Abstract
Interactions between smoking and neighborhood-level socioeconomic status (SES) as risk factors for higher polycyclic aromatic hydrocarbon (PAH) DNA adduct levels in prostate tissue were investigated. PAH-DNA adducts were measured by immunohistochemistry with staining intensity measured in optical density units by semiquantitative absorbance image analysis in tumor adjacent tissue from 400 prostatectomy specimens from the Henry Ford Health System in Detroit. For each subject, their U.S. Census tract of residence was classified as being of higher or lower SES using the median value of the distribution of the proportion of tract residents with a high-school education. Generalized estimating equation models were used to assess interactions between neighborhood-level SES and smoking status, adjusting for race, age, education level, tumor volume, primary Gleason grade and prostate specific antigen (PSA) at diagnosis. There was a statistical interaction (P = 0.004) between tract-level SES and smoking status. In lower SES tracts smoking status was not associated with adduct staining, but in higher SES tracts adduct staining intensity was 13% (P = 0.01) higher in ever-smokers as compared to never-smokers. Among never-smokers, living in a lower SES tract was associated with a 25% higher mean staining intensity (P < 0.001). Neighborhood SES modifies the association between individual smoking status and PAH-DNA adduct levels in prostate tissue.
Keywords: prostate, PAH-DNA adducts, neighborhood study, socioeconomic status, smoking
INTRODUCTION
Over the past two decades prostate cancer has become the most common nonskin malignancy in men and the second leading cause of cancer death among men in the United States [Parkin et al., 2001; Jemal et al., 2006]. Following up on data from occupational studies, we have been investigating possible environmental causes of prostate cancer, documenting the presence and correlates of polycyclic aromatic hydrocarbon-DNA (PAH-DNA) adducts in prostate tumor and tumor adjacent tissue from prostatectomy patients at the Henry Ford Health System, in Detroit. PAH are a family of environmental carcinogens resulting from incomplete combustion processes and are found, among other places, in air pollution, cigarette smoke, and diesel exhaust [Baek et al., 1991; Harrison et al., 2009]. The human prostate expresses Phase I metabolism enzymes required to bioactivate PAH to reactive metabolites capable of covalently binding to DNA and forming PAH-DNA adducts [John et al., 2009; Hruba et al., 2010; Martin et al., 2010]. PAH-DNA adducts represent a DNA damaging event and serve as a biomarker of the biologically effective dose of PAH [Perera and Weinstein, 1982]. We have documented associations between: adduct levels and pathological features of prostate cancer, between adduct levels and genetic polymorphisms in genes coding for enzymes that metabolize PAH, and between adduct levels and biochemical recurrence after prostatectomy [Rybicki et al., 2004; Nock et al., 2007; Tang et al., 2007; Rybicki et al., 2008]. However, a strong, overall relationship between PAH-DNA adduct levels in prostate tissues and cigarette smoking, a major source of PAH exposure, has not been observed. Associations between smoking status and adduct levels were observed in tumor, but not tumor adjacent tissue, among Caucasians but not among African Americans [Nock et al., 2007].
Here, we use an approach known as “eco-epidemiology” to further explore individual and neighborhood-level determinants of PAH-DNA adduct levels in prostate tissue [Susser and Susser, 1996; Merlo et al., 2005]. This approach attempts to incorporate multiple levels of organization—societal, neighborhood, individual, and molecular—into study design and analysis, acknowledging that social or group-level processes and individual-level processes can independently or jointly predict exposure and/or health [Susser and Susser, 1996; Willis et al., 2003]. Here we focus on the question of whether indicators of individual and neighborhood-level socioeconomic status (SES) are associated with adduct levels and modify associations between smoking status and adduct levels.
Phelan and Link have suggested that lower SES is a fundamental cause of health disparities that operates through multiple pathways [Link and Phelan, 1995; Phelan et al., 2004]. One pathway, as suggested by the literature on environmental justice/inequalities, is that poor and minority neighborhoods have disproportionately higher concentrations of environmentally polluting industrial sites and poor and minority populations face higher levels of pollution from all sources [Brulle and Pellow, 2006]. Across the United States, the Environmental Protection Agency’s (EPA’s) National Air Toxics Assessment found that cancer risk from ambient air toxics were highest in Census tracts located in metropolitan areas that were highly racially segregated [Morello-Frosch and Jesdale, 2006]. In the greater Detroit metropolitan area, neighborhood poverty tracks with the presence of older and more polluting industrial sites, highways and truck routes, all of which are sources of PAH [Schulz et al., 2002; Downey, 2005] and the EPA Detroit Multipollutant Pilot Project found higher ambient air pollution levels in lower income areas in Detroit [US Environmental Protection Agency, 2008]. Recent spatial analyses of pollution exposures from EPA’s Toxics Release Inventory sites in Detroit finds that Census tracts with higher median house hold incomes and a higher proportion of residents who completed High School have significantly lower exposures [Downey, 2006].
