Abstract
Background Chronic exposure to toxic metals such as arsenic and cadmium has been implicated in the development of kidney and cardiovascular diseases but few studies have directly measured exposure during inutero and early child development.
Methods We investigated the impact of exposure to arsenic (mainly in drinking water) and cadmium (mainly in rice) during pregnancy on blood pressure and kidney function at 4.5 years of age in rural Bangladesh. The effect of arsenic exposure in infancy was also assessed.
Results Within a cohort of 1887 children recruited into the MINIMat study, exposure to arsenic (maternal urinary arsenic, U-As), but not cadmium, during in utero development was associated with a minimal increase in blood pressure at 4.5 years. Each 1 mg/l increase in pregnancy U-As was associated with 3.69 mmHg (95% CI: 0.74, 6.63; P: 0.01) increase in child systolic and a 2.91 mmHg (95% CI: 0.41, 5.42; P: 0.02) increase in child diastolic blood pressure. Similarly, a 1 mg/l increase in child U-As at 18 months of age was associated with a 8.25 mmHg (95% CI: 1.37, 15.1; P: 0.02) increase in systolic blood pressure at 4.5 years. There was also a marginal inverse association between infancy U-As and glomerular filtration rate at 4.5 years (−33.4 ml/min/1.72 m2; 95% CI: −70.2, 3.34; P: 0.08). No association was observed between early arsenic or cadmium exposure and kidney volume at 4.5 years assessed by ultrasound.
Conclusions These modest effect sizes provide some evidence that arsenic exposure in early life has long-term consequences for blood pressure and maybe kidney function.
Keywords: Bangladesh, arsenic, cadmium, blood pressure, kidney function
Introduction
Exposures experienced in utero and early post-natal development may be important triggers for disease susceptibility in later life.1 Birth weight is a commonly examined marker of early-life conditions and has been shown by some studies to be inversely associated with important disease risk factors such as blood pressure2 and kidney function.3 A lack of adequate nutrition has often been studied within this context, but other environmental exposures may also be important.
Arsenic is commonly ingested from drinking water and food sources in many parts of the world. Bangladesh is one of the most affected countries with more than 50% of the 6–11 million tube-wells containing arsenic concentrations above the World Health Organization guideline value of 10 μl/l (British Geological Survey; www.bgs.uk). In addition, the rice-based diet contributes to elevated exposure to arsenic4 and cadmium.5 Both arsenic and cadmium are known carcinogens that may also damage various organs and functions including the kidney, bone and cardiovascular system.6–9 Although there is some evidence that chronic arsenic exposure may be associated with increased cardiovascular risk,10 the evidence base is often hampered by weak study designs.11
Exposure to toxic agents during in utero development has the potential to cause severe impairment of organ function and could represent a critical window of exposure. Arsenic easily crosses the placenta12 and has been shown to adversely affect birth outcomes such as foetal loss and birth weight.13–15 Cadmium, on the other hand, accumulates in the placenta with limited transfer to the foetus16 although effects on birth size17–19 and child growth have been observed.20 Studies of the effect of chronic metal exposure on cardiovascular health rarely investigate the impact of exposure during development with a prospective design. Here we present an analysis of the effect of arsenic and cadmium exposure during pregnancy and at 18 months of age on blood pressure and kidney function at 4.5 years, from a prospective birth cohort study in rural Bangladesh.
Methods
This study was part of a larger follow-up study of the MINIMat (Maternal and Infant Nutrition Interventions, Matlab) trial conducted in rural Bangladesh between 2000 and 2004 and coordinated by the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B). The MINIMat trial investigated the effectiveness of a variety of food and multi-micronutrient supplements in pregnancy aimed at improving birth outcomes and neonatal health and has been described in detail elsewhere.21 Assessment of kidney function and blood pressure was part of a follow-up undertaken when the children born into the trial were 4.5 years old. The MINIMat trial was conducted in an area with frequently elevated arsenic concentrations in well water, which is the drinking water source of more than 95% of the population. The trial subjects and their offspring therefore form an important birth cohort in which to study the potential health effects of early-life exposure. For the present study we had data on arsenic and cadmium exposure during pregnancy and child’s arsenic exposure at 18 months of age.
All of the 3267 individuals who were live singleton births during the original MINIMat trial were eligible for the follow-up at 4.5 years of age. This age point was chosen to investigate the impact of supplementation on early childhood growth, the results of which will be published elsewhere. Full informed consent was obtained from the parents or guardians of eligible children prior to enrolment. Blood pressure was assessed on all recruited individuals. Glomerular filtration rate was assessed on a sub-sample of individuals born during the first year of the MINIMat trial (June 2002–June 2003) due to cost considerations. The organ biometric study by ultrasound was restricted to a different sub-sample of individuals, born during the second half (June 2003–June 2004) of the trial, in order to minimize participant burden. Urinary arsenic (U-As) during pregnancy was measured on all available urine samples provided by pregnant women enrolled into the trial. Urinary cadmium (U-Cd) in pregnancy and U-As in infancy (18 months) was measured in a sub-sample of mothers who were recruited into the first half of the trial and gave birth up to December 2003. Further details of samples sizes and loss to follow-up are provided in the results.
