Abstract
Efavirenz diminishes methadone plasma concentrations, an effect attributed to CYP3A induction, but actual mechanisms are unknown. This investigation determined the effects of two weeks of efavirenz (600 mg daily) on hepatic and intestinal CYP3A4/5 (probed with intravenous and oral alfentanil), hepatic CYP2B6 (oral efavirenz hydroxylation) and intestinal transporter (oral fexofenadine) activities, and on methadone pharmacokinetics and pharmacodynamics in healthy volunteers. It also assessed efavirenz effects on CYP expression and activity in human hepatocytes. Efavirenz significantly induced systemic and oral alfentanil clearance 2- to 5-fold, increased alfentanil hepatic and intestinal extraction ratios, and significantly induced apparent 8-hydroxyefavirenz formation clearance. Efavirenz also moderately decreased fexofenadine plasma concentrations, suggesting decreased intestinal uptake and/or increased P-glycoprotein-mediated efflux. Efavirenz induced CYP2B6 and CYP3A4 expression, activity, and methadone metabolism in human hepatocytes. Efavirenz coinduces hepatic CYP2B6 and CYP3A4/5 activities, coinduces hepatic and intestinal CYP3A4/5, and coinduces gastrointestinal CYP3A and xenobiotic efflux transporters.
Keywords: cytochrome P450 3A, CYP3A, cytochrome P450 2B6, CYP2B6, efavirenz, methadone, alfentanil, in vivo probe, phenotyping
Efavirenz is a first-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), used for over a decade as an essential component of highly-active antiretroviral therapy.1,2 Efavirenz is a first-line drug used worldwide in the treatment of HIV and postexposure prophylaxis, and the preferred NNRTI. It is recommended in the United States as a third antiretroviral added to an initial two-drug regimen,3 and in the United Kingdom as initial therapy.4 Efavirenz is one of three drugs coformulated together (with emtricitabine and tenofovir), which comprises the most popular antiretroviral regimen due to the convenience of a single pill taken once daily and resultantly high treatment adherence.
Efavirenz is a well-known victim and perpetrator of drug-drug interactions. It is extensively metabolized, with cytochrome P450 (CYP) CYP2B6-catalyzed primary (to 8-hydroxyefavirenz) and secondary hydroxylation the principal (>90%) route of inactivation and systemic clearance.5,6 A minor route, CYP2A6-catalyzed 7-hydroxylation, may also influence disposition under certain circumstances.5,7 While a few drugs induce (rifampin) or inhibit (voriconazole) efavirenz elimination,8,9 drug interactions perpetrated by efavirenz are more numerous and clinically significant. For example, efavirenz decreased the area under the curve of several HIV protease inhibitors, anticonvulsants, steroids, buprenorphine, and statins,2,10,11 increased the metabolism of oral erythromycin and midazolam,12,13 and induced oral bupropion hydroxylation and clearance.14 Of significant importance is the reduction of methadone plasma concentrations by efavirenz, which can precipitate opioid withdrawal.15–17 In addition to causing drug-drug interactions, efavirenz also induces its own metabolism and clearance.18–20 Apparent transporter-mediated efavirenz drug-drug interactions have also been reported, such as the reduction in plasma concentrations of the non-CYP3A4-metabolized pravastatin.11
The mechanism of efavirenz-mediated drug interactions in general is incompletely understood. In human hepatocytes, efavirenz upregulated CYP3A mRNA and/or protein expression21–23 and activity.21 Efavirenz also upregulated CYP2B6 mRNA and protein expression in human hepatocytes,23 but not LS180 human colon cancer cells.22 Efavirenz has been described as both an inhibitor and inducer of CYPs 2B6, 2C9, 2C19, and CYP3A.10,12,13,2,14 In addition, efavirenz caused dose-dependent, tissuespecific induction of hepatic but not intestinal CYP3A4,12 an apparent paradox which has remained unexplained. Efavirenz was also reported to influence the expression or activity of several transporters, although the data have been characterized as sparse and conflicting.24 In vitro, efavirenz induced P-glycoprotein (P-gp, ABCB1)22,24–26 and breast cancer resistance protein (BCRP, ABCG2) expression,22 variably induced expression of multidrug resistance proteins 1–6 (MRP1-6, ABCC1-6),22,24 and inhibited activity of MRPs 1–3.27 However efavirenz did not affect P-gp activity in cultured cells or rat intestine.28,29 Little or no information is available on the clinical effect of efavirenz on transporters.
The specific mechanism of efavirenz reductions in methadone plasma concentrations and opioid withdrawal described above is unidentified.15–17 CYP2B6 and CYP3A4 have the greatest activity towards methadone N-demethylation in vitro, while CYP3A5 is comparatively inactive,30–34 and these two CYPs are considered to mediate methadone clearance with disputed degrees of relative importance. Antiretroviral drug effects on methadone clearance have traditionally been attributed to alterations in CYP3A4 activity, although this concept has been challenged, and greater importance of CYP2B6 suggested.35–37 Methadone is also a substrate for P-gp, which influences methadone intestinal absorption in animals,38 although the role of P-gp in human methadone disposition and clinical effects is poorly understood. Therefore, efavirenz effects on methadone disposition and clinical effects may involve modulation of CYP2B6, CYP3A, and/or drug transporters.
A combined laboratory and clinical investigation was performed to determine (i) mechanism(s) of efavirenz alterations in methadone disposition and clinical effect, including altered CYP2B6-, CYP3A4/5- and/or P-gp-mediated methadone bioavailability, first-pass metabolism, and systemic clearance; (ii) effects on CYP2B6 and CYP3A expression and activity and methadone metabolism in human hepatocytes; (iii) efavirenz influence on methadone pharmacodynamics; (iv) efavirenz effects on hepatic CYP2B6 and CYP3A4/5, first-pass CYP3A4/5, and intestinal transporter activities, using validated in vivo probes; and (v) the ability of a noninvasive in vivo CYP3A4/5 probe to detect efavirenz drug interactions and predict methadone disposition.