For studies in which measures of individual-level SES are not available researchers have often used place of residence to link study subjects to area-based measures of SES and used these data as a proxy for individual-level status [for instance see, Gorey and Vena, 1995; Breen and Figueroa, 1996; Krieger et al., 2002; McBride et al., 2010]. However, area-based measures of SES should be viewed as community or neighborhood-level influences, which may impact exposure, health, disease prognosis, and disease treatment independently from individual-level socioeconomic characteristics or may interact with them to predict health [Krieger et al., 1997; Rundle et al., 2008].
Here, we consider whether measures of individual-level and neighborhood-level SES are associated with adduct levels in prostate specimens and also consider whether there are interactions between neighborhood SES and smoking status on PAH-DNA adduct levels. To our knowledge this is the first application of multilevel or “eco-epidemiology” approaches to the study of the distribution and determinants of adduct levels in any tissue type. The work demonstrates the utility of these approaches and provides novel insights into the risk factors and correlates of PAH-DNA adduct levels in prostate tissue.
METHODS
Study Data
Study subjects whose prostate cancer had been treated by prostatectomy were drawn from a larger case–control study of prostate cancer that enrolled subjects between July 1 2001 and December 31, 2004 and has been described extensively elsewhere [Rybicki et al., 2006; Nock et al., 2007]. Study subjects completed a questionnaire that gathered data on sociodemographic characteristics and health behaviors. Data on income were not collected; however, data on educational attainment were collected and serve as the measure of individual-level SES used in this study. Study subjects were coded into three educational attainment groups, less than high-school graduation, having graduated high-school and having attended some college or more. Data on the prostate specific antigen (PSA) levels at prostatectomy and on the pathology of the tumor were retrieved from study subject’s medical records.
Paraffin embedded tumor and tumor adjacent tissue sections from 400 study subjects were stained for PAH-DNA adducts using immunohistochemistry as described in detail previously [Rundle et al., 2000; Rybicki et al., 2004]. The immunohistochemical assay uses the monoclonal 5D11 antibody, which in cell culture studies has been shown to produce staining intensity levels strongly correlated with treatment dose of benzo(a)-pyrene-diolepoxide. Although the 5D11 antibody was produced in response to the adduct of the carcinogenic intermediate of benzo(a)pyrene (BPDE-DNA), it cross reacts with various affinities with other structurally related PAH; hence the terminology “PAH-DNA” is commonly used [Rundle et al., 2000; Rybicki et al., 2004]. Tissue staining for PAH-DNA adducts was measured by semiquantitative absorbance image analysis with a Cell Analysis System 200 microscope (Becton-Dickinson, San Jose, CA) running the Cell Measurement Program and staining intensity is reported in optical density (OD) units [Rundle et al., 2000; Rybicki et al., 2004].
Study subjects home addresses were geocoded to their Census tracts, spatial units defined by the U.S. Census Bureau for the Decennial Census population enumeration and commonly used population health research to designate neighborhood areas [Krieger et al., 2002]. Data on a series of tract-level indicators of SES from the 2000 Decennial Census Summary File 3 database including; median house hold income, per capita income, the percent of the population living below the Federal poverty level, the percent of the population receiving public assistance income, aggregated total public assistance income, the percent of households headed by single mothers and the proportion of the population 25 years or older that had completed high-school.
Statistical Analyses
Spearman rank correlation analyses were conducted to determine the extent to which the tract-level SES measures correlated across tracts in which study subjects lived. Initial analyses of associations between neighborhood SES and adduct levels focused on tract-level educational attainment so that the individual and neighborhood-level SES measures paralleled one another. For each Census tract the proportion of residents 25 years or older who had completed high-school was calculated. The proportion who had completed high-school was considered both as a continuous variable and as a dichotomous variable using the median value (median = 0.84) of the distribution across tracts to categorize men as living in higher and lower SES tracts. Linear generalized estimating equations (GEE) were used to determine whether individual educational attainment and Census tract-level proportion of residents who completed high-school were associated with adduct levels in tumor adjacent prostate tissue after control for other covariates. The analyses controlled for individual-level demographic and pathology variables previously shown to be associated with adduct levels in this study population, including; age, race, tumor volume, Primary Gleason grade, and PSA at diagnosis. Analyses were also replicated in African American and Caucasian subjects separately.
To assess whether there was a statistical interaction between individual-level SES and smoking status, men were stratified into two educational groups, those with a high-school education or less and those with more than a high-school education. Within each of the two strata of educational attainment, a GEE model was fit to determine whether smoking status (ever verses never) was associated with adduct staining intensity controlling for individual-level demographic and pathology variables previously shown to be associated with adduct staining in this study population. A formal test for statistical interaction was performed by fitting a model that included a dichotomous variable to indicate the two educational attainment groups, a variable for smoking status, and an interaction term for educational attainment and smoking status. Similar analyses were performed to test whether neighborhood SES interacted modified associations between smoking status and adduct levels. Men were stratified as to whether they lived in high or low SES tracts using the median value (median = 0.84) of the proportion of residents who completed high-school to categorize tracts as being high or low SES. Analyses were replicated using the other tract-level SES indicator variables and using adduct staining intensity data from tumor tissue as the outcome.
