Abstract
The General Population Cohort (GPC) was set up in 1989 to examine trends in HIV prevalence and incidence, and their determinants in rural south-western Uganda. Recently, the research questions have included the epidemiology and genetics of communicable and non-communicable diseases (NCDs) to address the limited data on the burden and risk factors for NCDs in sub-Saharan Africa. The cohort comprises all residents (52% aged ≥13years, men and women in equal proportions) within one-half of a rural sub-county, residing in scattered houses, and largely farmers of three major ethnic groups. Data collected through annual surveys include; mapping for spatial analysis and participant location; census for individual socio-demographic and household socioeconomic status assessment; and a medical survey for health, lifestyle and biophysical and blood measurements to ascertain disease outcomes and risk factors for selected participants. This cohort offers a rich platform to investigate the interplay between communicable diseases and NCDs. There is robust infrastructure for data management, sample processing and storage, and diverse expertise in epidemiology, social and basic sciences. For any data access enquiries you may contact the director, MRC/UVRI, Uganda Research Unit on AIDS by email to mrc@mrcuganda.org or the corresponding author.
Keywords: Data resource profile, general population cohort, communicable and non-communicable diseases, Uganda
Data Resource basics
Rationale, collaborations and funding
The General Population Cohort (GPC) is a population-based open cohort study established in 1989 by the Medical Research Council (MRC) UK in collaboration with the Uganda Virus Research Institute (UVRI) to examine trends in prevalence and incidence of HIV infection and their determinants. This cohort is funded by MRC UK and the UK Department for International Development.
Since 2010, the scientific research questions have incorporated the epidemiology and genetics of both communicable and non-communicable diseases (NCDs). Although NCDs are projected to become the most common causes of death in Africa by 2030,1 their magnitude, distribution and risk factors have not been fully studied in a large-scale epidemiological context in sub-Saharan Africa. The GPC provides a unique framework for building on a large-scale prospective cohort study in a sub-Saharan African population to examine a wide range of health indices. This provides the foundation for long-term studies providing evidence for health policy and public health programmes in Uganda and other countries in sub-Saharan Africa. Observational studies suggest that infectious diseases such as HIV and tuberculosis may be risk factors for type 2 diabetes.2 The collaboration with the University of Cambridge and the Wellcome Trust Sanger Institute has strengthened the GPC platform and enabled both NCDs and infectious diseases to be studied in parallel, improving efficiency while allowing investigation of reciprocal relationships between communicable and non-communicable diseases.
Genetic studies
The introduction of genetic studies into the GPC was driven by the dearth of genetic epidemiological studies in sub-Saharan African populations. Most of the studies have focused on populations of European descent.3–5 To generalize findings from genetic studies of complex diseases it is important to examine genetic susceptibility in a global context, with studies in sub-Saharan African populations, where genetic diversity is greatest,6,7 forming an integral component of this effort. The complex patterns of genetic diversity and gene expression in modern human populations are the result of varying evolutionary histories shaped by differing environmental and biological factors.8,9 This diversity might account for some differences in the prevalence of complex diseases and distribution of risk factor traits among populations.10,11 Genetic analysis has the potential to: discover novel disease susceptibility loci and variants; assess the structure of association signals; and refine (fine-map) association signals at new and existing disease and trait loci.12
Data Resource area and population coverage
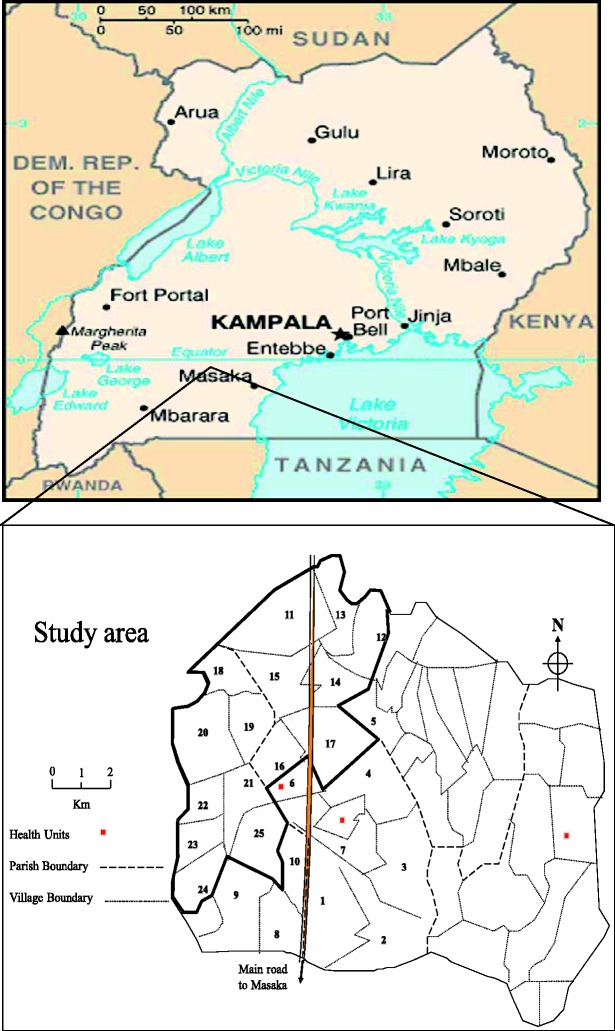
The study area is located in south-western Uganda in Kyamulibwa sub-county of Kalungu district, approximately 120 km from Entebbe town (where the MRC/UVRI office and central laboratories are located). The study area is divided into villages defined by administrative boundaries varying in size from 300 to 1500 residents. From 1989–99, the initial study population of about 10 000 residents comprised a cluster of 15 neighbouring villages and a pilot village for pre-testing study tools and procedures. From 1999, ten more adjacent villages with comparable characteristics,13 were added to the cohort, doubling the population. At the centre of the study area (village 17; Figure 1) is the MRC/UVRI field station with administrative offices, laboratories and study clinics.
Figure 1.
Map showing study villages in Kyamulibwa sub-county, Uganda
Survey frequency
The study population is recruited through annual house-to-house ‘rounds’ of census through which participants for the medical surveys are selected. To be eligible for the census, an individual must have spent or be planning to spend at least 3 months in a household within the study area. All residents aged 13 years and above were included in all the medical survey rounds 1–22 (1989–2011). For the first seven medical survey rounds (1989–96) all residents (all ages) were eligible for the survey, although children under 13 years were only surveyed for de-worming in rounds 5–7 (1993–96). In rounds 8–10 (1996–99) children under 13 years were excluded. From survey rounds 11–22 (1999–2011) all children aged 0–2 years were included in the medical survey but all the children aged 3–12 years were only surveyed in rounds 11(1999–2000), 13 (2001–02), 16 (2004–05), 19 (2007–08), and 22 (2010–11). In the rest of the rounds, only children aged 3–12 years exposed to maternal HIV infection or with previous positive HIV- tests or those missed in the previous round to be surveyed were included (Table 1). All these exclusions were driven by study themes and resources.
Table 1.