Measurements
Exposure assessment
Urinary measures were chosen as the most informative as they reflect exposure from all sources including food.4,5,22,23 Spot urine samples were collected from participating women in early (median: week 8; 10th, 90th percentile: week 7, 12) and also in late (week 30; week 29, 33) gestation.24 Urine samples were also collected from the children at 18 months of age (10th, 90th percentile: 17.9, 18.2 months) using plastic bags placed in potties;25 only arsenic measurements were conducted for this time point. Samples were transferred to polyethylene containers and stored at −70°C before analysis at Karolinska Institute, Sweden. Maternal urine was analysed for the sum concentration of metabolites of inorganic arsenic (inorganic arsenic, methylarsonic acid and dimethylarsinic acid), using hydride generation atomic absorption spectroscopy24 and for cadmium using inductively coupled mass spectrometry (ICPMS).5 Concentrations of arsenic metabolites in children’s urine were measured using high pressure liquid chromatography on line with hydride generation and ICPMS.25 Application of quality control procedures showed excellent agreement with reference materials.5,24,25 As no certified reference samples for arsenic metabolites in urine were available, we also compared the concentrations of total urinary arsenic at gestational week 8 (measured using ICPMS) with concentrations obtained by adding the different U-As metabolites (urinary inorganic arsenic + urinary monomethylarsonic acid + urinary dimethylarsinic acid) and found a high correlation (R2 = 0.96) with a nearly 1:1 ratio and a linear regression slope of β1 = 0.97 (P < 0.001). U-As and U-Cd concentrations were adjusted for variation in urine dilution by specific gravity (average 1.012 g/ml for mother’s urine and 1.009 g/ml for children’s urine), which were found to be less dependent on age, nutritional status and arsenic exposure than was creatinine adjustment.26
Outcome assessment
Measurements during the 4.5-year follow-up were conducted by fully trained fieldworkers in sub-centre health clinics in the Matlab study area covered by ICDDR,B. Blood pressure was measured in triplicate using an automated oscillometric device (Omron 705IT, Morton Medical Ltd) under standard conditions: the first measurement was taken after the subject had been seated at rest for 5 min, with 1 min between each subsequent measurement. The mean of the three readings was used as an estimate of average blood pressure.
Kidney function was assessed by calculation of glomerular filtration rate (GFR) estimated from plasma cystatin C (CysC), analysed from stored frozen samples using the immunoturbidimetric analysis,27 in Uppsala University Hospital, Sweden. GFR was calculated from CysC using a prediction equation generated in Swedish patients and applicable for use in children.28 Kidney volume was assessed by ultrasonography conducted using a convex scanner (Toshiba SSA 510 A Famio-5, Toshiba Medical Systems, Japan) at a frequency of 3.75 Mhz. Two medical doctors conducted all measurements after extensive training and standardization. Right and left kidney volumes were calculated by fitting a best-fit ellipsoid shape that was converted into an estimate of volume using internal software. This method was highly correlated with the alternative length, width, depth method of assessing volume. The average of right and left kidney volume was then adjusted for body surface area calculated from the Haycock formula.29
Potential confounders and covariates
Maternal height and weight were measured under standard conditions at enrolment into the trial (week 8 of gestation). Maternal blood pressure was measured by a single mercury sphygmomanometer reading at four time points in pregnancy; 8–10, 14–15, 19–20 and 30–31 weeks of gestation. Birth weight and length were measured within 72 h of birth by trained fieldworkers and season of birth was defined by fitting Fourier terms.30 At the 4.5-year follow-up a variety of anthropometric measurements were conducted including weight (digital scale: Tanita, Chasmors Ltd) and height (stadiometer: Chasmors Ltd). Total body composition was assessed using a foot-to-foot bioelectrical impedance analyser (Tanita TBF-300MA, Chasmors Ltd) applying equations previously generated in this population using deuterium dilution as the reference method.31 Parental socio-economic status (SES) was assessed by a continuous wealth index previously generated in this population and which included data on land ownership, the construction materials of house walls, ownership of household assets, ownership of sarees or shalwer-kameez for ceremonial use and pairs of shoes or sandals owned.32
Statistical analysis
Data were analysed using STATA 10.0 (Stata Corporation, College Station, TX, USA) and all outcome variables were normally distributed. U-Cd and U-As at all time points had highly skewed distributions and residuals that were not normally distributed, requiring log-transformation to meet linear regression assumptions. Simple regression analysis was used to assess the association between potential confounding variables and the urinary metals. The association between early-life toxic metal exposure and later blood pressure/kidney function was assessed by fitting linear regression models; for this analysis U-Cd and U-As were fitted as untransformed terms. Quadratic exposure terms were fitted in order to test whether the relationship was curvilinear but were not found to be significant and are not reported. Variables were considered to be confounders if they were found to be related to both the exposure and outcome variables and these were included in final models; sex, age and height were included in all final models as they were related to the outcomes under study. We evaluated potential effect modification by fitting interaction terms into the adjusted models between the exposure variables (U-As and U-Cd) and various potential effect modifiers: MINIMat trial intervention group (food and micronutrient interventions separately), child sex, parental SES and maternal early pregnancy BMI.