RESULTS
As expected the tract-level SES indicator variables were highly correlated with each other, Spearman correlations between proportion of residents who completed high-school and the other tract-level measures of SES were all strong; −0.71 to −0.90 for inverse correlates and 0.75–0.91 for positive correlates. The correlation closest to the null was between the proportion of residents who completed high-school and the proportion of households headed by a single mother, with a correlation of −0.71.
Table I shows descriptive statistics for the individual sociodemographic variables and across tracts for the proportion of residents who completed high-school. Table II presents results for the overall study population, prior to stratification by tract-level SES, for analyses of associations between PAH-DNA adduct staining levels in tumor adjacent tissue and smoking status, individual-level education and tract-level high-school completion. In the overall study population, PAH-DNA adduct staining intensity in tumor adjacent tissue was not associated with the subject’s educational attainment nor with smoking status. However, increasing proportion of residents in the Census tract who completed high-school was nonsignificantly (P = 0.08) associated with lower staining intensity for PAH-DNA adducts. When tracts were classified into higher or lower SES categories based on the median value for proportion of residents completing high-school, staining intensity for adducts in tumor adjacent tissue was significantly higher in lower SES tracts (0.02 OD units lower, P = 0.03).
TABLE I.
Socio-Demographic Characteristics of the Study Population
| Categorical variables | N | % |
|---|---|---|
| Race | ||
| White | 223 | 56.2 |
| Black | 174 | 43.8 |
| Education | ||
| Less than high-school | 67 | 17 |
| High-school graduate | 193 | 48 |
| Education beyond high-school | 140 | 35 |
| Smoking status | ||
| Nonsmoker | 147 | 36.8 |
| Ex-smoker | 210 | 52.5 |
| Current smoker | 43 | 10.8 |
| Continuous variables | Mean | SD |
|
| ||
| PAH Adduct Staining Intensity | ||
| Tumor tissue (OD Units) | 0.15 | 0.05 |
| Normal tissue (OD Units) | 0.25 | 0.08 |
| Age (years) | 61.0 | 6.7 |
| Tract-level proportion of residents who completed high-school | 0.83 | 0.11 |
TABLE II.
Associations Between PAH-DNA Adduct Staining Intensity in Prostate Tumor Adjacent Tissue, Smoking Status, and Measures of Individual and Neighborhood-Level SES
| Model 1 Beta coefficient, P-value | Model 2 Beta coefficient, P-value | |
|---|---|---|
| Individual-level measures | ||
| Smoking Status | ||
| Never | Ref | Ref |
| Ever | 0.009, 0.28 | 0.009, 0.27 |
| Education | ||
| Less than high-school | Ref | |
| High-school graduate | −0.002, 0.85 | −0.002, 0.89 |
| Education beyond high-school | −0.001, 0.92 | −0.001, 0.95 |
| Tract-level measures | ||
| Proportion of residents who completed high-school | −0.08, 0.08 | – |
| Tract-level SES categories | ||
| Lower (less than the median proportion of residents who completed high-school) | – | Ref |
| Higher (at or above the median proportion of residents who completed high-school) | – | −0.02, 0.03 |
Beta coefficients in models 1 and 2 are from GEE regression models and are mutually adjusted for the other variables in the table and for age, race, PSA at prostatectomy, tumor Gleason score and tumor volume. Model 1 treats tract-level proportion of residents ≥25 years of age who completed high-school as a continuous variable. Model 2 treats tract-level SES as a dichotomous variables where higher SES tracts are defined as those where the proportion of residents ≥25 years of age who completed high-school is ≥0.84 (the median of the distribution across tracts).
When separate analyses were conducted among men with a high-school education or less and among men with at least some college education, smoking status was not associated with staining intensity for adducts in tumor adjacent tissue in either group of men. However, when men were stratified into whether or not they lived in a higher or lower SES tract, significant differences in associations between smoking and PAH-DNA adduct levels were apparent by tract-level SES status. In a multivariable model, smoking status was not associated with adduct staining intensity in men living in lower SES tracts (tracts below the median for the proportion of residents who completed high-school). However, among men living in higher SES tracts, in a multivariable model, DNA adduct staining intensity was 0.03 OD units higher (13% higher) in ever-smokers as oppose to never-smokers (P = 0.008). The interaction term for smoking status and higher versus lower tract-level SES was statistically significant (P = 0.004). Table III shows the covariate adjusted mean PAH-DNA adduct staining intensity among ever-, versus never-smokers living in higher and lower SES tracts. Among nonsmokers, adduct staining intensity was 0.05 OD units higher (25% higher) for men living in lower SES as compared to higher SES tracts (P < 0.001). Similar associations between smoking status and PAH-DNA adducts across strata of tract-level SES were seen when African American and Caucasian men were analyzed separately. There was also an interaction between smoking status and tract-level SES on PAH-DNA adduct levels measured in tumor tissue (see Table IV).