This article reports a comprehensive crossover investigation in healthy volunteers to assess efavirenz effects on CYP2B6, CYP3A4/5 and transporter activities. Hepatic and first pass CYP3A4/5 activities were evaluated using intravenous and oral alfentanil (a nonselective CYP3A4/5 substrate, henceforth collectively referred to as CYP3A) as the in vivo probe.39,40 Fexofenadine, a substrate for P-gp and other transporters, was the in vivo transporter probe.41,42 Pupil diameter change (miosis) was used as a surrogate for alfentanil plasma concentrations to noninvasively estimate alfentanil clearance and hence CYP3A activity. Efavirenz effects on CYP induction and activity in human hepatocytes were also studied. An accompanying article describes efavirenz effects on methadone pharmacokinetics and pharmacodynamics.43
Results
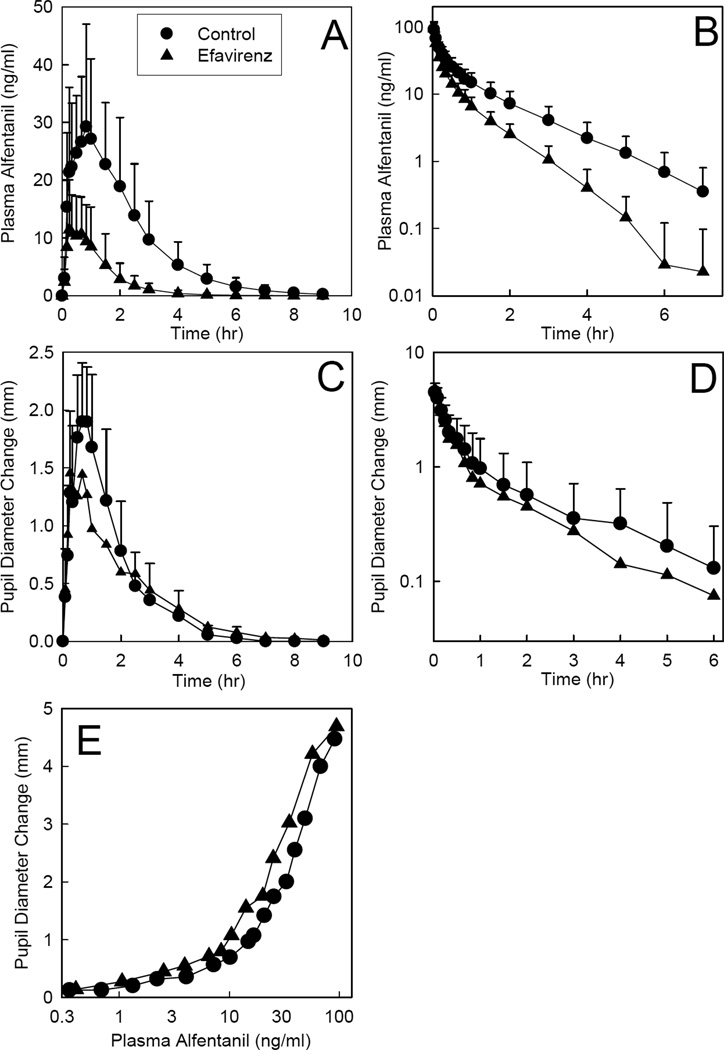
Efavirenz significantly induced hepatic and first-pass CYP3A activity. Efavirenz effects on alfentanil plasma concentrations are shown in Figures 1A–B, and pharmacokinetic parameters provided in Table 1. The AUC ratio (efavirenz/control) for IV alfentanil was reduced to 0.5 by efavirenz, and systemic clearance and the hepatic extraction ratio were both doubled, indicating 2-fold induction of hepatic CYP3A activity. Efavirenz reduced the AUC ratio (efavirenz/control) for oral alfentanil to 0.2, increased apparent oral clearance 5-fold, increased the intestinal extraction ratio by 50%, and decreased oral bioavailability in half, cumulatively indicating 5-fold induction of first-pass CYP3A activity.
Figure 1.
Efavirenz effects on first-pass and hepatic CYP3A activity, assessed using alfentanil as a CYP3A probe. Subjects received 43 µg/kg oral or 15 µg/kg intravenous alfentanil. Panels A and B show alfentanil plasma concentrations after oral and intravenous administration, respectively. Panels C and D show dark-adapted pupil diameter change from baseline (miosis), used as a surrogate for alfentanil plasma concentrations, oral and intravenous administration, respectively. Panel E shows the relationship between miosis and plasma concentration after intravenous alfentanil administration. Each data point is the mean ± SD (n = 12). Some SD are omitted for clarity.
Table 1.
Intravenous and oral alfentanil pharmacokinetics and effects
| Control | Efavirenz | |
|---|---|---|
| IV alfentanil | ||
| Cmax (ng/ml) | 92 ± 23 | 101 ± 32 |
| AUC0–∞ (ng •hr •ml−1) | 55 ± 22 | 30 ± 9* |
| AUC0–∞ ratio (efavirenz/control) | 0.54 (0.47, 0.62) | |
| CLIV (ml•kg−1•min−1) | 5.0 ± 1.5 | 9.3 ± 3.0* |
| Elimination t1/2 (hr) | 1.1 ± 0.2 | 0.85 ± 0.47* |
| EH | 0.32 ± 0.09 | 0.59 ± 0.17* |
| Maximum miosis (mm) | 4.6 ± 0.8 | 4.6 ± 0.7 |
| Miosis AUEC0–∞ (mm•hr) | 4.3 ± 2.8 | 3.2 ± 2.5* |
| Miosis AUEC0–∞ ratio (efavirenz /control) | 0.58 (0.38, 0.89) | |
| Oral alfentanil | ||
| Cmax (ng/ml) | 35 ± 17 | 15 ± 8* |
| AUC0–∞ (ng •hr •ml−1) | 75 ± 40 | 18 ± 13* |
| AUC0–∞ ratio (efavirenz/control) | 0.22 (0.16, 0.30) | |
| CL/F (ml•kg−1•min−1) | 12.4 ± 6.4 | 58.2 ± 32.6* |
| Elimination t1/2 (hr) | 1.1 ± 0.3 | 0.65 ± 0.18* |
| Foral | 0.46 ± 0.15 | 0.20 ± 0.09* |
| EG | 0.37 ± 0.15 | 0.55 ± 0.18* |
| Maximum miosis (mm) | 2.3 ± 0.5 | 1.6 ± 0.9 |
| Miosis AUEC0–∞ (mm•hr) | 4.2 ± 1.6 | 4.3± 4.6 |
| Miosis AUEC0–∞ ratio (efavirenz/control) | 0.63 (0.40, 1.01) |
Subjects received 15 µg/kg IV alfentanil and 43 µg/kg oral alfentanil. Results are the arithmetic mean ± SD (n=12), except the AUC and AUEC ratio (efavirenz/control), which is the geometric mean (90% CI). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CLIV, systemic clearance of IV alfentanil; CL/F, apparent oral clearance of alfentanil; EH, hepatic extraction ratio; EG, intestinal extraction ratio; Foral, bioavailability. AUEC, area under the effect (miosis)-time curve
Significantly different from control (p<0.05)
Pupil data were available before plasma alfentanil concentrations, and used as an early surrogate for alfentanil clearance to assess efavirenz effects on CYP3A activity (Figure 1C–D and Table 1). Efavirenz decreased the magnitude and duration of alfentanil miosis, and significantly decreased the AUEC ratio for intravenous but not oral alfentanil by approximately half. Efavirenz had no influence on alfentanil pharmacodynamics, measured as the concentration-miosis relationship (Figure 1E). The EC50 for miosis was approximately 40 ng/ml, maximum miosis was not reached at the highest alfentanil concentrations observed (100 ng/ml), and little miosis occurred at low alfentanil concentrations (<10 ng/ml).