Results
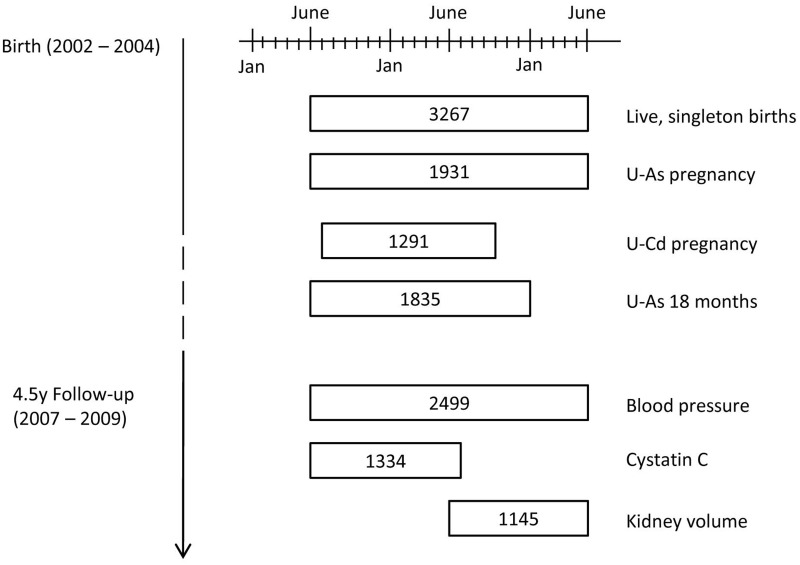
There were 3267 live singleton births during the MINIMat trial, representing the baseline for this follow-up study (Figure 1). Maternal U-Cd levels at 8 weeks’ gestation were available for 1291 individuals whereas U-As was measured in 1707 individuals at 8 weeks’ gestation and 1799 individuals at 30 weeks; 1575 individuals had U-As measurements at both time points. At 18 months of age, U-As concentrations were available for 1835 individuals. In total, 1488 individuals had U-As measurements at all three time points. At 4.5 years of age, 2499 individuals had blood pressure measurements recorded, representing 76% of the original birth cohort. Due to the design of the follow-up, fewer measurements of kidney function were conducted: 1334 and 1145 for CysC and kidney volume, respectively. There were no marked differences in available characteristics between individuals who were lost to follow-up and those who were recruited into the current study, or between the different sub-samples of kidney function assessment (Table 1). The main cause of loss to follow-up was that individuals could not be located during the follow-up study (39% of losses). Refusal by parents or guardians accounted for 28% of losses, out-migration for 22% and the remaining 11% of lost individuals had died between birth and 4.5 years of age.
Figure 1.
Flow diagram of recruitment into current follow-up study. Numbers represent sample sizes of various measurements and how they overlap with the original birth cohort (n = 3267) within the MINIMat trial. U-As pregnancy represents the number of individuals with urinary arsenic measurements from at least one time point in pregnancy: 8 weeks or 30 weeks of gestation. U-Cd refers to urinary cadmium measurement during pregnancy. U-As 18 months refers to child urinary arsenic measurements at 18 months of age
Table 1.
Characteristics of study cohort
| Lost to follow-upa |
Recruited into 4.5 y follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blood pressure sample |
GFR sample |
Kidney volume sample |
|||||||
| N | Mean (SD)b | N | Mean (SD)b | N | Mean (SD)b | N | Mean (SD)b | ||
| Maternal characteristics | |||||||||
| Age (y) | 768 | 26.1 (5.8) | 2499 | 26.6 (6.0) | 1334 | 26.7 (6.0) | 1145 | 26.6 (6.0) | |
| Wealth indexc | 725 | 0.63 (−1.76, 2.00) | 2386 | 0.38 (−1.65, 1.80) | 1332 | 0.10 (−1.93, 1.74) | 1038 | 0.68 (−1.26, 1.91) | |
| Early pregnancy (week 8) BMI (kg/m2) | 767 | 20.2 (2.7) | 2490 | 20.1 (2.7) | 1328 | 19.9 (2.6) | 1142 | 20.3 (2.8) | |
| Maternal urinary arsenic (µg/l) week 8 gest | − | − | 1692 | 80.5 (38, 208) | 1178 | 85 (38, 216) | 675 | 70 (37, 182) | |
| week 30 gest | − | − | 1780 | 82.5 (42, 228) | 1283 | 82 (41, 228) | 661 | 83 (44, 243) | |
| Maternal urinary cadmium(µg/l) week 8 gest | − | − | 1280 | 0.63 (0.38, 1.0) | 1159 | 0.63 (0.38, 1.01) | 280 | 0.63 (0.41, 1.06) | |
| Children characteristics, | |||||||||
| Infancy | |||||||||
| Gestational age (weeks) | 768 | 39.1 (1.9) | 2499 | 39.2 (1.6) | 1334 | 39.2 (1.7) | 1145 | 39.2 (1.6) | |
| Birth weight (kg) | 768 | 2.67 (0.45) | 2498 | 2.70 (0.40) | 1333 | 2.69 (0.40) | 1145 | 2.73 (0.40) | |
| Birth length (cm) | 767 | 48.0 (4.9) | 2497 | 47.86 (2.39) | 1332 | 48.0 (2.54) | 1145 | 47.74 (2.14) | |
| Breastfeeding pattern at 4 monthsd | 673 | 54.7% exclusive, 33.1% predominant | 2484 | 59.7% exclusive, 28.3% predominant | 1328 | 60.8% exclusive, 27.7% predominant | 1136 | 58.3% exclusive, 30.2% predominant | |
| Urinary arsenic at 18 months (µg/l) | − | − | 1816 | 33.9 (18.2, 77.4) | 1275 | 33.6 (18.1, 76.5) | 701 | 33.4 (17.9, 73.6) | |
| 4.5 y follow-up | |||||||||
| Age (y) | − | − | 2499 | 4.6 (0.14) | 1334 | 4.5 (0.14) | 1145 | 4.6 (0.10) | |
| Male (%) | − | − | 2499 | 51.6% | 1334 | 52.7% | 1145 | 50.9% | |
| Height (cm) | − | − | 2499 | 99.8 (4.3) | 1333 | 99.6 (4.7) | 1145 | 99.8 (4.1) | |
| Weight (kg) | − | − | 2499 | 13.76 (1.66) | 1334 | 13.67 (1.62) | 1145 | 13.81 (1.63) | |