TABLE III.
Adjusted Mean Staining Intensity for PAH-DNA Adducts in Prostate Tumor Adjacent Tissue by Individual Smoking Status and Tract-Level SES
| Smoking Status | Higher SESa tracts Adjusted mean optical density units, 95% CI, P-valueb |
Lower SESa tracts Adjusted mean optical density units, 95% CI, P-valueb |
P-value for interaction between smoking status and tract SES |
|---|---|---|---|
| Never-smoker | 0.220, (0.203, 0.237) | 0.262 (0.244, 0.280) | |
| Ref | Ref | ||
| Ever-smoker | 0.248 (0.235, 0.262) | 0.246 (0.235, 0.257) | 0.004 |
| P = 0.008 | P = 0.14 |
Higher SES tracts defined as tracts where the proportion of residents ≥25 years of age who completed high-school is ≥0.84 (the median of the distribution across tracts).
Compared with nonsmokers adjusting for the men’s race, age, educational attainment, tumor volume, primary Gleason grade, log PSA at diagnosis.
TABLE IV.
Adjusted Mean Staining Intensity for PAH-DNA Adducts in Prostate Tumor Tissue by Individual Smoking Status and Tract-Level SES
| Smoking status | Higher SESa tracts Adjusted mean optical density units, 95% CI, P-valueb |
Lower SESa tracts Adjusted mean optical density units, 95% CI, P-valueb |
P-value for interaction between smoking status and neighborhood |
|---|---|---|---|
| Never-smoker | 0.130 (0.119, 0.141) | 0.157 (0.144, 0.169) | |
| ref | ref | ||
| Ever-smoker | 0.155 (0.147, 0.164) | 0.149 (0.141, 0.157) | 0.002 |
| P < 0.001 | P = 0.33 |
Higher SES tracts defined as tracts where the proportion of residents ≥25 years of age who completed high-school is ≥0.84 (the median of the distribution across tracts).
Compared with nonsmokers adjusting for the men’s race, age, educational attainment, tumor volume, primary Gleason grade, log PSA at diagnosis.
As would be expected given the high correlation between the measures of tract-level SES considered here, when analyses were repeated using the other measures of tract-level SES to classify tracts as being of higher or lower SES status, significant interactions were observed with smoking status. In fully adjusted multivariable models, significant interactions (P < 0.05) between smoking and tract-level SES were observed when median house hold income, per capita income, the proportion of the population living below the Federal poverty level, the proportion of the population receiving public assistance income, and aggregated total public assistance income were each used as measures of tract-level SES, and an interaction that approached statistical significance was observed when the proportion of households headed by single mothers was used as a measure of tract-level SES (P = 0.07). In each of these analyses smoking status was significantly associated with adduct staining intensity in tracts categorized as being higher SES but not in tracts categorized as being lower SES.
DISCUSSION
These results suggest that neighborhood-level SES is associated with PAH-DNA adduct levels in prostate tissue and that neighborhood-level SES significantly modifies associations between individual-level smoking status and adduct levels. Self-reported educational attainment, used as a measure of the men’s own SES, did not explain the interactions between smoking and tract-level SES. In analyses that only consider individual-level variables, the cross-level interaction between smoking status and tract-level SES appears to obscure the association between smoking status and PAH-DNA adduct levels in prostate tissue. These results demonstrate the utility of multilevel analytical approaches to identifying environmental and behavioral correlates of PAH-DNA adduct levels.
Measurement of individual-level SES was somewhat limited because educational attainment was the only measure of SES available at the individual-level. However, we do not believe that the tract-level indicators of SES act merely as proxies for differences in individual’s SES not accounted for by educational attainment or that the observed cross-level interactions result merely from underlying associations between individual SES and individual-level smoking behavior [Krieger et al., 1997; Adler and Ostrove, 1999; Krieger et al., 2002]. Smokers in minority and poorer neighborhoods are expected to smoke more menthol and/or unfiltered roll-your-own cigarettes, smoking patterns associated with higher exposures to cigarette carcinogens and thus larger differences in adduct levels between smokers and nonsmokers [Azzi et al., 2006; Young et al., 2006; Yerger et al., 2007; Muscat et al., 2009]. However, differences in staining intensity by smoking status were seen in the higher SES tracts not the lower SES tracts, and in race specific analyses the findings were similar for African Americans and Caucasians. These findings suggest that the tract-level SES indicators reflect phenomena other than differences in individual-level SES.
