Abstract
The European Medicines Agency (EMA) and the Federation of Pharmaceutical Industries and Associations (EFPIA) hosted a workshop on modeling and simulation (M&S).1 Representatives from industry, academia, and regulatory agencies from Europe and beyond discussed the role of M&S in the development and registration of medicinal products within plenary and breakout sessions (BOS). This manuscript summarizes the plenary discussion (Table 1) focusing on the European perspective. Deliverables from each BOS are included in separate papers.
M&S Definition
In this Perspective, and the others following, we have reflected the workshop title and adopted the generic term M&S to capture a range of quantitative approaches, including pharmacometrics/systems pharmacology2 and other mathematical/statistical approaches. What we imply though could be best characterized by the more modern terminology, namely, model-based drug development3 and model-informed regulatory assessment (cf. Terry Shepard's presentation).
Problem Statement
The pharmaceutical industry is facing considerable challenges as shown by the ever-increasing attrition rates for new drug development projects across all R&D phases.4 Less than 10% of new compounds that enter clinical trials ultimately make it to the market and late-stage failure is high. This level of “efficiency” can be and should be addressed by drug developers and regulators. There is historical precedence that industry can identify and remedy significant causes of attrition; inadequate drug metabolism and pharmacokinetics are no longer the primary cause of compound attrition. Nowadays, the high failure rates in phases 2 and 3 are due to inadequate compound efficacy, and this is the most important determinant of overall drug development cost.5
Opportunities with M&S For Regulators, Industry, and Academia
European Regulators share the industry view that the high compound attrition rate presents a major issue and have called for innovative (improved) methods in drug development and evaluation, and more frequent and early dialogue with industry.6 Currently, across both regulators and industry, there is a belief that M&S can increase the robustness of both regulatory and industrial decision making. A systematic quantitative approach to underpin and explain the underlying scientific rationale for the selected pathways, target mechanisms, molecule attributes, experimental designs, dose regimes, and patient populations investigated can add considerable value in the characterization of a compound's benefit/risk profile. Through such a structured approach, it will be possible to constantly examine the robustness of each of these interdependent components in the light of a dynamic environment (in terms of emerging science and competitive/comparative landscape). This will enable the assessment of the potential “value” of drugs in development to be determined on the basis of current evidence.
Regulators are keen to access the integrative knowledge and the potential for more quantitative decision making that M&S can offer, when involved in early discussions with industry and when evaluating the benefit/risk of medicinal products. Historical examples have demonstrated that M&S can be influential when appropriately used, e.g., in pediatrics, anti-infectives and small populations.7,8,9 All parties believe that the appropriate use of M&S is an indicator of a more rational drug development and subsequently supports robust outcomes of clinical trial authorization, scientific advice, pediatric investigation plans, and benefit/risk decisions. Decisions arrived in the absence of M&S can miss an opportunity to quantify and, in some circumstances, to mitigate the uncertainty in the benefit/risk assessment with potential impact on labeling and postapproval burden (cf. Terry Shepard's presentation).
Regulators acknowledge that the development of medicinal products is evolving in terms of conduct and focus. Clinical trials are being conducted in an ever-increasing international environment. Generating sufficient evidence to characterize benefit/risk across a broad range of populations (pediatrics, geriatrics, genotype, ethnicity, etc.) is becoming increasingly challenging. Moreover, the transition of “Omics” from research to clinic calls for new methods for drug development and evaluation. In this setting, it is anticipated that the conduct of drug development can be better targeted when incorporating underlying scientific advances, including M&S. The systematic integration of compound specific and mechanism and disease area relevant information should help to create a comprehensive, complete, and contemporary body of evidence for well-informed decision both for the drug developer and for the regulator.
Challenges with M&S For Regulators, Industry, and Academia
It is evident that M&S activities are underreported in regulatory submissions, and a number of factors have contributed to this situation arising:
One of the biggest challenges will be to bridge the communication gap between modeling scientists and other disciplines both within industry and between industry and regulators. It is also evident that there is a misperception surrounding the level of interest from regulatory agencies in applying M&S within exploratory development and dose-finding contexts. In particular, a misperception exists that dose–response characterization and dose regimen selection are determined solely at the company's risk with regulators having little or no interest in these critical aspects of development. While continuing to advance compounds into the expensive/expansive phase 3 arena undoubtedly represents risk principally to the drug developer, this should not be confused with a lack of concern or scientific interest from regulators. The unsustainable failure rates in phase 3 can be most likely attributed to the robustness of decisions to progress to phase 3. Clearly, the ability (or capacity) of existing early-stage information to provide sufficient and adequate “learning” still has to be appropriately addressed if the high failure rate in late-stage development is to be reduced.