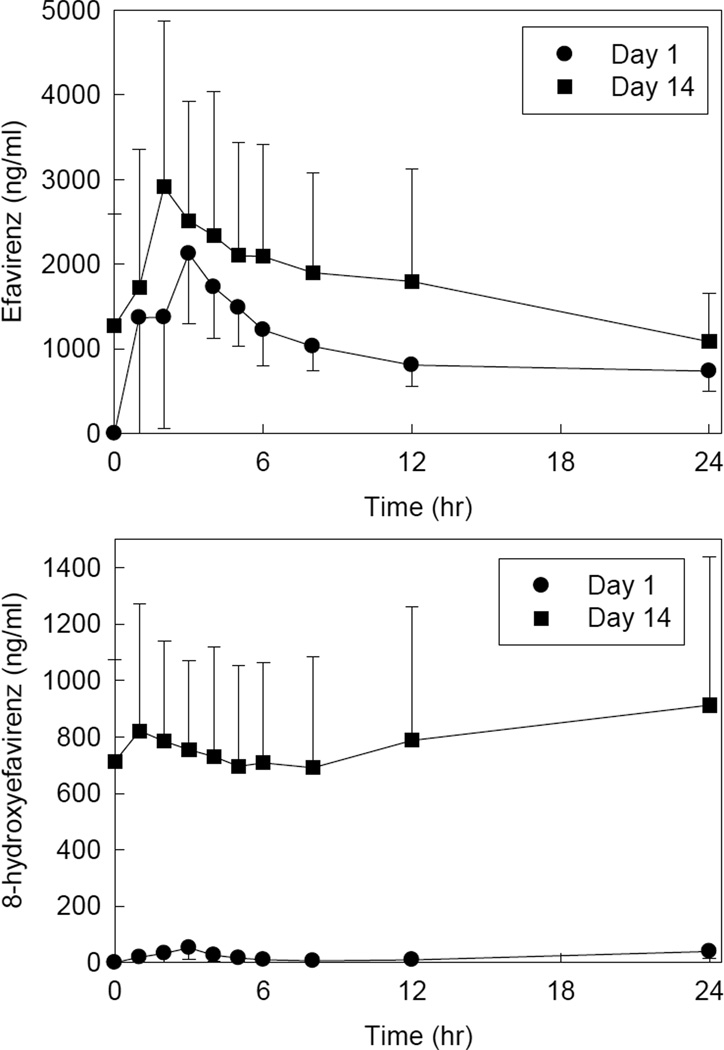
Metabolism of efavirenz was used to probe the activity of CYP2B6. Efavirenz and 8-hydroxyefavirenz plasma concentrations are shown in Figure 2, and pharmacokinetic parameters provided in Table 2. After two weeks of efavirenz, compared with the first dose, there were significant increases in efavirenz apparent oral clearance and 8-hydroxyefavirenz apparent formation clearance, and a significant decrease in the mid-dose interval efavirenz/8-hydroxyefavirenz plasma concentration ratio.
Figure 2.
Efavirenz effects on CYP2B6 activity, assessed using efavirenz disposition as a probe. Shown are efavirenz and 8-hydroxyefavirenz plasma concentrations after the first efavirenz dose and after 2 weeks of efavirenz. Each data point is the mean ± SD (n = 12).
Table 2.
Efavirenz pharmacokinetic parameters
| First dose | Two weeks | |
|---|---|---|
| CL/F (L/hr) | 12.3 ± 6.1 | 20.1 ± 9.5* |
| 12 hr efavirenz/8-hydroxyefavirenz concentration ratio | 152 ± 116 | 2.9 ± 2.5* |
| 8-hydroxyefavirenz apparent formation clearance (L/hr) | 1.0 ± 0.6 | 10.7 ± 5.7* |
CL/F, apparent oral clearance of fexofenadine.
Significantly different from control (p<0.05)
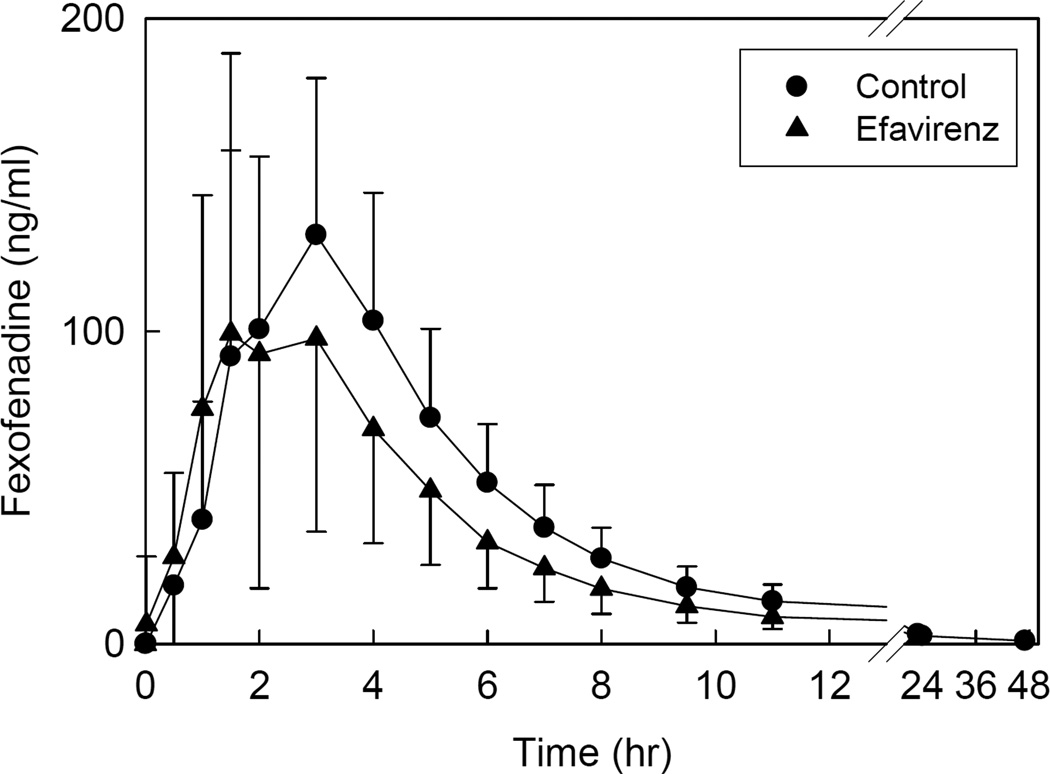
Disposition of oral fexofenadine was used to evaluate the activity of the intestinal efflux pump P-gp, and other intestinal transporters. Efavirenz had no effect on fexofenadine peak plasma concentrations, but decreased AUC (Figure 3, Table 3).
Figure 3.
Efavirenz effects on gastrointestinal transporter activity, assessed using fexofenadine as a transporter probe. Each subject received 60 mg oral fexofenadine on all occasions. Each data point is the mean ± SD (n = 12).
Table 3.