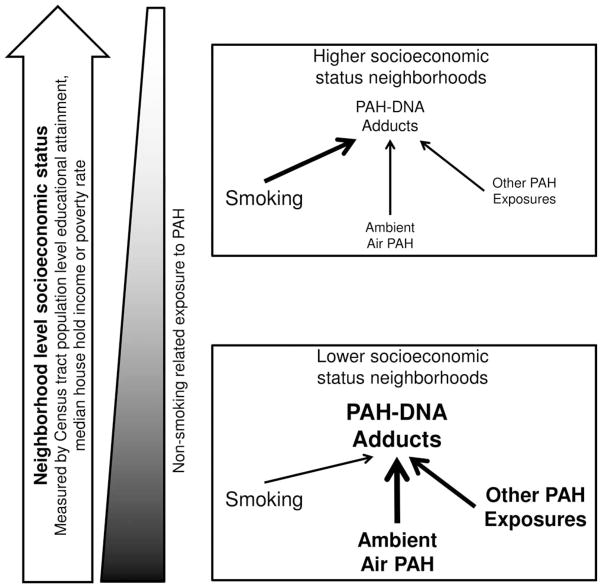
In the greater Detroit area tract-level SES tracks very closely with maps of estimated ambient pollution levels, including levels of benzene, which shares several important sources with PAH, including automobile transport [Baek et al., 1991; Kinney et al., 2002; US Environmental Protection Agency, 2008; Harrison et al., 2009; Weisel, 2010]. Furthermore, pollution sources in the Detroit area cluster in poorer neighborhoods [Schulz et al., 2002; Downey, 2005; Downey, 2006]. Therefore, in lower SES neighborhoods it may be more difficult to measure associations between individual behaviors and adduct levels because neighborhood environmental factors obscure these associations. We suggest that associations between tract-level measures of SES and ambient exposures to PAH may explain the interactions observed in this study. Figure 1 depicts the conceptual model underlying the observed interaction between smoking status and neighborhood-level SES.
Fig. 1.
A conceptual illustration for the observed interaction between smoking status and neighborhood level socioeconomic status (SES). Although ambient exposures were not measured in this study, prior EPA studies and other literature suggest that ambient exposures to PAH in the Detroit Metropolitan Area are lower in higher SES neighborhoods. Neighborhood SES is measured in the study using Census tract level data on educational attainment, median house hold income or poverty rate. In figure 1, higher exposures to ambient air PAH and perhaps other PAH exposures are indicated with a larger and darker typeface, as are higher levels of PAH-DNA adducts. In this manner, Figure 1 depicts higher PAH-DNA adduct levels in tumor adjacent tissue from subjects living in lower SES neighborhoods and higher ambient air exposures and perhaps other PAH exposures in these same neighborhoods. The higher ambient exposures are thought to contribute to the higher observed levels of PAH-DNA adducts in low SES neighborhoods. The higher relative contribution of these sources is indicated by a thicker arrow connecting ambient air and other exposures to PAH-DNA adducts. In higher SES neighborhoods, where ambient air and perhaps other PAH exposures are lower, smoking status has a relatively stronger association (indicated by a thicker arrow) with PAH-DNA adduct levels. It is thought that this differential distribution of exposures and their relative contributions to adduct levels by neighborhood SES, is reflected in the study data as higher levels of PAH-DNA adducts in lower SES neighborhoods (Table 2) and an interaction between smoking status and neighborhood SES on PAH-DNA adduct levels (Tables 3 and 4).
Phelan and Link have suggested that SES is a fundamental cause of health disparities operating through multiple pathways [Link and Phelan, 1995; Phelan et al., 2004]. Consistent with this idea, it is possible that the observed effects of neighborhood SES on adduct levels arise through mechanisms other than the differential distribution of ambient pollution sources. Residents in lower SES areas may work in occupations with higher exposures to PAHs such as, asphalt maintenance, welding, transportation and manufacturing. Second, access to alcohol or patterns of alcohol consumption may be related to neighborhood SES and alcohol consumption has been shown to modify association between GSTm1 deletion status and adduct levels [Morland et al., 2002; Rundle et al., 2003; Romley et al., 2007]. Alternately, lower income neighborhoods often have fewer supermarkets and produce markets than higher income neighborhoods and so study subjects from lower SES neighborhoods may have poorer diets, which could affect adduct levels [Morland et al., 2002; Zenk et al., 2005; Rundle et al., 2009]. Finally, the observed cross-level interaction could reflect a constellation of effects linked to lower neighborhood SES.
A limitation of this study is the use of Census tracts to spatially define the study subject’s neighborhood and link to Census data on neighborhood SES. Census tracts are administrative units designed for purposes other than health research [Diez Roux, 2001; Krieger et al., 2002]. They generally do not represent individual’s own conceptualizations of their neighborhood nor are they designed to represent spatial scales over which group-level characteristics might be hypothesized to affect health [Diez Roux, 2001; Krieger et al., 2002]. That is, Census tracts are not designed with biological, medical or social considerations in mind and as such analytical results from neighborhood studies using these spatial units may be biased. A second potential limitation of the study is that, while the half-life of adducts in prostate tumor and tumor adjacent tissues is unknown, data from a single time point, the 2000 Census, were used to describe socioeconomic conditions in the subject’s residential neighborhood at the time of diagnosis. However, adduct levels measured at diagnosis are expected to reflect exposures close to the time of diagnosis rather than exposures throughout the overall developmental history of the tumor. Research with other tumor types showing associations between adduct levels and exposures and behaviors measured at the time of diagnosis suggest that adduct levels in tissues reflect recent exposures [Cuzick et al., 1990; Garner et al., 1990; van Schooten et al., 1990; Butkiewicz et al., 1999; God-schalk et al., 2002; Benhamou et al., 2003; Rundle et al., 2003; Gyorffy et al., 2004]. Another concern is that the effects of tumor growth on adduct levels measured in tumor tissue are unknown; however, the associations with smoking and neighborhood SES observed in tumor tissue paralleled those observed in tumor adjacent tissue. Another limitation of this research is the semiquantitative nature of the immunohistochemical assay for PAH-DNA adducts, which does not allow for quantification of adduct levels in units of adducts per nucleotide. Recently developed immunohistochemical assays for PAH-DNA adducts are more fully quantitative [John et al., 2009].