Summary of GPC participation for rounds 1-22
| Medical survey participation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Census Participation |
0–2 yrs |
3–12 yrs |
13+ yrs |
All age groups |
|||||||
| Year(s) | Survey round | Households | (%) | n | (%) | n | (%) | n | (%) | Total | (%) |
| 2010/11 | 22 | 3771 | 95.4 | 1571 | 83.3 | 5975 | 90.8 | 7830 | 73.1 | 15 376 | 80.2 |
| 2009/10 | 21 | 3709 | 95.0 | 1318 | 72.4 | 922b | N/A | 7343 | 71.4 | 7343 | 71.4 |
| 2008/09 | 20 | 3698 | 99.9 | 1239 | 72.3 | 956b | N/A | 6686 | 72.4 | 6686 | 72.4 |
| 2007/08 | 19 | 3777 | 96.2 | 1138 | 62.4 | 4909 | 78.1 | 6220 | 63.0 | 12 267 | 68.2 |
| 2006/07 | 18 | 3732 | 99.9 | 1274 | 68.7 | 1738b | N/A | 6253 | 66.5 | 6253 | 66.5 |
| 2005/06 | 17 | 3705 | 97.0 | 1312 | 73.3 | 1400b | N/A | 6251 | 66.5 | 6251 | 66.5 |
| 2004/05 | 16 | 3666 | 99.9 | 1259 | 67.4 | 4757 | 76.7 | 6378 | 66.3 | 12 394 | 70.0 |
| 2003/04 | 15 | 3600 | 99.9 | 1373 | 72.0 | 805b | N/A | 6932 | 72.0 | 6932 | 72.0 |
| 2002/03 | 14 | 3644 | 99.8 | 1169 | 61.9 | 678b | N/A | 6517 | 68.2 | 6517 | 68.2 |
| 2001/02 | 13 | 3631 | 99.9 | 1567 | 79.7 | 5343 | 86.5 | 6709 | 71.4 | 13 619 | 77.7 |
| 2000/01 | 12 | 3536 | 97.5 | 1323 | 65.9 | 1293b | N/A | 6802 | 73.2 | 6802 | 73.2 |
| 1999/00 | 11 | 3558 | 96.8 | 1476 | 75.1 | 4728 | 80.8 | 6315 | 69.8 | 12 519 | 74.2 |
| 1998/99 | 10 | 2282 | 97.9 | 0 | 0.0 | 0 | 0 | 4294 | 75.0 | 4294 | 75.0 |
| 1997/98 | 9 | 2102 | 97.9 | 0 | 0.0 | 0 | 0 | 4121 | 75.2 | 4121 | 75.2 |
| 1996/97 | 8 | 2010 | 97.9 | 0 | 0.0 | 0 | 0 | 3279 | 62.6 | 3279 | 62.6 |
| 1995/96 | 7a | 1917 | 98.8 | 563 | 54.3 | 1970 | 56.9 | 3338 | 65.6 | 5871 | 61.2 |
| 1994/95 | 6a | 1970 | 98.7 | 555 | 53.9 | 2124 | 60.7 | 3340 | 65.1 | 6019 | 62.3 |
| 1993/94 | 5a | 2017 | 98.0 | 722 | 66.0 | 2580 | 73.2 | 3464 | 66.3 | 6766 | 68.8 |
| 1992/93 | 4 | 2006 | 97.2 | 880 | 76.8 | 2784 | 78.6 | 3116 | 59.0 | 6780 | 68.0 |
| 1991/92 | 3 | 2056 | 96.9 | 991 | 79.0 | 2897 | 80.3 | 3607 | 66.8 | 7495 | 73.0 |
| 1990/91 | 2 | 1995 | 98.6 | 994 | 88.6 | 2894 | 84.6 | 3956 | 76.2 | 7844 | 80.6 |
| 1989/90 | 1 | 1806 | 95.1 | 969 | 90.5 | 2996 | 90.7 | 4336 | 88.2 | 8301 | 89.4 |
aSurvey rounds during which children (0–12yrs) were only included for de-worming. No other survey information is available for this age group in these rounds.
bdenotes number of children aged 3–12 years with either previous maternal HIV exposure, or HIV positive test in the previous round or those who were missed in the previous round survey. They were excluded in the total participation because their denominator could not easily be estimated.
Demographic and social characteristics of the study population at round 22 (2010–11) show a relatively young population with about 90% of the population less than 50 years of age, predominantly engaged in subsistence farming. The major ethnic group are Baganda (75%), followed by immigrants from Rwanda (16%) and Burundi (3%) and other tribes from Uganda and Tanzania (6%) who initially came as casual labourers. Only about 13% of the residents attained education beyond primary level (not shown in tables).
Overall, over 95% of households approached for census participated. The medical survey participation varied from year to year. Participation for 13 medical survey rounds (1989–92, 1997–2002, 2003–05 and 2008–11) was consistently above 70% with the first two rounds and for the latest round above 80%. Participation in the rest of the rounds was above 60% (Table 1).
Data from round 22 shows that the surveyed population of children aged 0–12 years was representative of those in the census (Table 2). Adult survey characteristics showed a higher participation of women than men, and of those ever married (currently married, widowed and separated) than never married. Children, siblings and grandchildren to the household head were underrepresented in the survey in comparison with the spouse and parent of the household head. As expected, those usually resident had better participation than those who spent less time in their households. The Baganda had a lower participation than the other ethnic groups in the study population. Religion had no influence on participation (Table 2).
Table 2.
Comparison of medical survey responders and eligible population
| Aged 0–12 years old |
Aged 13 years and above |
|||||
|---|---|---|---|---|---|---|
| Variable | N | n(%) | p-value | N | n(%) | p-value |
| Sex | 0.57 | <0.01 | ||||
| Male | 4302 | 3760 (87.4) | 5144 | 3373 (65.6) | ||
| Female | 4351 | 3735 (85.8) | 5874 | 4352 (74.1) | ||
| Age group | 0.34 | <0.01 | ||||
| 0–2 yrs | 1932 | 1627 (84.2) | ||||
| 3–12 yrs | 6721 | 5868 (87.3) | ||||
| 13–24 yrs | 4923 | 3136 (63.7) | ||||
| 25–34 yrs | 1921 | 1366 (71.1) | ||||
| 35–44 yrs | 1529 | 1143 (74.8) | ||||
| 45+ yrs | 2645 | 2080 (78.6) | ||||
| Census status | 0.10 | 0.36 | ||||
| Newborn | 529 | 438 (82.8) | ||||
| Resident | 6642 | 5862 (88.3) | 9281 | 6555 (70.6) | ||
| Internal movers | 616 | 470 (76.3) | 817 | 569 (69.7) | ||
| Immigrant | 866 | 725 (83.7) | 920 | 601 (65.3) | ||
| Time spent in household | 0.14 | <0.01 | ||||
| Normally resident | 6423 | 5682 (88.5) | 8280 | 6112 (73.8) | ||
| 6–12 mths | 1226 | 1013 (82.6) | 1334 | 843 (63.2) | ||
| 0–5 mths | 919 | 733 (79.8) | 960 | 589 (61.4) | ||
| Comes and goes | 80 | 66 (82.5) | 429 | 177 (41.3) | ||
| Usually resident | 0.45 | <0.01 | ||||
| Yes | 8516 | 7387 (86.7) | 10 470 | 7491 (71.6) | ||
| No | 91 | 70 (76.9) | 452 | 178 (39.4) | ||
| Marital status | <0.01 | |||||
| Never married | 8412 | 7284 (86.6) | 4888 | 3033 (62.1) | ||
| Currently married | 4134 | 3131 (75.7) | ||||
| Widowed | 674 | 526 (78.0) | ||||
| Divorced/separated | 1197 | 949 (79.3) | ||||
| Relationship in household | <0.01 | <0.01 | ||||
| Household head | 3613 | 2640 (73.1) | ||||
| Spouse | 2057 | 1693 (82.3) | ||||
| Child | 6302 | 5458 (86.6) | 3313 | 2073 (62.9) | ||
| Parent | 73 | 51 (69.9) | ||||
| Sibling | 68 | 57 (83.8) | 347 | 221 (63.7) | ||
| Grandchild | 1809 | 2140 (79.4) | 894 | 564 (63.1) | ||