| Impedance indexe | − | − | 2266 | 0.13 (0.01) | 1210 | 0.13 (0.01) | 1026 | 0.13 (0.01) | |
| Systolic blood pressure (mmHg) | − | − | 2499 | 91.4 (7.6) | 1321 | 91.3 (7.9) | 1136 | 91.7 (7.5) | |
| Diastolic blood pressure (mmHg) | − | − | 2499 | 54.4 (6.5) | 1321 | 54.2 (6.8) | 1136 | 54.8 (6.1) | |
| Glomerular filtration ratef (ml/min/1.73 m2) | − | − | 1321 | 158.3 (35.2) | 1334 | 158.2 (35.2) | 150 | 164.0 (35.1) | |
| Kidney volume (cm3/m2)g | − | − | 1136 | 103.7 (15.5) | 150 | 104.7 (15.1) | 1145 | 103.8 (15.5) | |
Abbreviations: GFR, glomerular filtration rate; BMI, body mass index.
aLost to follow-up refers to individuals from the original trail births not recruited into current follow-up study.
bFor skewed variables, the median value (interquartile range: 25%, 75%) have been presented.
cWealth index presented as a continuous variable calculated from an asset index.
dBreastfeeding pattern reported from maternal interviews and categorized into exclusive (nothing in addition to breast milk), predominant (breast milk plus other liquids) or partial (breast milk plus food and other milk).
eImpedance index (100/impedance) utilised as an estimate of body fatness.
fGlomerular filtration rate calculated from plasma cystatin C using prediction equations.28
gAverage kidney volume from right and left kidney adjusted for body surface area.29
Pregnant women participating in the MINIMat trial were on average 26 years of age with a median parity of 1 (range: 0, 10). The majority of women were lean with 28% categorized as underweight in early pregnancy (BMI < 18.5 kg/m2). Mean birth weight of infants born during the trial was 2.7 kg and length was 47.9 cm. The prevalence of low birth weight (<2.5 kg) was 30%, and 7% of babies were born before 37 weeks’ gestation. Median maternal urinary arsenic was 80 µg/l (10th, 90th percentile: 24, 383 µg/l) at week 8 of gestation and 83 µg/l (10th, 90th: 26, 415 µg/l) at week 30. Median maternal urinary cadmium was 0.63 µg/l (10th, 90th: 0.25, 1.53 µg/l) at week 8 of gestation. At 18 months, median urinary arsenic was 34 µg/l (10th, 90th: 12, 154 µg/l). Urinary concentrations of arsenic in early and late pregnancy were relatively correlated (r = 0.56), whereas both the 8-week and 30-week measurements were less correlated with measurements in infancy (r = 0.41 and r = 0.34, respectively).
At the 4.5 year-follow-up, children had an average BMI-for-age z-score of −1.66 (SD: 0.94) and a height-for-age z-score of −1.50 (SD: 0.95) compared with UK reference data;33 28% of boys and 33% of girls were classified as stunted (<−2 z-scores). Average systolic blood pressure was 91.1 ± 7.5 mmHg for girls and 91.4 ± 7.7 mmHg for boys, whereas diastolic blood pressure was 55.0 ± 6.4 mmHg for girls and 53.8 ± 6.5 mmHg for boys. Applying height and age-specific cut-offs,34 3.4% of girls and 1.7% of boys were classified as hypertensive. Mean GFR was 158.2 ml/min/1.73 m2 with no difference between boys and girls (data not shown). Mean kidney volume adjusted for body surface area was 103.8 ± 15.5 cm3/m2 for boys and 104.0 ± 15.9 cm3/m2 for girls.
Maternal U-As in pregnancy (average concentration at gestational week 8 and 30) and U-Cd at week 8 of gestation were both inversely associated with parental wealth index in simple linear regression fitting loge dependent variables. Each increasing wealth index quintile was associated with a 6.7% decrease (95% CI: −9.6, −3.7; P: < 0.001) in U-As in pregnancy. For every quintile increase in parental wealth index, U-Cd at week 8 of gestation decreased by 9.6% (95% CI: −12.1, −7.2; P: < 0.001). There were similar associations for loge child U-As at 18 months of age and parental wealth index with a decrease of 5.2% (95% CI: −8.2, −2.1; P: 0.001) for each increase in parental wealth index quintile. U-As in pregnancy was also associated with season of birth and with maternal blood pressure at 8 weeks of gestation (data not shown). U-Cd in pregnancy was not associated with systolic or diastolic blood pressure in early pregnancy (data not shown).