Fexofenadine pharmacokinetics
| Control | Efavirenz | |
|---|---|---|
| Cmax (ng/ml) | 141 ± 56 | 118± 81 |
| AUC0–∞ (ng •hr •ml−1) | 716 ± 242 | 554 ± 302 |
| AUC0–∞ ratio (efavirenz/control) | 0.73 (0.59,0.91) | |
| CL/F (ml•kg−1•min−1) | 23.2 ± 12.6 | 33.2 ± 18.5* |
| Elimination t1/2 (hr) | 5.4 ± 0.8 | 5.3 ± 0.8 |
Results are the arithmetic mean ± SD (n=12), except the AUC0–∞ ratio (efavirenz/control), which is the geometric mean (90% CI). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CL/F, apparent oral clearance
Significantly different from control (p<0.05)
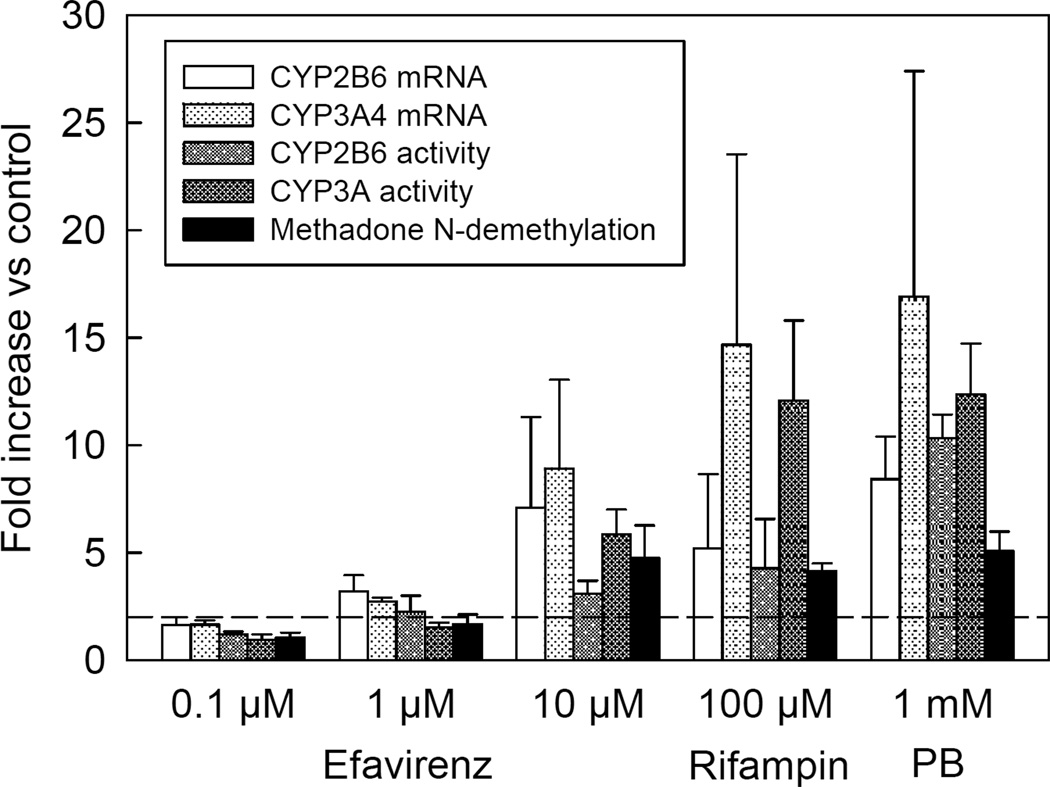
Efavirenz effects on CYP3A and CYP2B6, and the consequences for methadone metabolism, were measured in vitro using primary hepatocytes from three human livers. CYP3A activity was determined by metabolism of alfentanil to noralfentanil, and CYP2B6 activity was measured by the hydroxylation of bupropion. Efavirenz caused concentration-dependent induction of both CYP3A and CYP2B6 mRNA expression and catalytic activity (Figure 4). At 10 µM efavirenz, CYP3A and CYP2B6 mRNA expression were increased 9- and 7-fold, respectively, and CYP3A and CYP2B6 catalytic activity was increased 6- and 3-fold, respectively, compared with vehicle controls. Efavirenz also caused concentration-dependent induction of methadone N-demethylation (EDDP formation), which was induced 4-fold.
Figure 4.
Efavirenz effects on primary human hepatocyte CYP2B6 and CYP3A4 mRNA expression and catalytic activity, and methadone metabolism. Hepatocytes were incubated with efavirenz for 72 hr and then washed. CYP2B6 activity was determined by measuring bupropion hydroxylation, CYP3A activity was determined by measuring alfentanil dealkylation to noralfentanil, and methadone metabolism was determined by measuring EDDP formation. CYP2B6 and CYP3A4 mRNA was measured by RT-PCR. Rifampin (100 µM) and phenobarbital (PB, 1 mM) were used as positive controls. Hepatocytes were from a 51 year old male (nonsmoker, no ethanol use, taking escitalopram, 52 year old female (nonsmoker, no ethanol use, medication history unavailable), and 75 year old male (smoker, ethanol use, taking 25 duloxetine and cyclobenzaprine). Each data point is the mean ± SD of three livers, where mRNA and catalytic activity were determined in triplicate for each liver. The dotted line represents a 2-fold increase in expression or activity.
Discussion
One purpose of this investigation was to quantify effects of 2 weeks of oral efavirenz on hepatic and first-pass CYP3A activity. The major finding, as expected, was that efavirenz significantly induced hepatic CYP3A. For the hepatic CYP3A probe intravenous alfentanil, AUC was decreased to half that of control, while systemic clearance and the hepatic extraction ratio were doubled. Thus efavirenz caused a 2-fold induction of hepatic CYP3A activity. Efavirenz induction of hepatic CYP3A was nearly equivalent to the 2.5-fold increase in alfentanil clearance caused by rifampin (600 mg for 5d).39
The unexpected finding was that efavirenz also significantly induced intestinal CYP3A activity. For the first-pass CYP3A probe oral alfentanil, AUC was decreased to 22% of control, apparent oral clearance was increased 5-fold, and the intestinal extraction ratio increased 50%. Efavirenz induction of first-pass and intestinal CYP3A was similar to that by 75 mg rifampin for 5d,44 but less than by 600 mg for 6d, which decreased AUC to 5% of control, caused a 30-fold increase in apparent oral clearance, and a 75% increase in intestinal extraction ratio.39 Whether these differences in extent of rifampin and efavirenz induction reflect differences in the intrinsic efficacy or the time course of induction (maximal induction by rifampin and efavirenz occur after approximately 2 weeks and 3months, respectively19,45), cannot be determined from the present data.