| Other relative | 455 | 387 (85.1) | 594 | 392 (66.0) | ||
| Not related | 17 | 12 (70.6) | 122 | 87 (71.3) | ||
| Tribe | 0.85 | <0.01 | ||||
| Muganda | 6402 | 5551 (86.7) | 8413 | 5800 (68.9) | ||
| Rwandese/Burundi | 1256 | 1111 (88.5) | 1910 | 1438 (75.3) | ||
| Other | 466 | 395 (84.5) | 661 | 470 (71.1) | ||
| Religion | 0.79 | 0.31 | ||||
| Catholics | 4529 | 3946 (87.1) | 6371 | 4522 (71.0) | ||
| Protestant | 917 | 821 (89.5) | 1293 | 936 (72.4) | ||
| Muslim | 2373 | 2041 (86.0) | 2808 | 1878 (66.9) | ||
| Other | 324 | 265 (81.8) | 528 | 379 (71.8) | ||
Measures
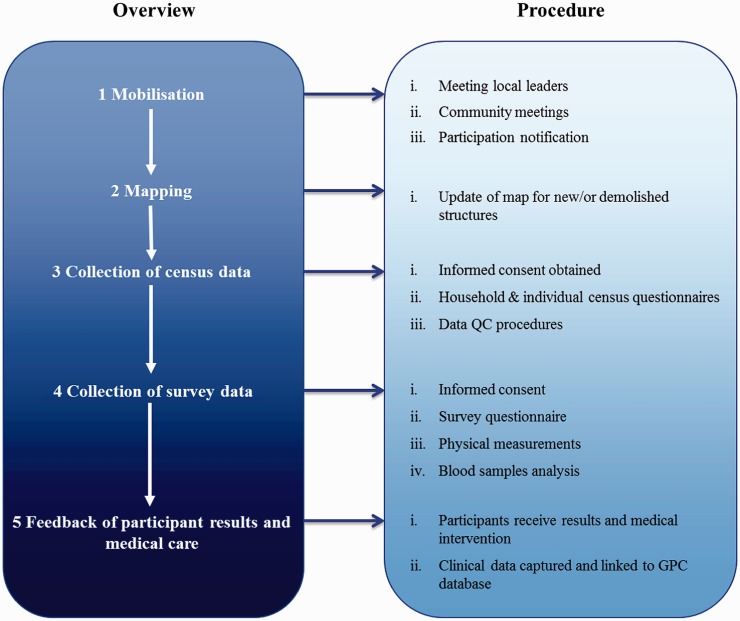
The recruitment and data collection process is broadly divided into five stages as outlined in Figure 2.
Figure 2.
GPC annual study round overview
Participant engagement and village mapping
Community mobilisation precedes the recruitment process and focuses on engaging with participants at both community and individual levels. Local leaders are first sensitised about study activities and their permission sought before holding community meetings. Community mobilisation is followed by mapping, then the census and medical survey. In 2008, hand-drawn maps were replaced by mapping using Geographical Positioning System (GPS) technology, precisely locating all dwellings and demarcating village boundaries and principal geographical features within the study area.
A census questionnaire is administered to a household head or adult representative to collect individual demographic and household socioeconomic data. For individuals who decline, reasons for non-participation are recorded. Where no respondent is available, three attempts are made to revisit the household.
Medical survey
Using the census database, eligible individuals are selected and requested to participate in the medical survey. Data on health and lifestyle are collected using a standard individual questionnaire, blood samples obtained and biophysical measurements taken, when necessary. In rounds 20 (2008–09) and 22 (2010–11) the original HIV risk questionnaire was adapted to obtain socio-demographic indices, sexual behaviour, lifestyle (diet, tobacco and alcohol consumption), medical history and biophysical measurements (height, weight, waist and hip circumferences and blood pressure) data in one interview session.
Blood samples are transported to MRC/UVRI laboratories in Entebbe. A portion of the venous blood sample is analysed according to protocol guidelines and the remaining portion is stored at –80°C. Remnant cells from serum samples are also collected and stored to provide DNA for future genetic analyses. Serum samples from participants over the previous 22 years are currently in storage. Such bio-banking of samples is invaluable for longitudinal and retrospective analysis. Most tests are done at the MRC/UVRI Uganda laboratories except for genetic tests, including tests for haemoglobinopathies, which are carried out at the Wellcome Trust Sanger Institute.
Clinical follow-up and care: integration and technology
A clinic located at the field station provides general health care to all study participants who present with acute medical conditions (malaria and acute respiratory tract infections among others) and chronic diseases such as HIV, hepatitis B and C, hypertension, diabetes and dyslipidaemia identified during medical surveys. The same participant identification numbers used in the census and survey are maintained in the clinic e-database making it possible to link these data to the survey data sets. When needed, clinicians can access previous clinical records and lifestyle and biophysical data collected during medical surveys to improve clinical assessments and medical decisions. The electronic system also ensures that data are organised and linked for research purposes.
Ethical considerations
Before all survey procedures including interviews, blood tests and sample storage for future use, written consent or assent in conjunction with parental/guardian consent for those less than 18 years of age, are obtained following Uganda National Council of Science and Technology (UNCST) guidelines.14 Written consent/assent is also obtained from participants on the use of their clinical records for research purposes. All study procedures including material transfer agreements are approved annually by the Uganda Virus Research Institute Science and Ethics Committee and the UNCST.
Data management and analysis
Households and individuals are assigned identification numbers during mapping and census. The GPS mapping data are downloaded onto a database and analysed using Arc-GIS software giving geographical and social details for spatial disease trends and risk analysis. Electronic data capture was introduced in 2009–10 to replace paper questionnaires. For the census and medical surveys, the pre-programmed e-questionnaire is prepared and loaded onto hand-held portable computers. The programme is linked to the central census database from previous rounds, allowing easy identification of participants. This ensures that data from previous rounds and between the census and survey are linked for each participant. The e-questionnaires for census and survey are also programmed to perform automatic data checks such as double entry of numbers, plausibility of answers (for instance ‘age of starting to smoke cannot be older than age of participant’), numerical limitations within ranges of plausibility and automatic question skips based on previous answers. The immediate availability of electronic data, without the need for separate data entry, has greatly increased the efficiency and effectiveness of data cleaning, checking and management. The availability of the electronic clinic database also provides an opportunity to link these data with the survey data during analysis of risk factors or disease outcomes and directly assess the prevalence of clinical disease.
Data available
As shown in Table 3, most of the survey rounds collected individual and household level demographic data, sexual behaviour and reproductive health.
Table 3.
