Abstract
The registration and approval of novel medicines have traditionally been based on evidence arising from large prospective trials. Such an approach is often not possible or unsuitable to evaluate the benefit-risk balance in special populations (e.g., children, ethnic groups, rare diseases). Inferences by modeling and simulation can play a major role in evidence synthesis. A framework is proposed that promotes its acceptability and the basis for decision making during development, registration, and therapeutic use of drugs.
M&S to Support Dose Finding in Special Populations and Ethnic Groups
The approval of medicines has been determined primarily by empirical evidence generation. By contrast, formal application of M&S approaches to support evidence synthesis as the basis for the evaluation of efficacy and safety has not been fully embraced in the regulatory approval process, despite its widespread application in other areas, such as the evaluation of effectiveness and/or cost–benefit of therapeutic interventions.1,2
Distinctly, further consideration of the requirements for pediatric drug development has led to the creation of various guidelines and introduction of a well-defined regulatory process in the European Union (e.g., Guideline on Clinical Trials in Small Populations, CHMP/EWP/83561/2005), which considers the benefit of M&S as a tool for characterizing pharmacokinetics, pharmacodynamics, efficacy, and safety in children.3
Obviously, situations exist in which complete data cannot be generated and inferences from, e.g., underpowered trials, single arm studies, surrogate end points, other populations will have to be made about the efficacy and safety of a compound to ensure access to treatment and availability of suitable therapeutic regimens to patients. In these circumstances, it has been demonstrated that the use of pharmacokinetic–pharmacodynamic relationships in conjunction with M&S concepts can support dose rationale as well as dose adjustment in specific subgroups of a population.
Given that M&S has been successfully used to assess the impact of clinically relevant differences in children (e.g., metabolic maturation and pharmacokinetics across different age groups), a similar approach could be conceived for ethnic groups and other special populations, which provides the basis for truly personalized medicines, including dosing algorithms.4,5
The Use of M&S to Extrapolate Across Different Populations and Diseases
As defined in the draft EMA concept paper, extrapolation may be generally defined as “extending information and conclusions available from studies in one or more subgroups of the patient population (source population), or in related conditions or with related medicinal products, to make inferences for another subgroup of the population (target population), or condition or product, thus reducing the need to generate additional information (types of studies, design modifications, number of patients required) to reach conclusions for the target population, or condition or medicinal product.”6
Similarly to the extrapolations typically used to describe pharmacokinetics, pharmacodynamics, efficacy, and safety in children and ethnic groups, many other situations exist for which inferences can be used to extrapolate and predict treatment response or characterize drug properties. Examples of the application of such a concept include the investigation of differences in population subsets (e.g., gender, comorbidities, impaired organ function) or the assessment of treatment response between health and disease conditions as well as across different diseases or age groups. Increasing interest in mechanism-based approaches has also prompted efforts towards model parameterization that enables the distinction between drug- and system or disease-specific properties, e.g., differences in the affinity to target receptors, maturational profiles and change in turnover rate of an enzyme with disease progression.7 Such a parameterization offers the basis for extrapolation and prediction between different drugs in the same disease or within and between drug classes.
As shown by the case studies (Table 1), a shift in paradigm can be observed in which evidence synthesis is favored, making evidence generation a confirmatory step in the continuum between assumptions and empirical evidence. Among the lessons learned from these examples, it is worth emphasizing that evidence generation without data integration, including systematic incorporation of prior knowledge leads to less than optimal experimental protocols and potentially inappropriate decision criteria. This aspect is often ignored in the rationale for mainstream clinical trials. Another important point arising from the same examples is that evidence synthesis can be far more powerful than evidence generation, as it gives insight into conditions that have not been evaluated experimentally. In addition, in conjunction with the appropriate clinical reasoning, evidence synthesis by inferential methods creates a more reliable foundation for decision making. It also enables one to account for practical and ethical constraints, e.g., trials in children or rare diseases. This is particularly relevant for low frequency events (e.g., polymorphisms) as well as when the onset of response is delayed relatively to the start of treatment and extrapolations must be made for prognosis and prediction of the outcome (e.g., in children where drug-induced changes in the maturation of physiological function may appear clinically late after treatment).