These analyses provide initial evidence for a cross-level interaction between a measure of neighborhood context and an individual risk behavior on the level of a biomarker of exposure. This work represents an initial step in applying the ideas of “eco-epidemiology” to the study of prostate cancer etiology and outcomes. Susser and Susser [1996] advanced this view of epidemiology that encompasses “many levels of organization—molecular and societal as well as individual..” and aims to “..integrate more than a single level in design, analysis, and interpretation”. In theory the risk of exposure to environmental carcinogens is mediated by individual and societal-level processes, as illustrated here, and eco-epidemiological or multilevel approaches may provide novel insights into the causes of interindividual variation in PAH-DNA adduct levels.
In conclusion, Census based indicators of neighborhood-level SES appear to modify associations between individual-level smoking status and PAH-DNA adduct levels in both prostate tumor adjacent and tumor tissue. The observed effect of neighborhood-level SES does not appear to merely represent neighborhood SES acting as a proxy for individual-level SES. The effect of neighborhood-level SES may reflect disparities in the distribution of other sources of PAH, or other social or behavioral phenomena that affect PAH-DNA adduct formation.
It is thought that this differential distribution of exposures and their relative contributions to adduct levels by neighborhood SES, is reflected in the study data as higher levels of PAH-DNA adducts in lower SES neighborhoods (Table II) and an interaction between smoking status and neighborhood SES on PAH-DNA adduct levels (Tables III and IV).
Acknowledgments
Grant sponsor: NIEHS; Grant Number R01 ES011126.
References
- Adler NE, Ostrove JM. Socioeconomic status and health: What we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Azzi C, Zhang J, Purdon CH, Chapman JM, Nitcheva D, Hebert JR, Smith EW. Permeation and reservoir formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and benzo[a]pyrene (B[a]P) across porcine esophageal tissue in the presence of ethanol and menthol. Carcinogenesis. 2006;27:137–145. doi: 10.1093/carcin/bgi173. [DOI] [PubMed] [Google Scholar]
- Baek S, Field R, Goldstone M, Kirk P, Lester J, Perry R. A review of atmospheric polycyclic aromatic hydrocarbons: Sources, fate and behavior. Water Air Soil Pollution. 1991;60:279–300. [Google Scholar]
- Benhamou S, Laplanche A, Guillonneau B, Mejean A, Desgrandchamps F, Schrameck C, Degieux V, Perin F. DNA adducts in normal bladder tissue and bladder cancer risk. Mutagenesis. 2003;18:445–148. doi: 10.1093/mutage/geg020. [DOI] [PubMed] [Google Scholar]
- Breen N, Figueroa JB. Stage of breast and cervical cancer diagnosis in disadvantaged neighborhoods: A prevention policy perspective. Am J Prev Med. 1996;12:319–326. [PubMed] [Google Scholar]
- Brulle RJ, Pellow DN. Environmental justice: Human health and environmental inequalities. Annu Rev Public Health. 2006;27:103–124. doi: 10.1146/annurev.publhealth.27.021405.102124. [DOI] [PubMed] [Google Scholar]
- Butkiewicz D, Cole KJ, Phillips DH, Harris CC, Chorazy M. GSTM1, GSTP1, CYP1A1 and CYP2D6 polymorphisms in lung cancer patients from an environmentally polluted region of Poland: Correlation with lung DNA adduct levels. Eur J Cancer Prev. 1999;8:315–323. doi: 10.1097/00008469-199908000-00008. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Routledge MN, Jenkins D, Garner RC. DNA adducts in different tissues of smokers and non-smokers. Int J Cancer. 1990;45:673–678. doi: 10.1002/ijc.2910450417. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey L. The unintended significance of race: Environmental racial inequality in Detroit. Soc Forces. 2005;83:971–1007. doi: 10.1353/sof.2005.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey L. Environmental racial inequality in Detroit. Soc Forces. 2006;85:771–796. doi: 10.1353/sof.2007.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner RC, Cuzick J, Jenkins D, Phillips DH, Hewer A, King MM, Routledge MN. Linear relationship between DNA adducts in human lung and cigarette smoking. IARC Sci Publ. 1990;104:421–426. [PubMed] [Google Scholar]
- Godschalk R, Nair J, van Schooten FJ, Risch A, Drings P, Kayser K, Dienemann H, Bartsch H. Comparison of multiple DNA adduct types in tumor adjacent human lung tissue: Effect of cigarette smoking. Carcinogenesis. 2002;23:2081–2086. doi: 10.1093/carcin/23.12.2081. [DOI] [PubMed] [Google Scholar]