Multiple linear regression analysis of the association between early-life toxic metal exposure and later kidney function revealed no association between U-As or U-Cd and child kidney volume (Table 2). In addition, maternal U-Cd was associated with neither child blood pressure nor GFR. However, in the adjusted analysis, maternal U-As in pregnancy was positively associated with both systolic and diastolic blood pressure at 4.5 years and U-As in infancy was also positively associated with systolic blood pressure at 4.5 years. In the adjusted analysis, exposure to U-As in late (week 30) pregnancy appeared to be the important time point for the association with blood pressure at 4.5 years (Table 3). U-As at 18 months, but not average maternal U-As in pregnancy, was inversely associated with GFR at 4.5 years in the adjusted analysis. However, early pregnancy (week 8) U-As was found to be inversely associated with GFR at 4.5 years in both the unadjusted and adjusted analyses (Table 3). There was no interaction between either the prenatal food or multiple-micronutrient intervention and the effect of urinary arsenic and cadmium on any of the outcomes studied (data not shown). Similarly there was no interaction between child sex, parental wealth index, maternal early pregnancy (week 8) BMI or maternal blood pressure and the effect of U-As and U-Cd on blood pressure or kidney function at 4.5 years (data not shown).
Table 2.
Association between urinary arsenic and cadmium in pregnancy and 18 months and child blood pressure or kidney function at 4.5 y
| Blood pressure (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Systolic |
Diastolic |
Glomerular filtration rate (ml/min/1.72 m2) |
Kidney volume (cm3/m2) |
||||||
| βa (95% CI) | R2 (%)b | β (95% CI) | R2 (%) | β (95% CI) | R2 (%) | β (95% CI) | R2 (%) | ||
| Maternal exposure | |||||||||
| U-As combined exposurec (mg/l) | Model 1d | 2.90 | 0.21 | 2.64 | 0.34 | −23.3 | 0.49 | 3.37 | 0.29 |
| (−0.06, 5.87) | (0.14, 5.15) | (−41.4, −5.3) | (−5.86, 12.60) | ||||||
| Model 2e | 3.69 | 0.28 | 2.91 | 0.45 | −14.2 | 0.02 | 2.89 | 0.002 | |
| (0.74, 6.63) | (0.41, 5.42) | (−32.2, 3.7) | (−6.17, 11.96) | ||||||
| U-Cd 8 weeks (µg/l) | Model 1 | −0.66 | 0.22 | −0.27 | 0.03 | −0.77 | 0.29 | 0.47 | 0.07 |
| (−1.62, 0.29) | (−1.07, 0.54) | (−5.18, 3.64) | (−3.33, 4.28) | ||||||
| Model 2 | −0.49 | 0.14 | −0.21 | 0.06 | −0.17 | 0.20 | 0.79 | 0.06 | |
| (−1.44, 0.45) | (−1.02, 0.59) | (−4.54, 4.20) | (−2.99, 4.57) | ||||||
| Infant exposure | |||||||||
| U-As 18 months (mg/l) | Model 1 | 6.05 | 0.23 | 1.90 | 0.07 | −44.7 | 0.61 | −7.15 | 0.85 |
| (−0.09, 13.0) | (−3.96, 7.76) | (−81.7, −7.8) | (−32.93, 18.63) | ||||||
| Model 2 | 8.25 | 0.36 | 2.75 | 0.08 | −33.4 | 0.36 | −1.90 | 0.77 | |
| (1.37, 15.1) | (−3.09, 8.59) | (−70.2, 3.34) | (−23.45, 27.26) | ||||||
aβ: regression coefficients from linear regression analysis fitting untransformed exposure variables.
bR2 value refers to total R2 for unadjusted models (model 1) and partial R2 for adjusted models (model 2).
cExposure defined as average pregnancy value (week 8 and week 30 of gestation combined).
dModel 1: unadjusted association.
eModel 2: adjusted for sex, age, parental wealth index, height at 4.5 years, season of birth and maternal early pregnancy BP (blood pressure models only).
Table 3.
Timing of urinary arsenic exposure in pregnancy and effect on child blood pressure and kidney function at 4.5 y
|
Blood pressure (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Systolic |
Diastolic |
Glomerular filtration rate(ml/min/1.72 m2) |
Kidney volume (cm3/m2) |
||||||
| βa (95% CI) | R2 (%)b | β (95% CI) | R2 (%) | β (95% CI) | R2 (%) | β (95% CI) | R2 (%) | ||
| Maternal exposure | |||||||||
| U-As 8 weeks (mg/l) | Model 1c | 0.50 | 0.04 | 1.59 | 0.10 | −25.9 | 0.67 | −0.37 | 0.32 |
| (−2.47, 3.47) | (−0.89, 4.07) | (−44.1, −7.7) | (−9.82, 9.09) | ||||||
| Model 2d | 1.45 | 0.06 | 1.75 | 0.15 | −21.2 | 0.44 | 0.75 | 0.04 | |
| (−1.51, 4.41) | (−0.73, 4.22) | (−39.2, −3.2) | (−8.59, 10.08) | ||||||
| U-As 30 weeks (mg/l) | Model 1 | 2.96 | 0.35 | 1.96 | 0.37 | −12.0 | 0.21 | 6.68 | 0.38 |
| (0.02, 5.90) | (−0.52, 4.44) | (−28.7, 4.7) | (−2.59, 15.94) | ||||||
| Model 2 | 3.56 | 0.42 | 2.45 | 0.46 | 0.51 | 0.01 | 6.04 | 0.26 | |
| (0.62, 6.50) | (−0.03, 4.94) | (−16.2, 17.2) | (−3.11, 15.20) | ||||||
aβ: regression coefficients from linear regression analysis fitting untransformed exposure variables
bR2 value refers to total R2 for unadjusted models (model 1) and partial R2 for adjusted models (model 2).
cModel 1: unadjusted association.
dModel 2: adjusted for sex, age, parental wealth index, height at 4.5 y, season of birth and maternal early pregnancy BP (blood pressure models only).