Brief overview of data collected during the GPC rounds
| Study rounds |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of information | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| Demographics | ||||||||||||||||||||||
| Age | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Sex | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Marital status | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Education level | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Past illness, treatment and immunisation | ||||||||||||||||||||||
| Recent severe illness | X | X | X | X | ||||||||||||||||||
| Hospital admissions | X | X | X | X | X | |||||||||||||||||
| Mental health | X | |||||||||||||||||||||
| Blood transfusions | X | X | X | X | X | X | X | |||||||||||||||
| History of immunisation | X | |||||||||||||||||||||
| Sexual and reproductive health and behaviour | ||||||||||||||||||||||
| Sex education | X | |||||||||||||||||||||
| Sexual partners and behaviour | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Condom use | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Family planning | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Pregnancy and outcomes | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Number of children | X | X | X | X | X | X | X | X | X | |||||||||||||
| Failed pregnancy attempts | X | X | ||||||||||||||||||||
| Menstruation | X | X | X | X | ||||||||||||||||||
| Genital symptoms and treatment | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| HIV | ||||||||||||||||||||||
| Prevention of mother to child transmission | X | X | X | X | ||||||||||||||||||
| Service knowledge, access and use | X | X | X | X | X | X | X | |||||||||||||||
| Testing and status disclosure | X | X | X | X | X | X | X | X | ||||||||||||||
| Circumcision | X | X | ||||||||||||||||||||
| Lifestyle and non-communicable diseases | ||||||||||||||||||||||
| Smoking | X | X | ||||||||||||||||||||
| Alcohol | X | X | X | X | ||||||||||||||||||
| Physical activity | X | |||||||||||||||||||||
| Diet | X | X | X | |||||||||||||||||||
| Treatment-seeking history | X | |||||||||||||||||||||
| Physical examination | ||||||||||||||||||||||
| Signs of infectious diseases | X | X | X | X | X | X | X | |||||||||||||||
| Eye health and vision | X | X | X | |||||||||||||||||||
| Blood pressure | X | X | ||||||||||||||||||||
| Weight and height | X | X | X | X | X | X | X | |||||||||||||||
| Waist and Hip circumference | X | X | ||||||||||||||||||||
| Laboratory examination of blood samples | ||||||||||||||||||||||
| HIV | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Glucose | X | |||||||||||||||||||||
| HbA1c | X | |||||||||||||||||||||
| Hepatitis B and C | X | |||||||||||||||||||||
| Lipids | X | |||||||||||||||||||||
| Liver function tests | X | |||||||||||||||||||||
| Haemoglobinopathies | X | |||||||||||||||||||||
| Full blood cell count | X | |||||||||||||||||||||
| Genetic tests | X | |||||||||||||||||||||
Limited data on NCD risk were collected in earlier survey rounds, smoking in rounds 5 and 21 and alcohol in rounds 8 and 12. Weight and height were measured annually in the first four rounds and round 6. In round 20, the following data were collected on NCD risk: weight, height, waist and hip circumference, blood pressure, blood glucose and smoking. During round 22 all the major modifiable cardiometabolic risk factors were assessed together with infectious biomarkers (HIV, hepatitis B and C) (Table 3).
Blood samples for DNA extractions are available for more than 8000 participants. To date, genotype data have been generated for more than 5000 participants using the Illumina HumanOmni2.5 BeadChip. These extensive genotype data combined with the NCD and additional health data collected from the participants constitute a unique resource to understand human genome diversity in sub-Saharan Africa and the aetiology of communicable and non-communicable diseases. Using the familial study design, up to 100 founders within each family have undergone whole genome sequencing. Additional whole genome sequencing studies are now under way.
Data resource use
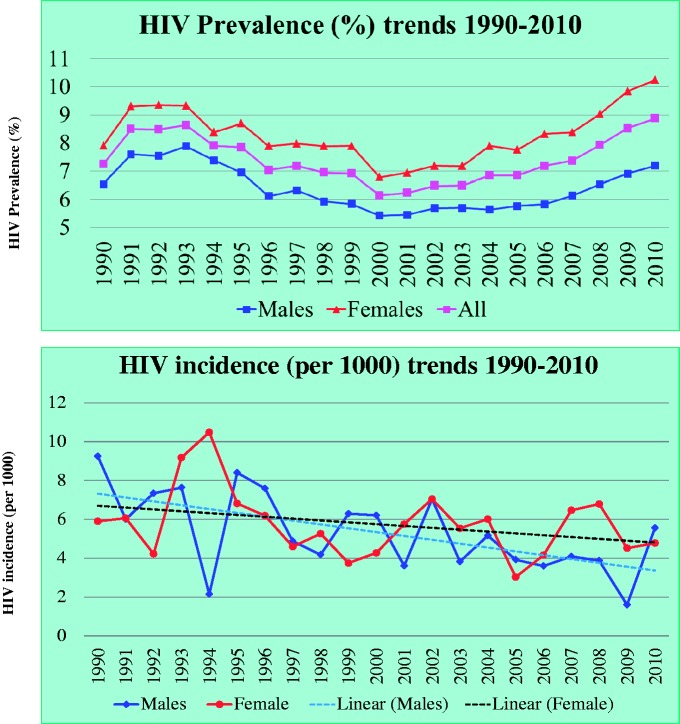
The GPC has made substantial contributions on trends of HIV prevalence and incidence that have been used for planning national HIV/AIDS programmes in Uganda, and globally. As shown in Figure 3, HIV prevalence decreased from 8.5% in 1991 to 6.1% in 2000 and remained fairly the same until 2005 when it steadily rose to 8.9% in 2010 in the presence of a steadily declining HIV incidence. This trend is attributable to better survival due to improvements in HIV care from the introduction of ART, as shown by a significant drop in death rates after introduction of ART in a recently published work from the same population.15. The recent Uganda AIDS indicator survey results showed a rise in HIV prevalence up from 6.4% in 2005 to 7.3% in 2011.16 In the absence of national population-based mortality data in relation to ART, the GPC findings provide a possible reason for the rise in HIV prevalence.
Figure 3.
Trends of HIV prevalence and incidence in the GPC over 20 years
The key findings of the GPC have been published as scientific papers (approximately 90 original articles between 1991 and 2012, most of which are available on the MRC/UVRI website: http://www.mrcuganda.org/Publications.html). Some of the key publications are as follows:
Trends of HIV epidemic and risk factors in rural Uganda: changes in sexual behaviour and other risk factors shaping the epidemic in a rural African setting.17–35
HIV-associated mortality: HIV-associated mortality in a rural population in Uganda, largely before the introduction of anti-retroviral therapy including the survival of children born to HIV-infected mothers.36–42
HIV infection and effect on children in rural communities: the effects of HIV as seen in orphanhood and child nutrition.43–46
HIV infection and fertility: highlighting the impact of HIV infection on fertility.47
Sexually transmitted infections: prevalence and incidence of sexually transmitted infections in rural Uganda and the contribution of this to HIV infection.48–50
Eye and dental problems: exploration of the occurrence of visual and dental problems in this rural population.51–55
NCDs: epidemiology with specific emphasis on the prevalence of cardiovascular risk factors at population level has also been assessed.56,57
Social science contributions: in understanding the behavioural characteristics of the study population in relation to participation in research,58,59 HIV risks,60–65 uptake of health services66,67 and impact of the epidemic on households and individuals.68–70
Basic science: evaluation HIV testing algorithms,71 HIV sub-type distribution and its relationship to HIV progression,72–77 and the association between hepatitis G and HIV infection.78
Methodological studies: evaluation of respondent-driven sampling in the GPC by comparing estimates from a respondent-driven sampling survey with total population data.79–81
Older people studies: focusing on the health and functional status of older people affected by HIV.82,83
The involvement of the GPC in the ALPHA (Analysing Longitudinal Population-based HIV/AIDS data on Africa) network: has resulted in a considerable HIV epidemiological research output, both in terms of papers based on GPC data and collective ALPHA network papers which include GPC data.84
GPC contribution to international policy on NCDs: research in round 20 contributed to the development of international policy on NCD research.85–87
Main strengths and weaknesses
A major strength of the GPC is conducting frequent census rounds and high participation especially for the census, which provides a relevant sampling frame for the survey and a database for imputing survey data for non-participants. A further strength is the availability of information at community, household and individual levels, providing a framework for multiple-level determinants of disease outcomes. Having no upper age limit for the survey provided an opportunity to study disease trends in a largely neglected older population. An additional strength of this cohort is the statistical power afforded by the large sample size, allowing us to explore multiple determinants of disease outcomes. The multidisciplinary approach, encompassing epidemiology and social and basic science, allows investigators to put together expert knowledge from their disciplines to provide a thorough and comprehensive analysis of the study outcomes. An equally important strength is the support of the community to the research activities in this area. This support has been achieved through continuous dialogue with the communities, resulting in a high level of trust and response to potentially sensitive questions. The research clinic established to meet the basic health care needs of the study participants provides an opportunity to merge clinic data with survey data to explore disease trends and possible interventions. The long experience of staff and the introduction in 2009 of electronic data capture has provided high quality data and research of an international standard in a resource-constrained context.