This is the first investigation to demonstrate efavirenz clinical coinduction of both hepatic and intestinal CYP3A activity. Although previous studies showed that efavirenz decreased the AUC of several oral CYP3A drugs, including HIV protease inhibitors, anticonvulsants, steroids, and statins,2,10,11 and increased the metabolism of oral erythromycin and midazolam,12,13 the mechanism was unknown. The present results suggest that the mechanism is induction of both hepatic and intestinal CYP3A. Efavirenz co-induction of hepatic and intestinal CYP3A is different from the previous report,12 and general consideration that efavirenz induces hepatic but not intestinal CYP3A4.1,2 These differences may reflect dose- (or time-) dependency of intestinal induction, since the previous investigation,12 which measured CYP3A4 protein content in intestinal biopsies, evaluated 200 and 400 mg efavirenz (for 10d), while the present investigation studied the currently used clinical dose of 600 mg (for 2 weeks).
The second major finding of this investigation was that efavirenz induced hepatic CYP2B6 activity, based on efavirenz autoinduction. The phenomenon of efavirenz autoinduction is frequently mentioned, although it is less described quantitatively. Two approaches have been used previously to evaluate efavirenz autoinduction. One evaluated changes in apparent oral efavirenz clearance.18,19 After multiple doses, efavirenz plasma AUC decreased 22–42%, presumably reflecting a commensurate increase in clearance.18 After 14d of efavirenz, autoinduction caused a 32% increase in oral clearance.19 The estimated average time to 50% of maximal autoinduction was 10d, with 81% of steady-state clearance reached after 14d, and up to 3 months required to reach full steady-state induction.19 A second approach used the mid-dose interval efavirenz/8-hydroxyefavirenz metabolic ratio.20 Because efavirenz is typically taken at bedtime to minimize side effects, daytime (mid-dose interval) sampling is often used for convenience in clinical studies.7 Long-term (4 vs 1 month) efavirenz autoinduction was associated with a decreased efavirenz/8-hydroxyefavirenz metabolic ratio,20 although autoinduction beginning with initialization of efavirenz was not evaluated. In the present investigation, efavirenz autoinduction was clearly observed, evidenced by significant increases in both efavirenz apparent clearance and 8-hydroxyefavirenz apparent formation clearance, and by a significant decrease in the efavirenz/8-hydroxyefavirenz metabolic ratio. Efavirenz 8-hydroxylation, which accounts for more than 90% of overall efavirenz oxidation, is catalyzed principally by CYP2B6, is considered a CYP2B6-selective pathway, and suggested to be a phenotypic probe for clinical CYP2B6 activity.2,5,20 Therefore, the several metrics of increased efavirenz 8-hydroxylation and clearance observed in this investigation support the conclusion that efavirenz autoinduced CYP2B6 activity. Indeed, other studies have also shown efavirenz upregulation of CYP2B6, assessed by heteroinduction. For example, the AUC ratio of hydroxybupropion/bupropion, a specific probe for CYP2B6, was increased 2.3-fold after 2 weeks of efavirenz.14
This investigation is therefore the first to demonstrate efavirenz clinical coinduction of both hepatic CYP2B6 and CYP3A activities. Although efavirenz autoinduction of CYP2B6 could theoretically be attributed to upregulation of intestinal as well as hepatic CYP2B6 activity, little support exists for this hypothesis. For example, efavirenz clinically did not induce intestinal CYP2B6 expression, based on immunoblot analysis of intestinal biopsies, albeit at 200 and 400 mg (not 600 mg) efavirenz.12 In human LS180 cells, efavirenz upregulated CYP3A4 but not CYP2B6 mRNA expression.22 More generally, in human intestinal precision-cut slices, the pregnane X receptor (PXR) and/or constitutive androstane receptor (CAR) ligands rifampin and phenobarbital upregulated CYP3A4 activity but had no significant effect on CYP2B6 activity.46 Overall, CYP2B6 is considered to be negligibly if at all expressed in human intestine.
Coinduction of hepatic CYP2B6 and CYP3A clinically is consistent with the present and prior in vitro studies. Efavirenz caused concentration-dependent induction of both CYP2B6 and CYP3A4 mRNA and catalytic activity in primary hepatocytes (Figure 4). In the same model, efavirenz previously caused concentration-dependent induction of CYP2B6 and CYP3A4 mRNA and protein expression21,23,47 and CYP3A4 activity.21 Efavirenz was a more effective inducer of hepatocyte CYP2B6 compared with CYP3A4 mRNA expression.23,47 In contrast, it was reported to be a more effective inducer of CYP3A4 than CYP2B6 protein expression.23 Nevertheless, previous studies showed that Western blot analysis of hepatocyte CYP2B6 protein expression was not as sensitive or quantitative, and underestimated the degree of induction, compared with mRNA and enzyme activity.48 In the present investigation, efavirenz increased hepatocyte CYP3A activity more than CYP2B6, although there was considerable variability between livers. Efavirenz is a ligand for both PXR and CAR, and coinduction of hepatocyte CYP2B6 and CYP3A4 activities has been variably attributed to preferential activation of either PXR or CAR. While PXR-mediated reporter gene activation by efavirenz was similar for CYP2B6 and CYP3A4,23,47 induction of both isoforms was variably attributed to preferential activation of PXR23 or CAR.47 Regardless of the mechanism, efavirenz is an inducer of both hepatic CYP2B6 and CYP3A4 in vitro and in vivo. Efavirenz coinduction of hepatic CYP2B6 and CYP3A4 is similar to that seen with the PXR and CAR activators phenobarbital and carbamazepine.19 Efavirenz induction of hepatocyte methadone N-demethylation in this investigation is consistent with induction of either CYP3A and CYP2B6. Both expressed isoforms have the greatest catalytic activity towards methadone N-demethylation in vitro,30–34
Another major purpose of this investigation was to assess the ability of alfentanil miosis to noninvasively detect alterations in CYP3A activity and drug interactions. Efavirenz decreased alfentanil miosis, and the AUEC ratio by approximately half. The difference was statistically significantly for intravenous alfentanil but narrowly missed significance for oral alfentanil. Several reasons explain this observation. First, there was greater variability in the disposition of oral compared with intravenous alfentanil, resulting in a wider confidence interval for the geometric mean ratio of induced/control. Second, plasma concentrations were lower after oral compared with intravenous alfentanil. Previous results showed that the optimal (linear) range for measuring miosis is ~20–100 ng/ml alfentanil, and that changes in miosis underestimate the changes in plasma alfentanil at lower concentrations.39,40,44 In the present protocol, alfentanil concentrations were much lower after oral administration, and exceeded 20 ng/ml only briefly. Third, because of the concentration-response relationship, the sensitivity of alfentanil miosis for detecting CYP3A induction, where plasma concentrations decrease, is less than that for detecting CYP3A inhibition, where plasma concentrations increase.39,44 Nevertheless, alfentanil miosis did predict efavirenz effects on hepatic CYP3A activity, and with a somewhat larger sample size might have predicted efavirenz effects on first-pass CYP3A activity.