Discussion
The current study provides some evidence that exposure to arsenic in late pregnancy and in infancy may be associated with raised blood pressure in childhood. However, the observed association was very small considering the arsenic exposure experienced by the study population. The average arsenic exposure in pregnancy, i.e. close to 100 µg/l in maternal urine, was associated with an increase in systolic blood pressure of less than 0.4 mmHg at 4.5 years of age. There was also evidence for an inverse association between urinary arsenic in early pregnancy and at 18 months of age and GFR at 4.5 years, but again the small effect size suggests limited clinical or public health importance.
This is one of the first studies to investigate the impact of exposure to toxic metals during in utero and early post-natal development on later cardiovascular health in humans. A number of animal studies have demonstrated cardiovascular-related toxic effects of arsenic35 and cadmium36 exposure during pregnancy. The majority of research on the early-life programming of hypertension and renal function has focused on the impact of nutrition. In experimental studies it has been demonstrated that hypertensive offspring can be produced by a range of nutrient restrictions provided during pregnancy in a variety of species.37–39 One of the postulated mechanisms is a reduction in kidney nephron number, which may lead to hyperfiltration and ultimately to glomerulosclerosis.40,41 In humans, nephrogenesis occurs between weeks 8 and 36 of gestation with no potential for increase after birth, suggesting that pregnancy may be acritical window for development.40 However, data from human studies on the impact of environmental exposures during pregnancy, including diet, are scarce with the majority of evidence derived from studies of birth weight associations.2,3,42
The health effects of chronic arsenic exposure have been extensively studied, particularly in areas such as Bangladesh where contamination of drinking water is often particularly high, and have been found to increase all-cause10,43 and cardiovascular-related mortality.10 Recent data from the National Health and Nutrition Examination Survey (NHANES) in the US found no association in a cross-sectional analysis between urinary arsenic (much lower concentrations, approximately 10% of the present study levels) or cadmium levels and hypertension in US adults.44,45 There was, however, some evidence that blood cadmium levels may be associated with raised diastolic, but not systolic, blood pressure.45 In a recent systematic review of the effect of arsenic exposure on cardiovascular risk, the authors reported that the majority of studies used ecological exposure assessment (drinking water) and that most were not of sufficient quality to allow for a definitive interpretation.11
One of the strengths of the current study was the assessment of arsenic and cadmium at the individual level in contrast to ecological exposure assessment utilized by many studies in this field. As expected, U-As levels showed extremely large variation with a median in pregnancy of 96 μg/l and a maximum of 1632 μg/l. This should be compared with an average 8.3 μg/l in the US population.44 U-As levels in infancy were lower than pregnancy exposures, likely reflecting the fact that many infants were still partially breastfed at this time.25 In contrast to the arsenic exposure, U-Cd concentrations were similar to those found in pregnant Japanese women (U-Cd 0.77 µg/g),46 but around 2-fold higher than in women of corresponding age in Europe47 and the USA.48 Smoking is an important contributor to cadmium exposure in many populations but is less relevant in the current study where smoking among pregnant women is essentially non-existent.49 In addition to the relatively low levels of exposure, the lack of any observed effect of U-Cd may reflect the limited transfer to the foetus that occurs during pregnancy, with most cadmium accumulating in the placenta.5 Still, the pronounced placental cadmium accumulation could be expected to have negative effects on kidney development. We have observed an effect of maternal cadmium exposure on birth weight and child body weight within this cohort,19,20 which may suggest that cardiovascular-related effects could become apparent in later life.
Other important strengths of the current study were the large sample size and accurate outcome measurements. Blood pressure was assessed using an automated device under standardized conditions to minimize observer-related errors. GFR is a well-recognized marker of kidney function and was assessed from plasma cystatin C, which is known to be a more accurate estimation of GFR than plasma creatinine in pediatric populations.50 Kidney volume was chosen as an additional important marker of kidney function and was assessed by highly trained technicians, with good inter-observer agreement. However, the study was limited by the number of available kidney function measurements and lacked an assessment of tubular function. A comparison study of children from three European countries found that whereas urinary cadmium was not associated with GFR, markers of tubular function (including excretion of retinol-binding protein) were raised with increasing cadmium exposure.51 We have observed elevated U-Cd for infants at 3 months of age within a sub-sample of the current cohort that may suggest limited reabsorption by the kidneys, an effect that could be mediated by impaired or immature tubular function.52
A further limitation of the current study is that U-As and U-Cd measurements were not available for all individuals; U-As in infancy and U-Cd were measured on a sub-sample of individuals for financial and logistical considerations. The sub-sample represented a calendar year of recruitment and is representative of the trial participants as a whole. However, one consequence is the reduced sample size for the investigation of kidney volume effects. This may affect the interpretation of the null effect observed, although the confidence interval remained relatively narrow.
