years, maternal folate intake during pregnancy and methyltetrahydrofolate reductase (MTHFR) C677T genotype. Br J Nutr. 2009;102:493–96. doi: 10.1017/S0007114509231746. [DOI] [PubMed] [Google Scholar]
- 68.Howe L, Galobardes B, Tilling K, Lawlor DA. Does drop-out from cohort studies bias estimates of socioeconomic inequalities in health? J Epidemiol Community Health. 2011;65:A31. doi: 10.1097/EDE.0b013e31827623b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spratt M, Carpenter J, Sterne JA, et al. Strategies for multiple imputation in longitudinal studies. Am J Epidemiol. 2010;172:478–87. doi: 10.1093/aje/kwq137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.