Table 1. Presentations break-out Session 3: modeling and simulation as a tool to bridge efficacy and safety data in special populations.
Handling Model Uncertainty, Model Misspecification and Validity of Assumptions in the Use of M&S
The impact of uncertainty may be closely related to clinical consequences. The challenges for the systematic implementation of M&S in regulatory submissions have been illustrated with examples of successful and unsuccessful approaches across a range of diseases and conditions. Some of the recent experiences were also shared to bring in regulatory expectations. It is clear that the degree of regulators' scrutiny in the use of M&S depends on the impact it has on regulatory decision making, i.e., approval, label claims and implications for patients. A streamlined process and a framework to assess assumptions and consequences for patients and other stakeholders has been highlighted as a prerequisite for better understanding and acceptance of alternative approaches for evidence synthesis of the benefits and risks of an intervention.8
Therefore, assumptions underlying M&S need to be evaluated in a similar way one queries the validity and integrity of the evidence derived by empirical experimentation. Supposing that a research question has been clearly formulated, an experiment (clinical trial) can be designed to address and potentially answer it. Under most circumstances, assumptions will be made regarding different elements of the protocol, from the randomness of the sample to the biological mechanisms describing drug action. Once the experiment has been conducted, the research question may be answered, but conclusions and inferences from the analysis of the results will be made on the postulation that assumptions have not been violated. If there is doubt about the validity or integrity of the experiment, the research question may be revisited or a new hypothesis considered and tested in subsequent experiments.
What is important in making inferences by M&S is to be critical about the assumptions, understanding the impact of their violation and consequently the implications for the population or the individual patient. In fact, drug development and approval could be described as a process in which assumptions are replaced by evidence or inferences and consequently by knowledge (i.e., increased certainty).9 The ultimate goal is to achieve optimal treatment benefit as well as minimize the risk of harm and agree on mitigation measures if acceptable safety cannot be achieved otherwise, e.g., label restrictions.
In contrast to an empirical approach, M&S enables continuous and systematic assessment of the benefit–risk balance and its interpretation by relevant stakeholders. A potential downside of M&S is that it involves assumptions that cannot always be validated. Therefore, it is essential that models integrate pharmacological and physiological rationales to prevent bias and support the different assumptions used during the iterative cycles of model building and validation throughout drug development.
Common Objectives and Proposed Next Steps
The rationale for evidence synthesis by M&S is to address feasibility issues, avoid unnecessary studies in the target population as well as facilitate the interpretation of the limited evidence available or expected to be generated. The implementation of such principles within the regulatory context requires that stakeholders refrain from asking dogmatic questions, but rather present in an open dialogue the various positions and underlying assumptions. When making inferences from a model, instead of assessing the “correctness” of the assumptions, one should be pragmatic about which expectations can be met given clinical needs and available knowledge.10 Quantitative information about the outcomes as well as the potential for violation of the assumptions should be provided for decision-making purposes, including details on mitigation measures or the requirements for additional evidence generation.
The participants in this breakout session called for the development of a framework supporting the use of M&S in clinical development plans, which will facilitate the regulatory approval process and therapeutic use of medicines in special populations and in rare diseases. The main challenges remain, however, the lack of clarity about qualifying assumptions and clinical consequences of inaccuracies or biases in M&S. A standardized process to summarize assumptions and evaluate their impact was considered a prerequisite in order to establish (i) the adequacy of the inferences, (ii) the need for additional evidence, and (iii) the requirement for mitigation measures. Of particular importance was the assessment of the consequences of assumptions used in M&S: assumptions can be violated (e.g., addressed by additional evidence or a better model), mitigated (e.g., by label restriction, treatment regimen adjustment) or managed as potential risks for patients, regulators or sponsors.