In summary, the current study is one of the first to investigate blood pressure and kidney function in childhood in relation to early-life exposure to arsenic and cadmium. The findings suggest no effect of exposure in early gestation but a small positive association between urinary arsenic exposure in late gestation and offspring blood pressure. In contrast, exposure to arsenic in early pregnancy may be inversely associated with GFR in childhood. Exposure to arsenic in infancy was also positively associated with child blood pressure and inversely associated with GFR, albeit again with small effect sizes. Kidney volume was not affected by arsenic or cadmium exposure in this study. The observed small effects may have limited clinical or public health significance and require replication before further conclusions can be drawn on the effect of early-life arsenic and cadmium exposure and later cardiovascular disease risk. It will also be important to follow these same individuals into later life to assess further long-term consequences of exposure.
Funding
This work was supported by the UK Medical Research Council [G0501839] and the Swedish Research Council, the Swedish International Development Cooperation Agency, and Karolinska Institutet. Funds were also obtained via a Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science [#18256005].
Conflict of interest: None declared.
KEY MESSAGES.
Exposure to toxic agents during the critical window of early life may have long lasting impacts on health.
In some countries, including Bangladesh, ingestion of toxic metals such as arsenic and cadmium represent an important public health issue.
Few studies have investigated the impact of exposure to toxic metals during development on cardiovascular risk factors using a prospective design.
The extremely modest effect size of arsenic exposure during pregnancy on blood pressure and kidney function observed here is of limited public health significance.
References
- 1.Gluckman PD, Hanson MA. Developmental Origins of Health and Disease. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 2.Adair L, Dahly D. Developmental determinants of blood pressure in adults. Annu Rev Nutr. 2005;25:407–34. doi: 10.1146/annurev.nutr.25.050304.092538. [DOI] [PubMed] [Google Scholar]
- 3.White SL, Perkovic V, Cass A, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–61. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Gardner R, Hamadani J, Grander M, et al. Persistent exposure to arsenic via drinking water in rural Bangaldesh despite major mitigation efforts. Am J Public Health. 2011;101:S333–388. doi: 10.2105/AJPH.2010.300025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kippler M, Ekstrom EC, Lonnerdal B, et al. Influence of iron and zinc status on cadmium accumulation in Bangladeshi women. Toxicol Appl Pharmacol. 2007;222:221–26. doi: 10.1016/j.taap.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Rahman MM, Ng JC, Naidu R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ Geochem Health. 2009;31(Suppl 1):189–200. doi: 10.1007/s10653-008-9235-0. [DOI] [PubMed] [Google Scholar]
- 7.Schuhmacher-Wolz U, Dieter HH, Klein D, Schneider K. Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects. Crit Rev Toxicol. 2009;39:271–98. doi: 10.1080/10408440802291505. [DOI] [PubMed] [Google Scholar]
- 8.Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicol Sci. 2008;102:207–18. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–28. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Sohel N, Persson LA, Rahman M, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20:824–30. doi: 10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- 11.Navas-Acien A, Sharrett AR, Silbergeld EK, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162:1037–49. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- 12.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44:185–90. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 13.Rahman A, Vahter M, Smith AH, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangalesh. Am J Epidemiol. 2009;169:304–12. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 14.Hopenhayn C, Ferreccio C, Browning SR, et al. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14:593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- 15.von Ehrenstein OS, Guha Mazumder DN, Hira-Smith M, et al. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. Am J Epidemiol. 2006;163:662–29. doi: 10.1093/aje/kwj089. [DOI] [PubMed] [Google Scholar]
- 16.Kippler M, Lonnerdal B, Goessler W, Ekstrom EC, Arifeen SE, Vahter M. Cadmium interacts with the transport of essential micronutrients in the mammary gland–a study in rural Bangladeshi women. Toxicology. 2009;257:64–69. doi: 10.1016/j.tox.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Lin CM, Doyle P, Wang D, Hwang YH, Chen PC. Does prenatal cadmium exposure affect fetal and child growth? Occup Environ Med. 2011;68:641–46. doi: 10.1136/oem.2010.059758. [DOI] [PubMed] [Google Scholar]
- 18.Salpietro CD, Gangemi S, Minciullo PL, et al. Cadmium concentration in maternal and cord blood and infant birth weight: a study on healthy non-smoking women. J Perinat Med. 2002;30:395–99. doi: 10.1515/JPM.2002.061. [DOI] [PubMed] [Google Scholar]
- 19.Kippler M, Tofail F, Gardner R, et al. Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect. 2012;120:284–89. doi: 10.1289/ehp.1103711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner R, Kippler M, Tofail F, et al. Environmental exposures to metals and children's growth to five years: a prospective cohort study. Am J Epidemiol. doi: 10.1093/aje/kws437. 2012; doi:10.1093/aje/kws437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persson LA, Arifeen SE, Ekstrom EC, et al. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh. JAMA. 2012;307:2050–59. doi: 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- 22.Rahman MM, Owens G, Naidu R. Arsenic levels in rice grain and assessment of daily dietary intake of arsenic from rice in arsenic-contaminated regions of Bangladesh–implications to groundwater irrigation. Environ Geochem Health. 2009;31(Suppl 1):179–87. doi: 10.1007/s10653-008-9238-x. [DOI] [PubMed] [Google Scholar]