The last major finding of this investigation, which is the first to evaluate efavirenz effects on P-gp and other transporters, was that efavirenz appeared to moderately induce P-gp. Efavirenz decreased fexofenadine AUC, which was not due to enhanced elimination, suggesting increased fexofenadine intestinal efflux. When this investigation was designed, fexofenadine was used to probe intestinal P-gp-mediated interactions based on published recommendations.49 Originally considered selective for P-gp, fexofenadine is now known to be a substrate for multiple transporters. Intestinal fexofenadine absorption is influenced by both P-gp-mediated efflux and organic anion transporting polypeptide (OATP) 1A2-mediated uptake.50 Hepatic excretion of unchanged drug is the primary route of fexofenadine elimination. Hepatic uptake of fexofenadine involves OATPs 1B1, 1B3 and 2B1.51 Fexofenadine is a substrate for hepatic sinusoidal MRP3 (but not MRP4)-mediated efflux, and BSEP- (but not BCRP-, and possibly not MRP2-) mediated hepatic canicular efflux.51 Polymorphisms of P-gp, MRP2, OATP2B1 influence fexofenadine pharmacokinetics. Modeling and simulation studies suggested that hepatic uptake, rather than basolateral efflux or biliary excretion, is the principal determinant of fexofenadine clearance and systemic concentrations.52 Clinical evidence also supports the liver as a site of fexofenadine drug interactions.53 Together, therefore, drug interactions which change fexofenadine disposition may reflect altered intestinal permeability (OATP1A2-mediated uptake and/or P-gp-mediated efflux), or OATP 1B1-, 1B3- and/or 2B1-mediated hepatic uptake, and thus fexofenadine may more aptly be considered a more generalized probe for transporters function. Thus efavirenz effects may reflect decreased intestinal OATP1A2-mediated uptake and/or increased P-gp-mediated efflux, and/or increased OATP 1B1-, 1B3- and/or 2B1-mediated hepatic uptake. Since fexofenadine elimination rates were not affected, the present results suggest decreased intestinal uptake and/or increased P-gp-mediated efflux.
Some in vitro data corroborate the above considerations. In LS180 intestinal cells, efavirenz induced P-gp expression and activity,22,25 and 2 to 3-fold induction of BCRP, MRP3, and MRP5 mRNA.22 Efavirenz was not a substrate for P-gp, MRP1, MRP2, or BRCP,22 but inhibited BCRP and MRPs 1–326,27 but not P-gp,28 in cells overexpressing these transporters. Concomitant efavirenz upregulation of P-gp, CYP3A and CYP2B6 is consistent with its activation of PXR, and very high induction potential index (calculated based on potency for PXR activation and clinical concentration).54
There are potential limitations of this investigation. Efavirenz effects were evaluated in healthy volunteers rather than in HIV-infected patients. This was deliberate, because standard antiretroviral therapy involves several drugs, thereby precluding a careful mechanistic evaluation and attribution of results to any one specific drug. Pharmacogenetic influences on efavirenz induction of CYPs and transporters were not evaluated in this small investigation. Lastly, a longer duration of efavirenz administration may have somewhat greater effects on induction.19
In summary, efavirenz significantly induced hepatic, intestinal, and first-pass CYP3A4/5 activity, and significantly induced hepatic CYP2B6 activity. Efavirenz also altered the activity of intestinal transporters.
Methods
Clinical Protocol
Additional Methods information is provided in an accompanying article.43 The protocol was a two-session sequential crossover approved by the University of Washington Institutional Review Board. Twelve normal HIV-negative volunteers (22±4 yr, 71±12 kg) were studied before and after 2 weeks of oral efavirenz (600 mg nightly, continued for the duration of the study). Hepatic and first-pass CYP3A activities were evaluated using intravenous and oral alfentanil, and P-gp activity assessed using oral fexofenadine, as described previously.36,37,39,40,42,43 Plasma alfentanil and fexofenadine concentrations were simultaneously quantified using solid-phase extraction and electrospray liquid chromatography-mass spectrometry. Dark-adapted pupil diameter was measured just prior to blood sampling.
Plasma and urine efavirenz and 8-hydroxyefavirenz concentrations were determined by liquid-liquid extraction and liquid chromatography-mass spectrometry. To plasma (100µl) was added aprobarbital (62.5 ng) (Restek, Bellefonte, PA) and 30µl of 50 mM sodium carbonate, then twice extracted with 1 ml ethyl acetate:pentane (1:1). After centrifugation, organic layers were combined, evaporated under nitrogen (60°C), resuspended in 500µl acetonitrile, centrifuged, transferred to a new tube, evaporated to dryness, and resuspended in 250µl acetonitrile. Calibration standards contained 0, 5, 10, 25, 50, 100, 500, 1000, 2500 and 5000 ng/ml efavirenz (NIH AIDS Research and Reference Reagent Program) and 0, 0.5, 1, 2.5, 5, 10, 50, 100, 250 and 500 ng/ml 8-hydroxyefavirenz (Toronto Research Chemicals, Ontario, Canada) in blank plasma. Samples (40µl injection) were analyzed using an Agilent (Palo Alto, CA) 1100 series HPLC with vacuum degasser, binary solvent pump and 96-well plate auto sampler, and SunFire C18 column (4.6 × 150mm, 5µm) (Waters Corp., Milford, MA), interfaced to an Agilent 1100 series mass spectrometer operated in negative electrospray ionization mode. Mobile phase was (A) 0.04% formic acid in water and (B) 98% acetonitrile at 1 ml/min. Gradient conditions were initially 50% B, increased to 75% at 3 min, 100% at 4 min, held for 3.5 min, then decreased to 50% for 5 min re-equilibration. Parameters were: nitrogen drying gas (11 L/min, 350°C), nebulizer pressure 40 psi, capillary 3000 V, and fragmentor 120 V. Elution times for aprobarbital (m/z 209), 8-hydroxyefavirenz (m/z 330), and efavirenz (m/z 314) were 2.5, 4.8, and 5.4 min, respectively. Interday coefficients of variation were 9 and 10% for 10 and 1,000 ng/ml efavirenz, and 6% and 9% for 2.5 and 100 ng/ml 8-hydroxyefavirenz. For analysis of urine 8-hydroxyefavirenz, samples (10 µl) were hydrolyzed overnight at 37°C with 62.5 units β-glucuronidase, the internal standard aprobarbital (10 ng) added, diluted 1:50,000 with acetonitrile, and analyzed (20 µl) similar to plasma. Calibration standards contained 0, 10, 25, 50, 100, 200, 300, or 400 ng/ml 8-hydroxyefavirenz. Interday coefficients of variation were 8% and 6% for 25 and 300 ng/ml 8-hydroxyefavirenz.
Pharmacokinetic data were analyzed using noncompartmental methods.43 Formation clearance of 8-hydroxyefavirenz was the product of the molar fraction of dose recovered in urine and efavirenz apparent oral clearance. Paired t-tests assessed differences between groups for pharmacokinetic and effect parameters. Non-normal data were log transformed for analysis but reported as nontransformed results (arithmetic mean ± SD). Statistical significance was assigned at p< 0.05. Plasma AUC and urine data were also assessed as ratios (efavirenz/control) and the geometric mean and 90% confidence interval.