A potential limitation of the GPC is that this population has, to some extent, been ‘sensitised to epidemiological research’, especially on HIV and sexuality, leading to refusals and influencing participant behaviour, in some cases. However, participation has been greatly improved with the change of focus to NCDs. Although only limited longitudinal data on NCDs has been collected in this cohort, there is a unique opportunity to use stored samples that could potentially provide important insights into NCDs and their risk factors and also chronic infection, including hepatitis virus infections. Being a relatively homogeneous rural sub-Saharan African population, genetic findings may not be generalisable to all populations in the region where there are multiple ethnic differences. This highlights the importance of conducting studies across sub-Saharan Africa to fully understand differences in chronic disease risk attributable to environmental and genetic determinants.
Data resource access
The GPC database is rich, with 22 years of longitudinal data sets on demographics and disease surveillance. All data (census, survey and laboratory) generated through the cohort are stored and curated at the MRC/UVRI Uganda Research Unit on AIDS. For any data access inquiries you may contact the director, MRC/UVRI, Uganda Research Unit on AIDS by email to mrc@mrcuganda.org or the corresponding author. Genomic data are stored at the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) in the European Genome-phenome Archive (EGA). Requests for access to genomic data in the EGA are managed by the data access committee of the African Partnership for Chronic Disease Research (www.apcdr.org).
Funding
This work was supported by the Medical Research Council UK core funds through all the years, by a Wellcome Trust grant 2009–11 ‘Improving access and quality of data from a longitudinal HIV cohort in Uganda’ and [grant number MSU-G0901213] for the NCD (GPC round 22) by support from Wellcome Trust Sanger Institute for genomic studies. D.M. was a co-applicant on the Wellcome Trust grant from 2009–11 ‘Improving access and quality of data from a longitudinal HIV cohort in Uganda’, which was before he joined the Wellcome Trust in July 2012.
Acknowledgements
We gratefully acknowledge this funding. We thank the participants, community leaders, the GPC study teams and investigators for their tremendous contributions in various ways. Specifically we recognise the contribution of and thank the following project leaders: H.U. Wagner, Sam Mwiidu Mbulaiteye, June Businge and the GPC team members listed below. We recognise the contribution of Elizabeth Anderson in data analysis for this paper
The GPC study team: Andrew Bbuye, Ben Kivumbi (deceased), Hussein Ssessanga, Herman Bikaali, Patrick Kalule (deceased), Edward Senyondo (deceased), Lucian Mwesigye, Vincent Lubwama, Hamidu Ddamulira, Victoria Mayanja (deceased), Hafsa Nakaweesi (deceased), Zakaria Sekawutu, Musa Kweta, Lucy Nakayiza, Robert Kizza, Imelda Sabiiti, Dorothy Kato, Stephen Katwiita, Florence Abigaba, Elon Sematiko, Safina Ssessanga, Ruth Nyanzi, Pelegrino Mbabazi, Charles Dickens Mweruka, Sulainah Nakassagga, Sarah Namuganga, Agnes Nalwoga, Edward Lubowa, Josephine Naluwugge, Victoria Nakibirango, Mathias Sekitoleko, Norah Nalweyiso, Rose Lubega, Teopista Kabalisa, George Mondo, Nobert Kalinzi (deceased), Phoebe Kasubo, Gertrude Nazziwa, Christine Kangave (deceased), Reuben Bya-mugisha (deceased), Charles Muganzi (deceased), Moses Senkubuge (deceased), Dan Serugo (deceased), Justine Katende, Josephine Nakitto, Julius Arinaitwe, Harriet Nansubuga, Sarah Kiyimba, Jane Nakayiza, Christine Musoke, Sebastian Kazibwe, Victo Nanono, Chaddress Kabagenyi, Joseph Kibuuka, Mary Nanteza, Annet Musoke, Mariam Namagembe, Joseph Kitumba, Leo Kibirige, Levokata Nandawula, Gerald Senyomo, Abdulla Mubiru (deceased), Abbas Mawanda (deceased), Evah Mubiru, Ruth Senyonga, Ben Kiwanuka, Anthony Ruberantwari, Duncan Ssematimba, Kenneth Babigumira, Joseph Ouma, Brian Ajuna, Sebastian Owilla, Justin Okello, Joseph Kahwa, Patrick Tabuga, Henry Eotu, Edward Muhigirwa, Tobias Vudriko, Margaret Nabankema and Jackson Were.
Conflict of interest: None declared.
KEY MESSAGES.
The GPC has provided HIV incidence and prevalence trends, HIV infection determinants, and HIV effects on mortality, fertility, and orphanhood for planning national HIV/AIDS programmes in Uganda and globally by UNAIDS.
It has also provided a wealth of data on the health and well-being of children and older people in HIV endemic settings.
HIV prevalence decreased from 8.5% in 1991 to 6.1% in 2000, remained stable until 2005 then rose to 8.9% in 2010 while HIV incidence declined. This is partly explained by access to free anti-retroviral therapy (ART).
Recent survey data show an emerging burden of NCDs with relatively high prevalence of hypertension (22%) and central obesity in women (31.2%) in a young rural population.
