Abstract
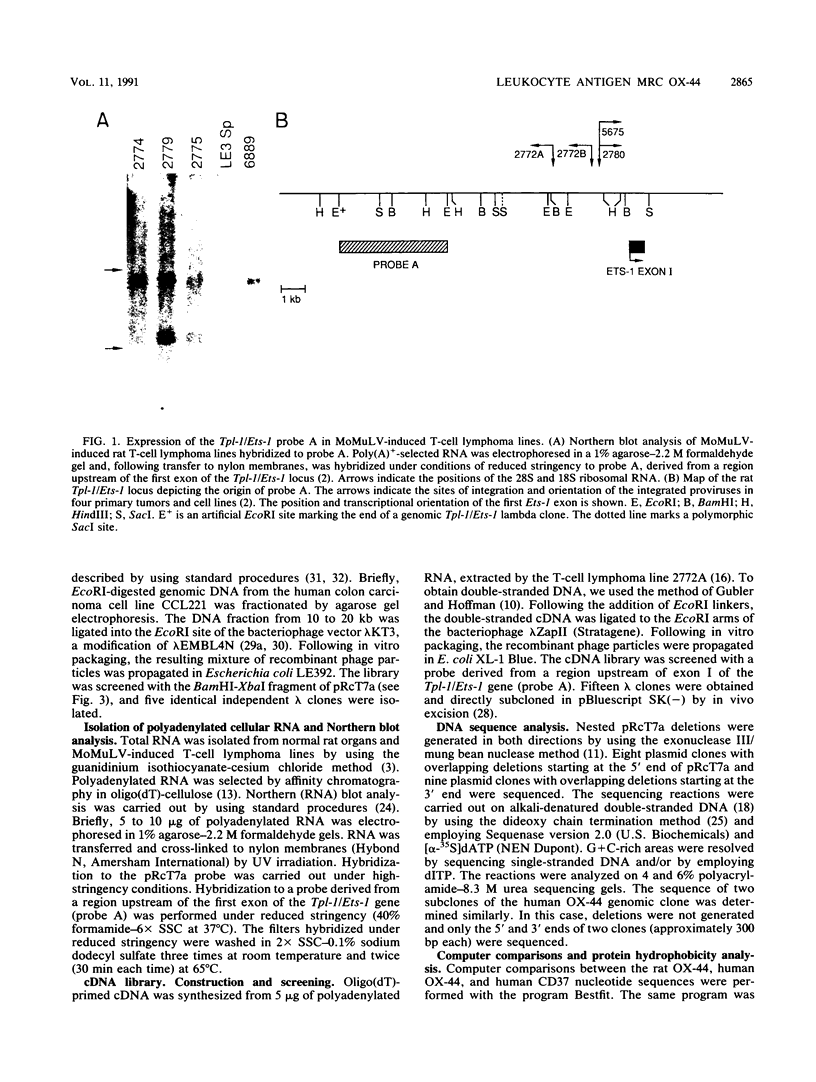
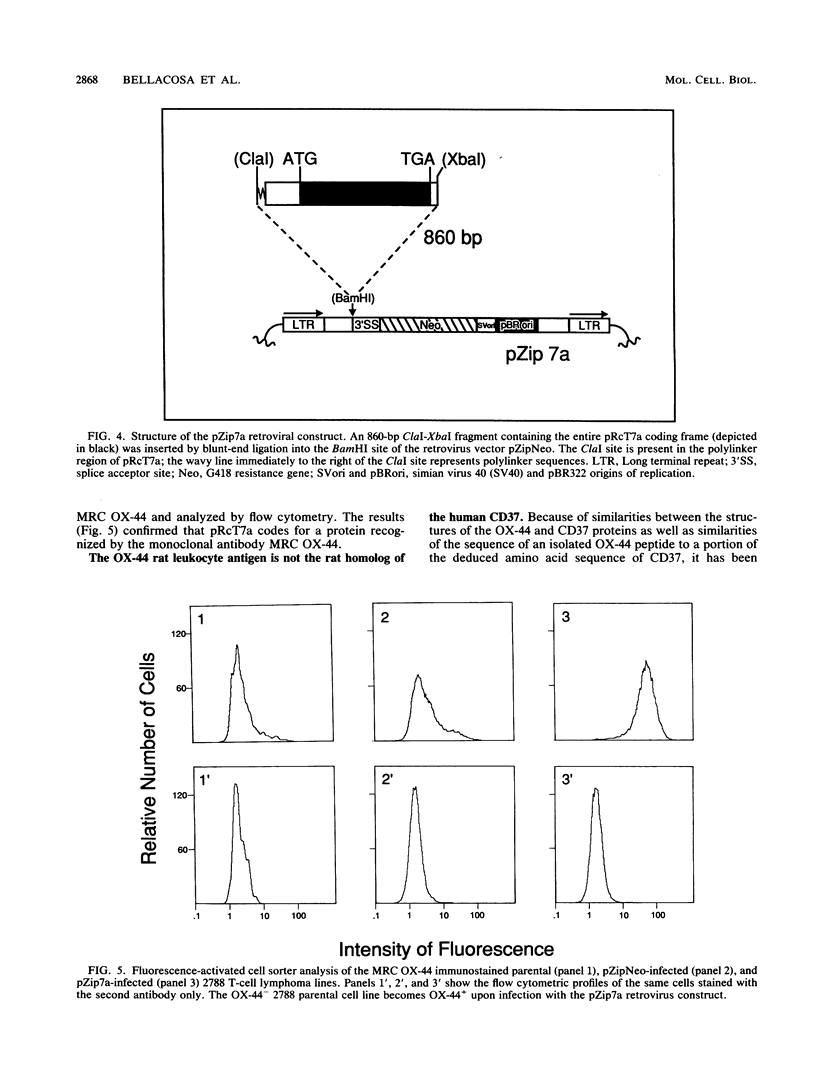
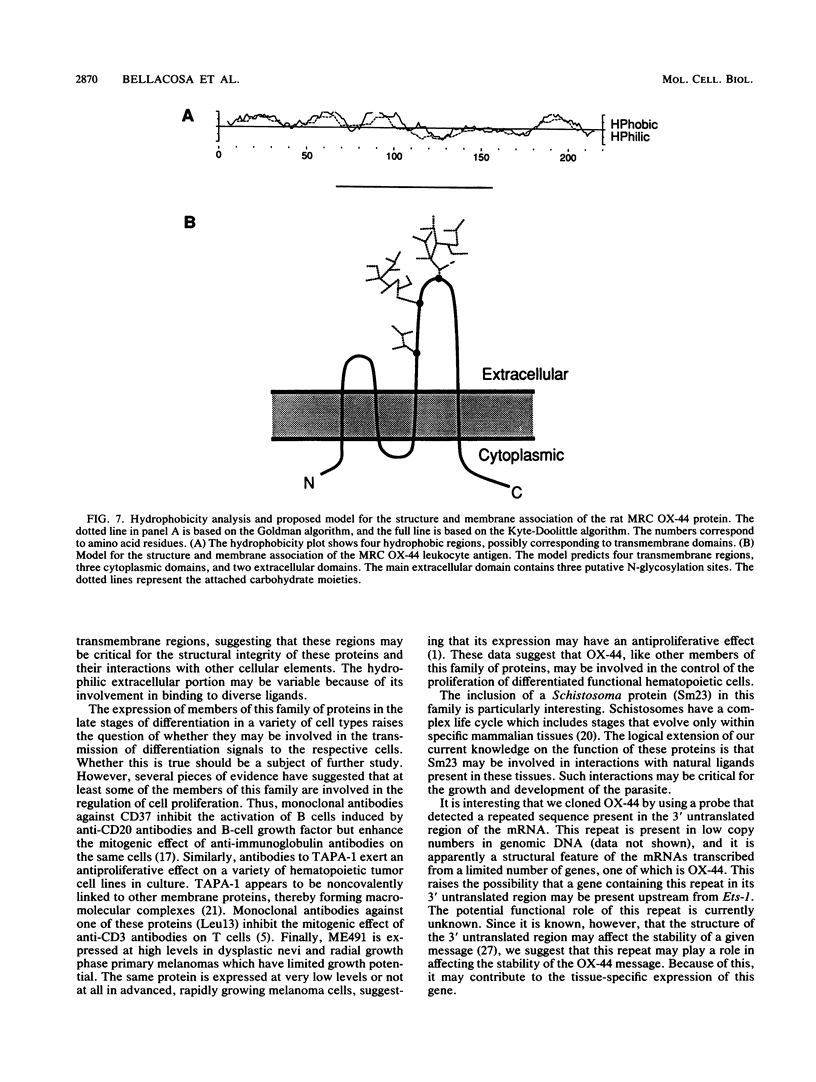
Moloney murine leukemia virus (MoMuLV)-induced rat T-cell lymphomas express discrete 1.8-, 2.2-, and 4-kb mRNA transcripts hybridizing under conditions of reduced stringency to a probe derived from a region upstream of the first exon of the Tpl-1/Ets-1 gene. Screening a cDNA library from one rat T-cell lymphoma with this genomic probe yielded 15 cDNA clones which were derived from 10 different genes. One of these genes, defined by the cDNA clone pRcT7a, was expressed as a 1.8-kb mRNA transcript in spleen and thymus but not in other normal rat tissues. Expression of the gene defined by the pRcT7a cDNA clone in a series of MoMuLV-induced rat T-cell lymphomas showed a perfect correlation with the expression of the rat leukocyte antigen MRC OX-44. Because of this observation, the pRcT7a clone was sequenced and it was shown to identify a gene coding for a 219-amino-acid protein. The homology between pRcT7a and the Tpl-1 probe used for its detection mapped within the 3' untranslated region of the pRcT7a cDNA clone. The pRcT7a protein, which exhibits four putative transmembrane regions and three putative glycosylation sites, contains a region which is nearly identical in sequence to a peptide derived from the rat leukocyte antigen MRC OX-44. This finding suggested that the pRcT7a cDNA clone defines the gene coding for OX-44. To confirm this finding, a pRcT7a construct in the retrovirus vector pZipNeo was introduced into the OX-44- T-cell lymphoma line 2788. Immunostaining with the MRC OX-44 monoclonal antibody followed by flow cytometry revealed that following gene transfer, the 2788 cells became OX-44+. Sequence comparisons revealed that pRcT7a/MRC OX-44 is a member of a family of genes which includes the melanoma-specific antigen ME491; the human leukocyte antigen CD37; the protein TAPA-1, which is expressed on the surface of human T cells and appears to be involved in growth regulation; the human gastrointestinal tumor antigen CO-029; and the Schistosoma mansoni-associated antigen Sm23.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson B., Ernst C. S., Ghrist B. F., Ross A. H., Clark W. H., Herlyn M., Herlyn D., Maul G., Steplewski Z., Koprowski H. Monoclonal antibody to a highly glycosylated protein reacts in fixed tissue with melanoma and other tumors. Hybridoma. 1985 Fall;4(3):243–255. doi: 10.1089/hyb.1985.4.243. [DOI] [PubMed] [Google Scholar]