Hepatocyte Experiments
Alfentanil and methadone were from the National Institutes of Drug Abuse through Research Triangle Institute (Research Triangle Park, NC). Efavirenz was from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Noralfentanil-d5, d3-EDDP, and d6-hydroxybupropion were from Cerilliant (Round Rocks, TX). Fresh plated human hepatocytes and Hepatocyte Supplement were from Cellzdirect/Invitrogen (Durham, NC). Williams E media was from Lonza (Walkersville, MD). Bis-tris acrylamide gels (4–12%) and nitrocellulose membranes were from Invitrogen (Carlsbad, CA). Mouse antihuman monoclonal CYP3A4, rabbit antihuman polyclonal CYP2B6 and mouse antihuman monoclonal β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-rabbit and goat anti-mouse secondary antibodies, and SDS-cell lysis and blocking buffers were from Li-COR (Lincoln, NE). Other reagents were from Sigma (St. Louis, MO).
Upon hepatocyte arrival, media was changed to supplemented Williams E and cells allowed to equilibrate for 24 hr at 37°C/5% CO2/95% humidity. Cells were incubated in supplemented Williams E media containing drugs (efavirenz, phenobarbital, rifampin) or vehicle control for 72 hr, with media/drug changed daily. Efavirenz and phenobarbital were dissolved in DMSO (final concentration 0.1%). Prior to testing CYP activity cells were incubated with drug-free media for 1 hr. Media was then changed to that containing 500 µM bupropion and cells incubated for 40 min at 37°C with shaking, to determine CYP2B6 activity. Media was removed, analyzed for hydroxybupropion, then replaced with drug-free media for a 1 hr wash-out period. Media was replaced with that containing 500 µM racemic methadone and cells incubated for 40 min. Media was removed, analyzed for EDDP, and replaced with drug-free media for a 1 hr wash-out. To determine CYP3A4/5 activity, media was replaced with that containing 200 µM alfentanil, cells incubated for 40 min, and media analyzed for noralfentanil. Each determination was performed in triplicate using hepatocytes from 3 livers. After each experiment, hepatocytes were frozen for later quantification of mRNA.
Metabolite analysis was performed on an API 3200 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA)-Shimadzu LC-20AC HPLC (Shimadzu, Columbia, MD) (EDDP and noralfentanil) or an API 4000 QTRAP mass spectrometer-Agilent 1100 HPLC (hydroxybupropion). Both mass spectrometers were equipped with a Turbo Ion Spray ionization source and a Waters T3 HPLC column (50 × 2.1mm, 3.5µm). Injection volume was 20 µl and the oven temperature was 25°C. The HPLC mobile phase (0.3 ml/min) was (A) 0.1% formic acid and (B) 0.1% formic acid in acetonitrile. The gradient program for EDDP was 35% B for 0 min, linear to 60% B over 1.0 min, held at 60% until 2 min, linear to 100% until 3 min, 100% B until 4 min, then re-equilibrated to initial conditions for 1 min; for noralfentanil it was 35% B for 0 min, linear to 40% B until 0.5 min, held at 40% until 2.5 min, linear to 100% until 3 min, held at 100% B until 4 min, then re-equilibrated to initial conditions over 1.5 min; for hydroxybupropion it was 10% B for 0 min, linear to 30% B over 1.0 min, held at 30% until 1.5 min, linear to 100% until 2 min, held at 100% B until 2.5 min, then re-equilibrated to initial conditions over 3.5 min. Retention times were 2.7, 2.9 and 1.5 min, respectively, for EDDP, noralfentanil and hydroxybupropion. Mass spectrometers were operated in positive-ion mode, ion spray voltage 5500, curtain gas at 20, ion source gas 1 at 30, ion source gas 2 at 30 and collision gas at 5, and unit mass resolution. Multiple reaction monitoring transitions for analytes and standards were m/z 278.2→234.2 and 281.2→234.2 for EDDP and d3-EDDP; m/z 277.0→128.0 and 282.0→128.0 for noralfentanil and d5-noralfentanil; m/z 256.1→238.1 and 262.2→167.2 for 8-hydroxybupropion and d6-8-hydroxybupropion. Metabolites were quantified using area ratios and standard curves prepared using calibration standards in blank media.
Hepatocyte mRNA expression was determined by real-time reverse transcription polymerase chain reaction using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA), according to the manufacturers instructions.
Acknowledgements
This investigation was supported by National Institutes of Health grants R01-GM63674, R01-DA14211, and K24-DA00417 (to E.D.K.) and M01-RR00037 (to the University of Washington General Clinical Research Center).
Footnotes
No author has any conflict of interest.
This investigation was conducted before the requirement for clinical trials registration
References
- 1.Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother. 2009;64:910–928. doi: 10.1093/jac/dkp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakhmanina NY, van den Anker JN. Efavirenz in the therapy of HIV infection. Expert Opin Drug Metab Toxicol. 2010;6:95–103. doi: 10.1517/17425250903483207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson MA, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 4.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 5.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 6.Rotger M, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kwara A, Lartey M, Sagoe KW, Kenu E, Court MH. CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients. AIDS. 2009;23:2101–2106. doi: 10.1097/QAD.0b013e3283319908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Cortes LF, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–690. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 9.Damle B, LaBadie R, Crownover P, Glue P. Pharmacokinetic interactions of efavirenz and voriconazole in healthy volunteers. Br J Clin Pharmacol. 2008;65:523–530. doi: 10.1111/j.1365-2125.2007.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith PF, DiCenzo R, Morse GD. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin. Pharmacokinet. 2001;40:893–905. doi: 10.2165/00003088-200140120-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gerber JG, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 12.Mouly S, et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin. Pharmacol. Ther. 2002;72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 13.Fellay J, et al. Variations of CYP3A activity induced by antiretroviral treatment in HIV-1 infected patients. Eur J Clin Pharmacol. 2005;60:865–873. doi: 10.1007/s00228-004-0855-8. [DOI] [PubMed] [Google Scholar]
- 14.Robertson SM, Maldarelli F, Natarajan V, Formentini E, Alfaro RM, Penzak SR. Efavirenz induces CYP2B6-mediated hydroxylation of bupropion in healthy subjects. J Acquir Immune Defic Syndr. 2008;49:513–519. doi: 10.1097/QAI.0b013e318183a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzolini C, Troillet N, Telenti A, Baumann P, Decosterd LA, Eap CB. Efavirenz decreases methadone blood concentrations. AIDS. 2000;14:1291–1292. doi: 10.1097/00002030-200006160-00036. [DOI] [PubMed] [Google Scholar]