References
- 1.World Health Organization. Global Burden of Disease Projections, 2009. http://www.who.int/healthinfo/global_burden_disease/projections/en/index.html (31 August 2011, date last accessed) [Google Scholar]
- 2.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher D, Sekajugo J, Harries AD, Grosskurth H. Research needs for an improved primary care response to chronic non-communicable diseases in Africa. Trop Med Int Health. 2010;15:176–81. doi: 10.1111/j.1365-3156.2009.02438.x. [DOI] [PubMed] [Google Scholar]
- 4.De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D.A.D) study. Diabetes Care. 2008;31:1224–29. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown TT, Cole SR, Li X, Kingsley LA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–86. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 7.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–33. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakobsson M, Scholz SW, Scheet P, et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature. 2008;451:998–1003. doi: 10.1038/nature06742. [DOI] [PubMed] [Google Scholar]
- 9.Conrad DF, Jakobsson M, Coop G, et al. A worldwide survey of haplotype variation and linkage disequilibrium in the human genome. Nat Genet. 2006;38:1251–60. doi: 10.1038/ng1911. [DOI] [PubMed] [Google Scholar]
- 10.Spielman RS, Bastone LA, Burdick JT, Morley M, Ewens WJ, Cheung VG. Common genetic variants account for differences in gene expression among ethnic groups. Nat Genet. 2007;39:226–31. doi: 10.1038/ng1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stranger BE, Nica AC, Forrest MS, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–24. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis JP, Palmer ND, Hicks PJ, et al. Association analysis in African- Americans of European-derived type 2 diabetes single nucleotide polymorphisms from whole-genome association studies. Diabetes. 2008;57:2220–25. doi: 10.2337/db07-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbulaiteye SM, Mahe C, Ruberantwari A, Whitworth JAG. Generalizability of population-based studies on AIDS: a comparison of newly and continuously surveyed villages in rural southwest Uganda. Int J Epidemiol. 2002;31:961–67. doi: 10.1093/ije/31.5.961. [DOI] [PubMed] [Google Scholar]
- 14.Uganda National Council of Science and Technology (UNCST) National Guidelines for Research Involving Human Subjects as Research Participants. March 2007. http://www.uncst.go.ug/dmdocuments/Guideline,Human%20Subjects%20Guidelines%20Marc.pdf (25 July 2012, date last accessed) [Google Scholar]
- 15.Kasamba I, Baisley K, Mayanja BN, Maher D, Grosskurth H. The impact of antiretroviral treatment on mortality trends of HIV-positive adults in rural Uganda: a longitudinal population-based study, 1999–2009. Trop Med Int Health. 2012;17:e66–e73. doi: 10.1111/j.1365-3156.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uganda Ministry of Health and ICF International 2012. 2011 Uganda AIDS Indicator Survey. Key findings. Calverton, MA: MOH and ICF International. http://health.go.ug/docs/UAIS_2011_KEY_FINDINGS.pdf (7 November 2012, date last accessed) [Google Scholar]
- 17.Nunn AJ, Kengeya-Kayondo JF, Malamba S, Seeley JA, Mulder DW. Risk factors for HIV-1 infection in adults in a rural Ugandan community: a population study. AIDS. 1994;8:81–86. doi: 10.1097/00002030-199401000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Mulder D, Nunn A, Kamali A, Kengeya-Kayondo J. Decreasing HIV-1 seroprevalence in young adults in a rural Ugandan cohort. BMJ. 1995;311:833–36. doi: 10.1136/bmj.311.7009.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunn AJ, Wagner HU, Kamali A, Kengeya-Kaoyndo JF, Mulder DW. Migration and HIV-1 seroprevalence in a rural Ugandan population. AIDS. 1995;9:503–06. [PubMed] [Google Scholar]
- 20.Nunn AJ, Mulder DW, Kamali A, Kengeya-Kayondo JF, Whitworth JAG. HIV-1 incidence in sub-Sahara Africa. Lancet. 1996;348:833–34. doi: 10.1016/s0140-6736(05)65261-0. [DOI] [PubMed] [Google Scholar]
- 21.Kengeya-Kayondo JF, Kamali A, Nunn AJ, Ruberantwari A, Wagner HU, Mulder DW. Incidence of HIV-1 infection in adults and socio demographic characteristics of seroconverters in a rural population in Uganda, 1990–1994. Int J Epidemiol. 1996;25:1077–82. doi: 10.1093/ije/25.5.1077. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter LM, Kamali A, Ruberantwari A, Malamba SS, Whitworth JAG. Rates of HIV-1 transmission within marriage in rural Uganda in relation to the HIV sero-status of the partners. AIDS. 1999;13:1083–89. doi: 10.1097/00002030-199906180-00012. [DOI] [PubMed] [Google Scholar]
- 23.Kamali A, Carpenter LM, Whitworth JAG, Pool R, Ruberantwari A, Ojwiya A. Seven-year trends in HIV-1 infection rates, and changes in sexual behaviour among adults in rural Uganda. AIDS. 2000;14:427–34. doi: 10.1097/00002030-200003100-00017. [DOI] [PubMed] [Google Scholar]
- 24.Mbulaiteye SM, Ruberantwari A, Nakiyingi JS, Carpenter LM, Kamali A, Whitworth JAG. Alcohol and HIV: a study among sexually active adults in rural south west Uganda. Int J Epidemiol. 2000;29:911–15. doi: 10.1093/ije/29.5.911. [DOI] [PubMed] [Google Scholar]
- 25.Mbulaiteye SM, Mahe C, Whitworth JAG, et al. Declining HIV-1 incidence and associated prevalence over 10 years in a rural population in south-west Uganda: a cohort study. Lancet. 2002;360:41–46. doi: 10.1016/s0140-6736(02)09331-5. [DOI] [PubMed] [Google Scholar]
- 26.Whitworth JAG, Mahe C, Mbulaiteye SM, et al. HIV-1 epidemic trends in rural south-west Uganda over a 10 year period. Trop Med Int Health. 2002;7:1047–52. doi: 10.1046/j.1365-3156.2002.00973.x. [DOI] [PubMed] [Google Scholar]
- 27.Mbulaiteye SM, Mahe C, Whitworth JAG, Nakiyingi JS, Kamali A. HIV-1 incidence and prevalence trends in Uganda. Lancet. 2002;360:1788–89. doi: 10.1016/s0140-6736(02)09331-5. [DOI] [PubMed] [Google Scholar]
- 28.Biraro S, Morison L, Nakiyingi J, Whitworth JAG, Grosskurth H. The role of vertical transmission and health care related factors in HIV infection in children: a community study in rural Uganda. J Acquir Immune Defic Syndr. 2007;44:222–28. doi: 10.1097/QAI.0b013e31802e2954. [DOI] [PubMed] [Google Scholar]
- 29.Biraro S, Shafer LA, Kleinschmidt I, et al. Is sexual behaviour changing in rural southwest Uganda? Behaviour trends in a rural population cohort 1993–2006. Sex Transm Infect. 2009;(Suppl 1):i3–i11. doi: 10.1136/sti.2008.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafer LA, Biraro S, Nakiyinji-Miiro J, et al. HIV prevalence and incidence are no longer falling in southwest Uganda: evidence from a Rural Population Cohort 1989–2005. AIDS. 2008;22:1641–49. doi: 10.1097/QAD.0b013e32830a7502. [DOI] [PubMed] [Google Scholar]
- 31.Slaymaker E, Bwanika JB, Kasamba I, Lutalo T, Maher D, Todd J. Trends in age at first sex in Uganda: evidence from Demographic and Health Survey data, and longitudinal cohorts in Masaka and Rakai. Sex Transm Infect. 2009;85(Suppl 1):i12–i19. doi: 10.1136/sti.2008.034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafer LA, Nsubuga RN, Mayanja BM, et al. Antiretroviral therapy and sexual behaviour in Uganda: a cohort study. AIDS. 2011;25:671–78. doi: 10.1097/QAD.0b013e328341fb18. [DOI] [PubMed] [Google Scholar]