- Gorey KM, Vena JE. The association of near poverty status with cancer incidence among black and white adults. J Community Health. 1995;20:359–366. doi: 10.1007/BF02283060. [DOI] [PubMed] [Google Scholar]
- Gyorffy E, Anna L, Gyori Z, Segesdi J, Minarovits J, Soltesz I, Kostic S, Csekeo A, Poirier MC, Schoket B. DNA adducts in tumour, normal peripheral lung and bronchus, and peripheral blood lymphocytes from smoking and non-smoking lung cancer patients: Correlations between tissues and detection by 32P-post-labelling and immunoassay. Carcinogenesis. 2004;25:1201–1209. doi: 10.1093/carcin/bgh131. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Delgado-Saborit JM, Baker SJ, Aquilina N, Meddings C, Harrad S, Matthews I, Vardoulakis S, Anderson HR. Measurement and modeling of exposure to selected air toxics for health effects studies and verification by biomarkers. Res Rep Health Eff Inst. 2009;143:3–96. discussion 97–100. [PubMed] [Google Scholar]
- Hruba E, Trilecova L, Marvanova S, Krcmar P, Vykopalova L, Milcova A, Libalova H, Topinka J, Starsichova A, Soucek K, Vondracek J, Machala M. Genotoxic polycyclic aromatic hydrocarbons fail to induce the p53-dependent DNA damage response, apoptosis or cell-cycle arrest in human prostate carcinoma LNCaP cells. Toxicol Lett. 2010;197:227–235. doi: 10.1016/j.toxlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- John K, Ragavan N, Pratt MM, Singh PB, Al-Buheissi S, Matanhelia SS, Phillips DH, Poirier MC, Martin FL. Quantification of phase I/II metabolizing enzyme gene expression and polycyclic aromatic hydrocarbon-DNA adduct levels in human prostate. Prostate. 2009;69:505–519. doi: 10.1002/pros.20898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney PL, Chillrud SN, Ramstrom S, Ross J, Spengler JD. Exposures to multiple air toxics in New York City. Environ Health Perspect. 2002;110(Suppl 4):539–546. doi: 10.1289/ehp.02110s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;(Spec No):80–94. [PubMed] [Google Scholar]
- Martin FL, Patel, Sozeri O, Singh PB, Ragavan N, Nicholson CM, Frei E, Meinl W, Glatt H, Phillips DH, Arlt VM. Constitutive expression of bioactivating enzymes in normal human prostate suggests a capability to activate pro-carcinogens to DNA-damaging metabolites. Prostate. 2010;70:1586–1599. doi: 10.1002/pros.21194. [DOI] [PubMed] [Google Scholar]
- McBride RB, Lebwohl B, Hershman DL, Neugut AI. Impact of socioeconomic status on extent of lymph node dissection for colon cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:738–745. doi: 10.1158/1055-9965.EPI-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo J, Chaix B, Yang M, Lynch J, Rastam L. A brief conceptual tutorial on multilevel analysis in social epidemiology: Interpreting neighbourhood differences and the effect of neighbourhood characteristics on individual health. J Epidemiol Community Health. 2005;59:1022–1028. doi: 10.1136/jech.2004.028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Jesdale BM. Separate and unequal: Residential segregation and estimated cancer risks associated with ambient air toxics in U.S. metropolitan areas. Environ Health Perspect. 2006;114:386–393. doi: 10.1289/ehp.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med. 2002;22:23–29. doi: 10.1016/s0749-3797(01)00403-2. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie JP., Jr Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiol Biomarkers Prev. 2009;18:35–41. doi: 10.1158/1055-9965.EPI-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock NL, Tang D, Rundle A, Neslund-Dudas C, Savera AT, Bock CH, Monaghan KG, Koprowski A, Mitrache N, Yang JJ, Rybicki BA. Associations between smoking, polymorphisms in polycyclic aromatic hydrocarbon (PAH) metabolism and conjugation genes and PAH-DNA adducts in prostate tumors differ by race. Cancer Epidemiol Biomarkers Prev. 2007;16:1236–1245. doi: 10.1158/1055-9965.EPI-06-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Perera FP, Weinstein IB. Molecular epidemiology and carcinogen-DNA adduct detection: new approaches to studies of human cancer causation. J Chronic Dis. 1982;35:581–600. doi: 10.1016/0021-9681(82)90078-9. [DOI] [PubMed] [Google Scholar]
- Phelan JC, Link BG, Diez-Roux A, Kawachi I, Levin B. “Fundamental causes” of social inequalities in mortality: A test of the theory. J Health Soc Behav. 2004;45:265–285. doi: 10.1177/002214650404500303. [DOI] [PubMed] [Google Scholar]