- Bear S. E., Bellacosa A., Lazo P. A., Jenkins N. A., Copeland N. G., Hanson C., Levan G., Tsichlis P. N. Provirus insertion in Tpl-1, an Ets-1-related oncogene, is associated with tumor progression in Moloney murine leukemia virus-induced rat thymic lymphomas. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7495–7499. doi: 10.1073/pnas.86.19.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L. Preparation and characterization of RNA: overview. Methods Enzymol. 1987;152:215–219. doi: 10.1016/0076-6879(87)52022-5. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chen Y. X., Welte K., Gebhard D. H., Evans R. L. Induction of T cell aggregation by antibody to a 16kd human leukocyte surface antigen. J Immunol. 1984 Nov;133(5):2496–2501. [PubMed] [Google Scholar]
- Classon B. J., Williams A. F., Willis A. C., Seed B., Stamenkovic I. The primary structure of the human leukocyte antigen CD37, a species homologue of the rat MRC OX-44 antigen. J Exp Med. 1989 Apr 1;169(4):1497–1502. doi: 10.1084/jem.169.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classon B. J., Williams A. F., Willis A. C., Seed B., Stamenkovic I. The primary structure of the human leukocyte antigen CD37, a species homologue of the rat MRC OX-44 antigen. J Exp Med. 1990 Sep 1;172(3):1007–1007. doi: 10.1084/jem.172.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hotta H., Ross A. H., Huebner K., Isobe M., Wendeborn S., Chao M. V., Ricciardi R. P., Tsujimoto Y., Croce C. M., Koprowski H. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 1988 Jun 1;48(11):2955–2962. [PubMed] [Google Scholar]
- Jacobson A. Purification and fractionation of poly(A)+ RNA. Methods Enzymol. 1987;152:254–261. doi: 10.1016/0076-6879(87)52028-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo P. A., Klein-Szanto A. J., Tsichlis P. N. T-cell lymphoma lines derived from rat thymomas induced by Moloney murine leukemia virus: phenotypic diversity and its implications. J Virol. 