- 16.Clarke SM, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br. J. Clin. Pharmacol. 2001;51:213–217. doi: 10.1046/j.1365-2125.2001.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCance-Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J Addict. 2002;11:271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- 18.Barrett JS, Joshi AS, Chai M, Ludden TM, Fiske WD, Pieniaszek HJ., Jr Population pharmacokinetic meta-analysis with efavirenz. Int. J. Clin. Pharmacol. Ther. 2002;40:507–519. doi: 10.5414/cpp40507. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M, Kaul S, Nandy P, Grasela DM, Pfister M. A model-based approach to characterize efavirenz autoinduction and concurrent enzyme induction with carbamazepine. Antimicrob Agents Chemother. 2009;53:2346–2353. doi: 10.1128/AAC.01120-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngaimisi E, et al. Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther. 2010;88:676–684. doi: 10.1038/clpt.2010.172. [DOI] [PubMed] [Google Scholar]
- 21.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44:1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 22.Weiss J, Herzog M, Konig S, Storch CH, Ketabi-Kiyanvash N, Haefeli WE. Induction of multiple drug transporters by efavirenz. J Pharmacol Sci. 2009;109:242–250. doi: 10.1254/jphs.08209fp. [DOI] [PubMed] [Google Scholar]
- 23.Svärd J, Spiers JP, Mulcahy F, Hennessy M. Nuclear receptor-mediated induction of CYP450 by antiretrovirals: Functional consequences of NR1I2 (PXR) polymorphisms and differential prevalence in whites and sub-Saharan africans. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e3181f52f0c. in press. [DOI] [PubMed] [Google Scholar]
- 24.Bousquet L, Pruvost A, Guyot AC, Farinotti R, Mabondzo A. Combination of tenofovir and emtricitabine plus efavirenz: in vitro modulation of ABC transporter and intracellular drug accumulation. Antimicrob Agents Chemother. 2009;53:896–902. doi: 10.1128/AAC.00733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Störmer E, von Moltke LL, Perloff MD, Greenblatt DJ. Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res. 2002;19:1038–1045. doi: 10.1023/a:1016430825740. [DOI] [PubMed] [Google Scholar]
- 26.Weiss J, et al. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J Antimicrob Chemother. 2007;59:238–245. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- 27.Weiss J, Theile D, Ketabi-Kiyanvash N, Lindenmaier H, Haefeli WE. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug Metab Dispos. 2007;35:340–344. doi: 10.1124/dmd.106.012765. [DOI] [PubMed] [Google Scholar]
- 28.Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol. 2007;73:1573–1581. doi: 10.1016/j.bcp.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Berruet N, Sentenac S, Auchere D, Gimenez F, Farinotti R, Fernandez C. Effect of efavirenz on intestinal p-glycoprotein and hepatic P450 function in rats. J Pharm Pharm Sci. 2005;8:226–234. [PubMed] [Google Scholar]
- 30.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 31.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–399. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 33.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–374. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 34.Chang Y, Fang WB, Lin SN, Moody DE. Stereo-selective metabolism of methadone by human liver microsomes and cDNA-expressed cytochrome P450s: a reconciliation. Basic Clin Pharmacol Toxicol. 2010;108:55–62. doi: 10.1111/j.1742-7843.2010.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009;101:158–168. doi: 10.1016/j.drugalcdep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: Insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110:660–672. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega I, Rodriguez M, Suarez E, Perez-Ruixo JJ, Calvo R. Modeling methadone pharmacokinetics in rats in presence of P-glycoprotein inhibitor valspodar. Pharm Res. 2007;24:1299–1308. doi: 10.1007/s11095-007-9251-2. [DOI] [PubMed] [Google Scholar]
- 39.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass cytochrome P450 3A activity: Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004;76:452–466. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Kharasch ED, et al. Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther. 2007;82:410–426. doi: 10.1038/sj.clpt.6100237. [DOI] [PubMed] [Google Scholar]
- 41.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab. Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- 42.Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–512. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kharasch E, D. Whittington D, Ensign D, Hoffer C, Bedynek PS. Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics: I. Evidence for CYP2B6 mediation of methadone clearance. Clin Pharmacol Ther. 2011 doi: 10.1038/clpt.2011.276. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharasch ED, Francis A, London A, Frey K, Kim T, Blood J. Sensitivity of intravenous and oral alfentanil and pupillary miosis as minimal and noninvasive probes for hepatic and first-pass CYP3A induction. Clin Pharmacol Ther. 2011;90:100–108. doi: 10.1038/clpt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohnhaus EE, Park BK. Measurement of urinary 6-β-hydroxycortisol excretion as an in vivo parameter in the clinical assessment of the microsomal enzyme-inducing capacity of antipyrine, phenobarbitone and rifampicin. Eur. J. Clin. Pharmacol. 1979;15:139–145. doi: 10.1007/BF00609878. [DOI] [PubMed] [Google Scholar]
- 46.van de Kerkhof EG, de Graaf IA, Ungell AL, Groothuis GM. Induction of metabolism and transport in human intestine: validation of precision-cut slices as a tool to study induction of drug metabolism in human intestine in vitro. Drug Metab Dispos. 2008;36:604–613. doi: 10.1124/dmd.107.018820. [DOI] [PubMed] [Google Scholar]
- 47.Faucette SR, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang JG, Ho T, Callendrello AL, Crespi CL, Stresser DM. A multi-endpoint evaluation of cytochrome P450 1A2, 2B6 and 3A4 induction response in human hepatocyte cultures after treatment with β-naphthoflavone, phenobarbital and rifampicin. Drug Metab Lett. 2010;4:185–194. doi: 10.2174/187231210792928224. [DOI] [PubMed] [Google Scholar]
- 49.Tucker GT, Houston JB, Huang S-M. Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential-toward a consensus. Clin. Pharmacol. Ther. 2001;70:103–114. doi: 10.1067/mcp.2001.116891. [DOI] [PubMed] [Google Scholar]
- 50.Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 51.Matsushima S, et al. Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008;73:1474–1483. doi: 10.1124/mol.107.041459. [DOI] [PubMed] [Google Scholar]
- 52.Swift B, Tian X, Brouwer KL. Integration of preclinical and clinical data with pharmacokinetic modeling and simulation to evaluate fexofenadine as a probe for hepatobiliary transport function. Pharm Res. 2009;26:1942–1951. doi: 10.1007/s11095-009-9909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tannergren C, Petri N, Knutson L, Hedeland M, Bondesson U, Lennernas H. Multiple transport mechanisms involved in the intestinal absorption and first-pass extraction of fexofenadine. Clin. Pharmacol. Ther. 2003;74:423–436. doi: 10.1016/S0009-9236(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 54.Cui X, Thomas A, Gerlach V, White RE, Morrison RA, Cheng KC. Application and interpretation of hPXR screening data: Validation of reporter signal requirements for prediction of clinically relevant CYP3A4 inducers. Biochem Pharmacol. 2008;76:680–689. doi: 10.1016/j.bcp.2008.06.016. [DOI] [PubMed] [Google Scholar]