- Romley JA, Cohen D, Ringel J, Sturm R. Alcohol and environmental justice: the density of liquor stores and bars in urban neighborhoods in the United States. J Stud Alcohol Drugs. 2007;68:48–55. doi: 10.15288/jsad.2007.68.48. [DOI] [PubMed] [Google Scholar]
- Rundle A, Field S, Park Y, Freeman L, Weiss CC, Neckerman K. Personal and neighborhood socioeconomic status and indices of neighborhood walk-ability predict body mass index in New York City. Soc Sci Med. 2008;67:1951–1958. doi: 10.1016/j.socscimed.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle A, Neckerman KM, Freeman L, Lovasi GS, Purciel M, Quinn J, Richards C, Sircar N, Weiss C. Neighborhood food environment and walkability predict obesity in New York City. Environ Health Perspect. 2009;117:442–447. doi: 10.1289/ehp.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle A, Tang D, Hibshoosh H, Estabrook A, Schnabel F, Cao W, Grumet S, Perera FP. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis. 2000;21:1281–1289. [PubMed] [Google Scholar]
- Rundle A, Tang D, Mooney L, Grumet S, Perera F. The interaction between alcohol consumption and GSTM1 genotype on polycyclic aromatic hydrocarbon-DNA adduct levels in breast tissue. Cancer Epidemiol Biomarkers Prev. 2003;12:911–914. [PubMed] [Google Scholar]
- Rybicki BA, Neslund-Dudas C, Bock CH, Rundle A, Savera AT, Yang JJ, Nock NL, Tang D. Polycyclic aromatic hydrocarbon—DNA adducts in prostate and biochemical recurrence after prostatectomy. Clin Cancer Res. 2008;14:750–757. doi: 10.1158/1078-0432.CCR-07-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki BA, Neslund-Dudas C, Nock NL, Schultz LR, Eklund L, Rosbolt J, Bock CH, Monaghan KG. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with the GSTP1 Ile105Val polymorphism. Cancer Detect Prev. 2006;30:412–422. doi: 10.1016/j.cdp.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki BA, Rundle A, Savera AT, Sankey SS, Tang D. Polycyclic aromatic hydrocarbon-DNA adducts in prostate cancer. Cancer Res. 2004;64:8854–8859. doi: 10.1158/0008-5472.CAN-04-2323. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, Williams DR, Israel BA, Lempert LB. Racial and spatial relations as fundamental determinants of health in Detroit. Milbank Q. 2002;80:677–707. iv. doi: 10.1111/1468-0009.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser M, Susser E. Choosing a future for epidemiology: II. From black box to Chinese boxes and eco-epidemiology. Am J Public Health. 1996;86:674–677. doi: 10.2105/ajph.86.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Liu JJ, Bock CH, Neslund-Dudas C, Rundle A, Savera AT, Yang JJ, Nock NL, Rybicki BA. Racial differences in clinical and pathological associations with PhIP-DNA adducts in prostate. Int J Cancer. 2007;121:1319–1324. doi: 10.1002/ijc.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency. Detroit Multi-pollutant Pilot Project: Hybrid Approach Modeling for Understanding Urban Air Quality. 2008. [Google Scholar]
- van Schooten FJ, Hillebrand MJ, van Leeuwen FE, Lutgerink JT, van Zandwijk N, Jansen HM, Kriek E. Polycyclic aromatic hydrocarbon-DNA adducts in lung tissue from lung cancer patients. Carcinogenesis. 1990;11:1677–1681. doi: 10.1093/carcin/11.9.1677. [DOI] [PubMed] [Google Scholar]
- Weisel CP. Benzene exposure: An overview of monitoring methods and their findings. Chem Biol Interact. 2010;184:58–66. doi: 10.1016/j.cbi.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A, Krewski D, Jerrett M, Goldberg MS, Burnett RT. Selection of ecologic covariates in the American Cancer Society study. J Toxicol Environ Health A. 2003;66:1563–1589. doi: 10.1080/15287390306425. [DOI] [PubMed] [Google Scholar]
- Yerger V, Przewoznik J, Malone R. Racialized geography, corporate activity, and health disparities: Tobacco industry targeting of inner cities. J Health Care Poor Underserved. 2007;18:10–38. doi: 10.1353/hpu.2007.0120. [DOI] [PubMed] [Google Scholar]
- Young D, Borland R, Hammond D, Cummings KM, Devlin E, Yong HH, O’Connnor RJ. Prevalence and attributes of roll-your-own smokers in the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15(Suppl 3):iii76–iii82. doi: 10.1136/tc.2005.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenk SN, Schulz AJ, Hollis-Neely T, Campbell RT, Holmes N, Watkins G, Nwankwo R, Odoms-Young A. Fruit and vegetable intake in African Americans income and store characteristics. Am J Prev Med. 2005;29:1–9. doi: 10.1016/j.amepre.2005.03.002. [DOI] [PubMed] [Google Scholar]