- 33.Kasamba I, Sully E, Weiss HA, Baisley K, Maher D. Extra-spousal partnerships in a community in rural Uganda with high HIV prevalence: a cross-sectional population-based study using linked spousal data. J Acquir Immune Defic Syndr. 2011;58:108–14. doi: 10.1097/QAI.0b013e318227af4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maher D, Waswa L, Karabarinde A, Baisley K. Concurrent sexual partnerships and associated factors: a cross-sectional population-based survey in a rural community in Africa with a generalised HIV epidemic. BMC Public Health. 2011;11:651. doi: 10.1186/1471-2458-11-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafer LA, Maher D, Weiss HA, Levin J, Biraro S, Grosskurth H. Contribution of population factors to estimation of HIV prevalence trends: a cohort study in rural Uganda, 1989–2007. Am J Epidemiol. 2011;174:1175–82. doi: 10.1093/aje/kwr234. [DOI] [PubMed] [Google Scholar]
- 36.Kasamba I, Baisley K, Mayanja BN, Maher D, Grosskurth H. The impact of antiretroviral treatment on mortality trends of HIV-positive adults in rural Uganda: a longitudinal population-based study, 1999–2009. Trop Med Int Health. 2012 doi: 10.1111/j.1365-3156.2012.02841.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulder DW, Nunn AJ, Kamali A, Nakiyingi J, Wagner HU, Kengeya- Kayondo JF. Two-year HIV-1 associated mortality in a Ugandan rural population. Lancet. 1994;343:1021–23. doi: 10.1016/s0140-6736(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 38.Mulder DW, Nunn A, Wagner HU, Kamali A, Kengeya-Kayondo JF. HIV-1 incidence and HIV-1 associated mortality in a rural Ugandan population cohort. AIDS. 1994;8:87–92. doi: 10.1097/00002030-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Kamali A, Wagner HU, Nakiyingi J, Sabiiti I, Kengeya-Kayondo JF, Mulder DW. Verbal autopsy as a tool for diagnosing HIV-related adult deaths in rural Uganda. Int J Epidemiol. 1996;25:67984. doi: 10.1093/ije/25.3.679. [DOI] [PubMed] [Google Scholar]
- 40.Nunn AJ, Mulder DW, Kamali A, Ruberantwari A, Kengeya-Kayondo JF, Whitworth J. Mortality associated with HIV-infection over five years in a rural Ugandan population: a cohort study. BMJ. 1997;315:767–71. doi: 10.1136/bmj.315.7111.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakiyingi JS, Breacher M, Whitworht JAG, et al. Child survival in relation to mother’s HIV infection and survival: evidence from a Ugandan cohort study. AIDS. 2003;17:1827–34. doi: 10.1097/00002030-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 42.Kazooba P, Kasamba I, Baisley K, Mayanja BN, Maher D. Access to, and uptake of, ART in a developing country with high HIV incidence: a population-based cohort study in rural Uganda, 2004–2008. Trop Med Int Health. 2012 doi: 10.1111/j.1365-3156.2012.02942.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kengeya-Kayondo JF, Malamba SS, Nunn AJ, Seeley JA, Ssali A, Mulder DW. Human immunodeficiency virus (HIV-1) seropositivity among children in a rural population of south-west Uganda: probable routes of exposure. Ann Trop Paediatr. 1995;15:115–20. doi: 10.1080/02724936.1995.11747758. [DOI] [PubMed] [Google Scholar]
- 44.Mulder DW, Nunn A, Kamali A, Kengeya-Kayondo JF. Post-natal incidence of HIV-1 infection among children in a rural Ugandan population: no evidence for transmission other than mother to child. Trop Med Int Health. 1996;1:81–85. doi: 10.1046/j.1365-3156.1996.d01-12.x. [DOI] [PubMed] [Google Scholar]
- 45.Kamali A, Seeley JA, Nunn AJ, Kengeya-Kayondo JF, Ruberantwari A, Mulder DW. The orphan problem: experience of a sub-Saharan Africa rural population in the AIDS epidemic. AIDS Care. 1996;8:509–15. doi: 10.1080/09540129650125470. [DOI] [PubMed] [Google Scholar]
- 46.Nalwoga A, Maher D, Todd J, Karabarinde A, Biraro S, Grosskurth H. Nutritional status of children living in a community with high HIV prevalence in rural Uganda: a cross-sectional population based survey. Trop Med Int Health. 2010;15:414–22. doi: 10.1111/j.1365-3156.2010.02476.x. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter LM, Nakiyingi JS, Ruberantwari A, Malamba SS, Kamali A, Whitworth JAG. Estimates of the impact of HIV infection on fertility in a rural Ugandan population cohort. Health Transition Review. 1997;7(Suppl 2):113–26. [Google Scholar]
- 48.Wagner HU, Van Dyck E, Roggen E, et al. Seroprevalence and incidence of sexually transmitted diseases in a rural Ugandan Population. Int J STD AIDS. 1994;5:332–37. doi: 10.1177/095646249400500509. [DOI] [PubMed] [Google Scholar]
- 49.Kamali A, Nun AJ, Mulder DW, Van Dyck E, Dobbins JG, Whitworth JAG. Seroprevalence and incidence of genital ulcer infections in a rural Ugandan population. Sex Transm Infect. 1999;5:98–102. doi: 10.1136/sti.75.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biraro S. PhD Thesis: London School of Hygiene and Tropical Medicine; 2011. Herpes simplex virus type-2: Epidemiological trends and relation with trends in HIV incidence and HIV transmission in south western Uganda. [Google Scholar]
- 51.Kamali A, Whitworth JAG, Ruberantwari A, et al. Causes and prevalence of non-vision impairing ocular conditions among a rural adult population in SW Uganda. Ophthalmic Epidemiol. 1999;6:41–48. doi: 10.1076/opep.6.1.41.1572. [DOI] [PubMed] [Google Scholar]
- 52.Mbualaiteye SM, Reeves BC, Mulwanyi F, Whitworth JAG, Johnson G. Incidence of visual loss in rural southwest Uganda. Br J Ophthalmo. 2003;87:829–33. doi: 10.1136/bjo.87.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mbulaiteye SM, Reeves B, Karabalinde A, et al. Evaluation of E optotypes as a screening test and the prevalence and causes of visual loss in a rural population in SW Uganda. Ophthalmic Epidemiol. 2002;9:251–62. doi: 10.1076/opep.9.4.251.1509. [DOI] [PubMed] [Google Scholar]
- 54.Nalweyiso N, Busingye J, Whitworth JAG, Robinson PG. Dental treatment needs of children in a rural subcounty of Uganda. Int J Paediatr Den. 2004;14:27–33. doi: 10.1111/j.1365-263x.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 55.Robinson PG, Nalweyiso N, Busingye J, Whitworth JAG. Subjective dental impacts of caries and fluorosis in rural Uganda children. Community Dent Health. 2005;22:231–36. [PubMed] [Google Scholar]
- 56.Maher D, Waswa L, Baisley K, Karabarinde A, Unwin N, Grosskurth H. Distribution of hyperglycaemia and related cardiovascular disease risk factors in low-income countries: a cross-sectional population-based survey in rural Uganda. Int J Epidemiol. 2011;40:160–71. doi: 10.1093/ije/dyq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maher D, Waswa L, Baisley K, Karabarinde A, Unwin N. Epidemiology of hypertension in low-income countries: a cross-sectional population-based survey in rural Uganda. J Hyperten. 2011;29:1061–68. doi: 10.1097/HJH.0b013e3283466e90. [DOI] [PubMed] [Google Scholar]
- 58.Seeley JA, Kengeya-Kayondo JF, Mulder DW. Community-based HIV/AIDS research . whither community participation? Unsolved problems in a research programme in rural Uganda. Soc Sci Me. 1992;34:1089–95. doi: 10.1016/0277-9536(92)90282-u. [DOI] [PubMed] [Google Scholar]