- 23.Cascio C, Raab A, Jenkins RO, Feldmann J, Meharg AA, Haris PI. The impact of a rice based diet on urinary arsenic. J Environ Monit. 2011;13:257–65. doi: 10.1039/c0em00482k. [DOI] [PubMed] [Google Scholar]
- 24.Vahter ME, Li L, Nermell B, et al. Arsenic exposure in pregnancy: a population-based study in Matlab, Bangladesh. J Health Popul Nutr. 2006;24:236–45. [PubMed] [Google Scholar]
- 25.Fangstrom B, Hamadani J, Nermell B, Grander M, Palm B, Vahter M. Impaired arsenic metabolism in children during weaning. Toxicol Appl Pharmacol. 2009;239:208–14. doi: 10.1016/j.taap.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Nermell B, Lindberg AL, Rahman M, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106:212–18. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Flodin M, Jonsson AS, Hansson LO, Danielsson LA, Larsson A. Evaluation of Gentian cystatin C reagent on Abbott Ci8200 and calculation of glomerular filtration rate expressed in mL/min/1.73 m2 from the cystatin C values in mg/L. Scand J Clin Lab Invest. 2007;67:560–67. doi: 10.1080/00365510601187773. [DOI] [PubMed] [Google Scholar]
- 28.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–31. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 29.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 30.Fulford AJ, Rayco-Solon P, Prentice AM. Statistical modelling of the seasonality of preterm delivery and intrauterine growth restriction in rural Gambia. Paediatr Perinat Epidemiol. 2006;20:251–59. doi: 10.1111/j.1365-3016.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 31.Kahn AI, Hawkesworth S, Hossain D, et al. Body composition of Bangladeshi children: comparison and development of leg-to-leg bioelectrical impedance equation. J Health Popul Nutr. 2012;30:281–90. doi: 10.3329/jhpn.v30i3.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha KK, Frongillo EA, Alam DS, Arifeen SE, Persson LA, Rasmussen KM. Appropriate infant feeding practices result in better growth of infants and young children in rural Bangladesh. Am J Clin Nutr. 2008;87:1852–59. doi: 10.1093/ajcn/87.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–29. [PubMed] [Google Scholar]
- 34.NHBPEP. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98:649–58. [PubMed] [Google Scholar]
- 35.Srivastava S, D'Souza SE, Sen U, States JC. In utero arsenic exposure induces early onset of atherosclerosis in ApoE-/- mice. Reprod Toxicol. 2007;23:449–56. doi: 10.1016/j.reprotox.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacquillet G, Barbier O, Rubera I, et al. Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am J Physiol Renal Physiol. 2007;293:F1450–60. doi: 10.1152/ajprenal.00223.2007. [DOI] [PubMed] [Google Scholar]
- 37.Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol. 2005;16:2545–56. doi: 10.1681/ASN.2005030300. [DOI] [PubMed] [Google Scholar]
- 38.Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- 39.McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats – a review. Placenta. 2006;27(Suppl A):S56–60. doi: 10.1016/j.placenta.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. J Nutr. 2007;137:1066–72. doi: 10.1093/jn/137.4.1066. [DOI] [PubMed] [Google Scholar]
- 41.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? Am J Kidney Dis. 1995;26:91–98. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 42.Hawkesworth S, Walker CG, Sawo Y, et al. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in The Gambia. Am J Clin Nutr. 2011;94:S1853–60. doi: 10.3945/ajcn.110.000877. [DOI] [PubMed] [Google Scholar]
- 43.Argos M, Kalra T, Rathouz PJ, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376:252–58. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones MR, Tellez-Plaza M, Sharrett AR, Guallar E, Navas-Acien A. Urine Arsenic and Hypertension in US Adults: The 2003-2008 National Health and Nutrition Examination Survey. Epidemiology. 2011;22:153–61. doi: 10.1097/EDE.0b013e318207fdf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition Examination Survey (NHANES) Environ Health Perspect. 2008;116:51–56. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010;45:1468–74. doi: 10.1080/10934529.2010.500942. [DOI] [PubMed] [Google Scholar]
- 47.Akesson A, Berglund M, Schutz A, Bjellerup P, Bremme K, Vahter M. Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health. 2002;92:284–87. doi: 10.2105/ajph.92.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter PA, Bishop EE, Wang J, Swahn MH. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Int J Environ Res Public Health. 2009;6:1930–46. doi: 10.3390/ijerph6071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindberg AL, Ekstrom EC, Nermell B, et al. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res. 2008;106:110–20. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Narvaez-Sanchez R, Gonzalez L, Salamanca A, et al. Cystatin C could be a replacement to serum creatinine for diagnosing and monitoring kidney function in children. Clin Biochem. 2008;41:498–503. doi: 10.1016/j.clinbiochem.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 51.de Burbure C, Buchet JP, Leroyer A, et al. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006;114:584–90. doi: 10.1289/ehp.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kippler M, Nermell B, Hamadani J, Tofail F, Moore S, Vahter M. Burden of cadmium in early childhood: longitudinal assessment of urinary cadmium in rural Bangladesh. Toxicol Lett. 2010;198:20–25. doi: 10.1016/j.toxlet.2010.04.029. [DOI] [PubMed] [Google Scholar]