1990 Aug;64(8):3948–3959. doi: 10.1128/jvi.64.8.3948-3959.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. M., Pène J. J. Optimal conditions for supercoil DNA sequencing with the Escherichia coli DNA polymerase I large fragment. Gene Anal Tech. 1988 Mar-Apr;5(2):32–39. doi: 10.1016/0735-0651(88)90024-6. [DOI] [PubMed] [Google Scholar]
- Oren R., Takahashi S., Doss C., Levy R., Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990 Aug;10(8):4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D. J., Green J. R., Jefferies W. A., Puklavec M., Williams A. F. The MRC OX-44 antigen marks a functionally relevant subset among rat thymocytes. J Exp Med. 1987 Jan 1;165(1):1–13. doi: 10.1084/jem.165.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D. J., Williams A. F. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987 Nov 1;166(5):1603–1608. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz-Albiez R., Dörken B., Hofmann W., Moldenhauer G. The B cell-associated CD37 antigen (gp40-52). Structure and subcellular expression of an extensively glycosylated glycoprotein. J Immunol. 1988 Feb 1;140(3):905–914. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szala S., Kasai Y., Steplewski Z., Rodeck U., Koprowski H., Linnenbach A. J. Molecular cloning of cDNA for the human tumor-associated antigen CO-029 and identification of related transmembrane antigens. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6833–6837. doi: 10.1073/pnas.87.17.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K. D., Hobbs C. A. New cloning vectors and techniques for easy and rapid restriction mapping. Gene. 1988 Jul 30;67(2):169–182. doi: 10.1016/0378-1119(88)90394-0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Lohse M. A. Concerted DNA rearrangements in Moloney murine leukemia virus-induced thymomas: a potential synergistic relationship in oncogenesis. J Virol. 1985 Oct;56(1):258–267. doi: 10.1128/jvi.56.1.258-267.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M. D., Henkle K. J., Mitchell G. F. An immunogenic Mr 23,000 integral membrane protein of Schistosoma mansoni worms that closely resembles a human tumor-associated antigen. J Immunol. 1990 Apr 15;144(8):3195–3200. [PubMed] [Google Scholar]