- 59.Nakibinge S, Maher D, Katende J, Kamali A, Grosskurth H, Seeley J. Community engagement in health research: two decades’ experience from a research project on HIV in rural Uganda. Trop Med Int Health. 2008;14:190–95. doi: 10.1111/j.1365-3156.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 60.Seeley J, Wagner HU, Mulemwa J, Kengeya-Kayondo JF, Mulder DW. The development of a community based HIV/AIDS service in a rural area of Uganda. AIDS Care. 1991;3:207–17. doi: 10.1080/09540129108253064. [DOI] [PubMed] [Google Scholar]
- 61.Seeley JA, Kajura E, Bachengana C, Okongo M, Wagner HU, Mulder DW. The extended family and support for people with AIDS in a rural population in south west Uganda: a safety net with holes? AIDS Care. 1993;5:117–22. doi: 10.1080/09540129308258589. [DOI] [PubMed] [Google Scholar]
- 62.Seeley JA. Searching for indicators of vulnerability: a study of household coping strategies in rural south west Uganda—a background report: Uganda Virus Research Institute. 1993 [Google Scholar]
- 63.Seeley JA, Malamba SS, Nunn AJ, Mulder DW, Kengeya-Kayondo JF, Barton T. Socio economic status, gender and risk of HIV-1 infection in a rural community in south west Uganda. Med Anthropol Q. 1994;8:78–89. [Google Scholar]
- 64.Whitworth J, Pickering H, Morgan D. Disclosing HIV status to partners. Lancet. 1996;347:973. doi: 10.1016/s0140-6736(96)91462-2. [DOI] [PubMed] [Google Scholar]
- 65.Nabaitu J, Bachengana C, Seeley J. Marital instability in a rural population in south west Uganda: Implication for the spread of HIV-1 infection. Africa(Lond) 1994;64:243–51. [PubMed] [Google Scholar]
- 66.Kengeya-Kayondo JF, Seeley JA, Kajura-Bajenja E, et al. Recognition, treatment seeking behaviour and perception of cause of malaria among rural women in Uganda. Acta Trop. 1994;58:267–73. doi: 10.1016/0001-706x(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 67.Whitworth J, Pickering H, Mulwanyi F, Ruberantwari A, Dolin P, Johnson G. Determinants of attendance of patient satisfaction at eye clinics in south-western Uganda. Health Policy Plan. 1999;14:77–81. doi: 10.1093/heapol/14.1.77. [DOI] [PubMed] [Google Scholar]
- 68.Seeley J, Biraro S, Shafer LA, et al. Using in-depth qualitative data to enhance our understanding of quantitative results regarding the impact of HIV and AIDS on households in rural Uganda. Soc Sci Med. 2008;67:1434–1. doi: 10.1016/j.socscimed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Seeley J, Kabunga E, Tumwekwase G, Wolff B, Grosskurth H. The impact of the AIDS epidemic on the lives of older people in rural Uganda. Working Paper 4. School of Development Studies, University of East Anglia. http://www1.uea.ac.uk/polopoly_fs/1.73128!/dev%20wp%2004%20seeley%202008.pdf (2 November 2011, date last accessed) [Google Scholar]
- 70.Seeley J, Wolff B, Kabunga E, Tumwekwase G, Grosskurth H. “And this is where we’ve buried our sons” – the oldest old people coping with the impact of the AIDS epidemic in a resource-poor setting in rural Uganda. Ageing Soc. 2009;29:115–34. [Google Scholar]
- 71.Nunn AJ, Biryahwaho B, Downing RG, Goen G van der, Ojwiya A, Mulder DW. Algorithms for detecting antibodies to HIV-1: results from a rural Ugandan cohort. AIDS. 1993;7:1057–61. doi: 10.1097/00002030-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Kaleebu P, Whitworth J, Hamilton L, et al. Molecular epidemiology of HIV Type 1 in a rural community in southwest Uganda. AIDS Res Hum Retroviruses. 2000;16:393–01. doi: 10.1089/088922200309052. [DOI] [PubMed] [Google Scholar]
- 73.Kaleebu P, Ross A, Morgan D, et al. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Uganda cohort. AIDS. 2001;15:293–99. doi: 10.1097/00002030-200102160-00001. [DOI] [PubMed] [Google Scholar]
- 74.Yirrell DL, Kaleebu P, Morgan D, et al. Inter-and intra-genic intersubtype HIV-1 recombination in rural and semi-urban Uganda. AIDS. 2002;16:279–86. doi: 10.1097/00002030-200201250-00018. [DOI] [PubMed] [Google Scholar]
- 75.Yirrell DL, Kaleebu P, Morgan D, Hutchinson S, Whitworth JA. HIV-1 subtype dynamics over 10 years in a rural Ugandan cohort. Int J STD AIDS. 2004;15:103–06. doi: 10.1258/095646204322764299. [DOI] [PubMed] [Google Scholar]
- 76.Senkaali D, Muwonge R, Morgan D, Whitworth JAG, Kaleebu P. The relationship between HIV-1 disease progression and V3 serotype in a rural Ugandan cohort. AIDS Res Hum Retroviruses. 2004;20:932–37. doi: 10.1089/aid.2004.20.932. [DOI] [PubMed] [Google Scholar]
- 77.Gale CV, Yirrell DL, Campbell E, Vander Paal L, Grosskurth H, Kaleebu P. Genotypic variation in the pol gene of HIV-1 type 1 in an antiretroviral treatment-naïve population in rural southwestern Uganda. AIDS Res Hum Retroviruses. 2006;22:985–92. doi: 10.1089/aid.2006.22.985. [DOI] [PubMed] [Google Scholar]
- 78.Yirrell DL, Wright E, Shafer LA, et al. Association between active GBV-C (hepatitis G) infection and HIV-1 disease in Uganda. Int J STD AIDS. 2007;18:244–49. doi: 10.1258/095646207780659006. [DOI] [PubMed] [Google Scholar]
- 79.McCreesh N, Frost SD, Seeley J, et al. Evaluation of respondent-driven sampling. Epidemiology. 2012;23:138–47. doi: 10.1097/EDE.0b013e31823ac17c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCreesh N, Johnston LG, Copas A, et al. Evaluation of the role of location and distance in recruitment in respondent-driven sampling. Int J Health Geogr. 2011;10:56. doi: 10.1186/1476-072X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCreesh N, Nadagire TM, Seeley J, Katongole J, White RG. Community understanding of respondent- driven sampling in a medical research setting in Uganda: importance for the use of RDS for public health research. Int J Soc Res Methodol. 2012 doi: 10.1080/13645579.2012.661204. doi:10.1080/13645579.2012.661204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scholten F, Mugisha J, Seeley J, et al. Health and functional status among older people with HIV/AIDS in Uganda. BMC Public Healt. 2011;11:886. doi: 10.1186/1471-2458-11-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scholten F, Mugisha J, Seeley J, Zalwango F Stuart Wright. Direct and Indirect Effects of HIV/AIDS and Anti-Retroviral Treatment on the Health and Wellbeing of Older People. Geneva: SAGE; WHO www.cordaid.nl/nl/(15457)-well-being-of-older-people-study.pdf (24 July 2012, date last accessed) [Google Scholar]
- 84.Maher D, Biraro S, Hosegood V, et al. Translating global health research aims into action: the example of the ALPHA (Analysing Longitudinal Population-based HIV/AIDS data in Africa) network. Trop Med Int Health. 2010;15:321–28. doi: 10.1111/j.1365-3156.2009.02456.x. [DOI] [PubMed] [Google Scholar]
- 85.McCarthy M, Maher D, Ly A, Ndip A. Developing the agenda for European Union collaboration on non-communicable diseases research in Sub-Saharan Africa. Health Res Policy Syst. 2010;19:13. doi: 10.1186/1478-4505-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maher D, Smeeth L, Sekajugo J. Health transition in Africa: practical policy proposals for primary care. Bull World Health Organ. 2010;88:943–48. doi: 10.2471/BLT.10.077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maher D, Sekajugo J. Research on health transition in Africa: time for action. Health Res Policy Syst. 2011;9:5. doi: 10.1186/1478-4505-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]