Effective actions were considered to promote sharing of standards for evidence synthesis when deriving inferences from M&S (see Figure 1) in the concerned population(s), i.e., in rare diseases, pediatric indications, other special populations or across ethnic groups. In addition, it was clear that M&S approaches should be ranked according to their potential predictive or prognostic value. A proposal was made for the introduction of a “skepticism factor” during the regulatory evaluation process, which would allow one to weigh the assumptions underpinning the extrapolation or inferences made from a model taking into account the degree of confidence one has in those assumptions. The advantages and limitations of such an approach could then be monitored by a pilot project with examples, building upon those presented during the workshop. Obviously, the ownership of existing data and the standards required for data integration remains an important issue from a practical point of view. This issue is particularly important for small companies, which develop drugs for children and rare diseases, but have very limited opportunities to access historical data.
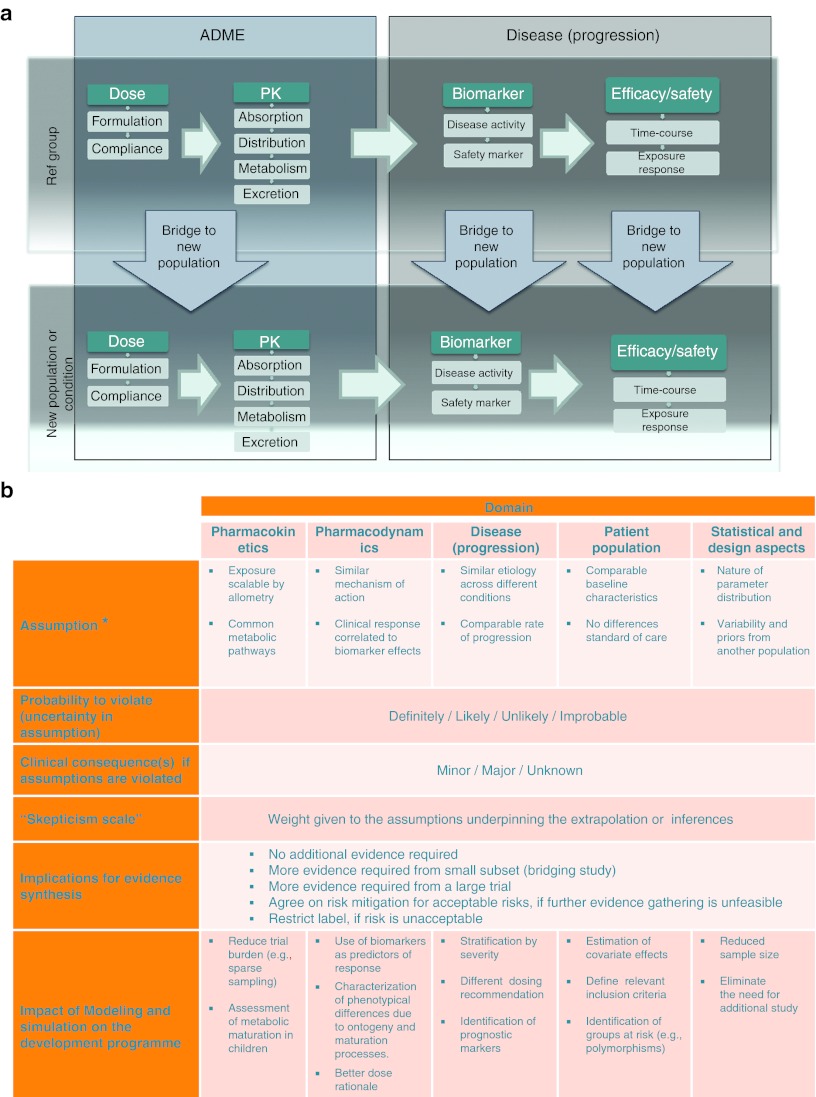
Figure 1.
(a) Schematic diagram for translating, extrapolating and bridging findings within (horizontal axes) and across (vertical axes) populations. The diagram depicts the evidence required as well as the assumption building process for assessing the differences and similarities in clinical, biological, pharmacological, and pharmaceutical, substrates which should be considered and presented in a systematic manner. Each arrow represents a different step in the translation, extrapolation, or bridging of available evidence. The validity of such an inferential exercise as well as the decision on the need for additional evidence is substantiated by the criteria presented in the framework shown in b. (b) Framework for evidence synthesis by modeling and simulation. The less evidence is available or generated, the higher the implications of inferences by modeling and simulation. Implementation of a model-based approach requires a clear description of the risk and corresponding clinical consequences, including mitigation measures. Assumptions required for inferential purposes should be characterized into at least five categories: pharmacokinetics, pharmacodynamics, disease, population characteristics, and design factors. Of importance is the possibility to assess risk as the probability of an assumption being violated (i.e., from unlikely to definitely) and the clinical consequences associated with such violation (i.e., from minor to major). *Exemplified for comparison of new vs. reference population.
A consensus was achieved regarding the need for a concerted effort on the points to consider for evidence synthesis based on inferential methods using M&S and on the availability of a common template for the assessment of model assumptions, clinical implications, risks, and mitigation measures.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the organizers, panelists and presenters for break out session 3: Huub-Jan Kleijn (MSD), Matts Kågedal (AZ), Chyi-Hung Hsu (J&J), Wolfgang Köpcke (EMA), Peter Bauer (University of Vienna), Martin Posch (EMA), Ralf Herold (EMA), Trevor Johnson (Simcyp), Joe Standing (GOSH), Stephanie Läer (Heinrich-Heine University) and Christophe Male (Medical University of Vienna).
References
- Hoaglin D.C.et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2 Value Health 14429–437.2011 [DOI] [PubMed] [Google Scholar]
- Jones B.et al.; PSI Health Technology Special Interest Group, Evidence Synthesis sub-team Statistical approaches for conducting network meta-analysis in drug development Pharm. Stat. 10523–531.2011 [DOI] [PubMed] [Google Scholar]
- Manolis E., &, Pons G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br. J. Clin. Pharmacol. 2009;68:493–501. doi: 10.1111/j.1365-2125.2009.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Gorter de Vries F., Burger D., Danhof M., &, Della Pasqua O. A model-based approach to dose selection in early pediatric development. Clin. Pharmacol. Ther. 2010;87:294–302. doi: 10.1038/clpt.2009.234. [DOI] [PubMed] [Google Scholar]
- Yuen E., Gueorguieva I., Wise S., Soon D., &, Aarons L. Ethnic differences in the population pharmacokinetics and pharmacodynamics of warfarin. J. Pharmacokinet. Pharmacodyn. 2010;37:3–24. doi: 10.1007/s10928-009-9138-4. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Human Medicines Development and Evaluation. Concept paper on extrapolation of efficacy and safety in medicine developmentEMA/129698/2012 (http://www.ema.europa.eu/docs/en_GB/document_library/ Scientific_guideline/2012/06/WC500129285.pdf ) ( 2012
- Bellanti F., &, Della Pasqua O. Modelling and simulation as research tools in paediatric drug development. Eur. J. Clin. Pharmacol. 2011;67 Suppl 1:75–86. doi: 10.1007/s00228-010-0974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolis E., Rohou S., Hemmings R., Karlsson M.O., &, Milligan P.A.How to use modelling and simulation in clinical development: Output from the EFPIA/EMA Modelling and Simulation Workshop CPT Pharmacometrics Syst. Pharmacol.this issue. [DOI] [PMC free article] [PubMed]
- Griffin S., Bojke L., Main C., &, Palmer S. Incorporating direct and indirect evidence using bayesian methods: an applied case study in ovarian cancer. Value Health. 2006;9:123–131. doi: 10.1111/j.1524-4733.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- Bauer P. Statistical methodology relevant to the overall drug development program. Drug Inf. J. 2003;37:81–89. [Google Scholar]