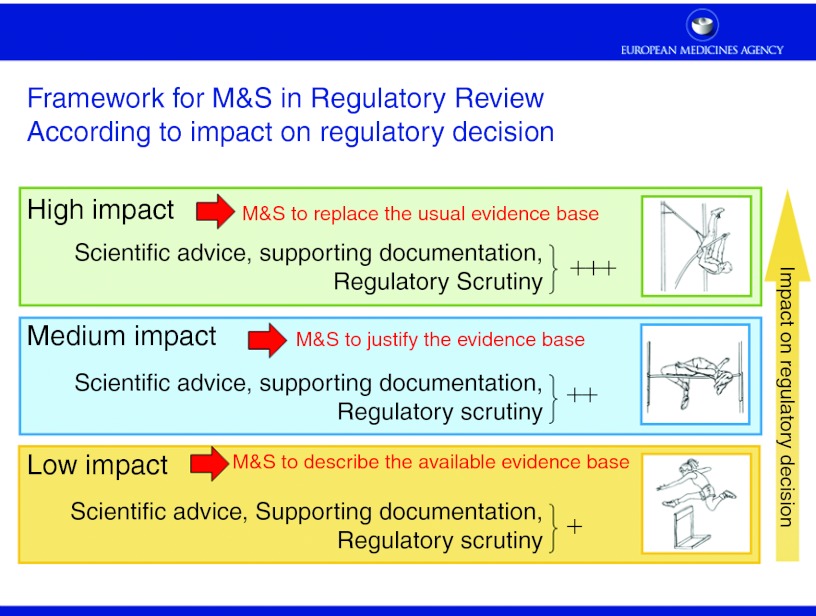
Other challenges are inherent to the M&S methodology per se. These include the need to expand the model building database and the need to standardize methods, review, and reporting. Standardized methods, accounting for issues such as internal, external validity, control of bias and type I error, and well-reported results (cf. Monica Edholm's presentation) are always favored by regulators for obvious reasons, especially in a confirmatory setting. An open question is how M&S can be customized to meet the regulatory requirements or, the other way around, how regulators can customize their thinking to adjust to the more probabilistic (through estimation and predictive/extrapolative) “mind-set” of M&S. It is acknowledged that a “one size fits all solution” is not possible given the diversity of modeling approaches (e.g., systems pharmacology, population, physiological, disease progression models). However, every effort should be made to standardize the modeling approach/workflow and establish good practice documents. A framework was proposed (Figure 1) where the degree of regulatory scrutiny, level of documentation, and the need for early dialogue is proportional to the weight (“impact”) of the M&S exercise in regulatory decision making. According to this framework and depending on the degree of impact, specific regulatory standards are suggested, and early discussion with regulators is becoming increasingly important to gain consensus. This was further illustrated by case studies where M&S results were either accepted by EMA as a basis for labeling of an unstudied dose (cf. Valerie Cosson's presentation) or rejected due to questionable extrapolation assumptions (cf. Oscar Della Pasqua's presentation and Chyi Hung Hsu's presentation).
Relevant data are the cornerstone of every model. Currently, data are segregated between different companies and organizations, or even between different products. As a consequence, current models have a potential to be built on incomplete data and limited in a broader context through being derived from potentially incomplete/inadequate data. Pooling precompetitive data (e.g., systems, placebo, failed drugs data) together from different (but relevant) sources would create an enormous potential for a more comprehensive understanding of the human (patho)physiology and pharmacology thereby establishing more “universal” mechanistic models with broader applicability. Approaches such as cross-validation between different sources of data will also raise the external validity of the models and minimize bias. Regulators encourage such academic–industry initiatives and are amenable to discuss the applicability of the resulting models/datasets in regulatory submissions.
The variable readiness within the current regulatory system to evaluate M&S and the capacity of the system to engage at a level of detail that the individual drug developer might find useful are other challenges.
M&S experts are not necessarily influential across the breadth of the drug development industry, leading to heterogeneity and inconsistency in practice within the industry.
The current electronic common technical document structure does not lend itself to detailed reporting of M&S activities of the types detailed above.
Figure 1.
Framework for M&S in regulatory review according to impact on regulatory decision. M&S, modeling and simulation.
Common Objectives and the Next Steps Proposed
All parties in attendance are committed to working together toward promoting model-based drug development and model-informed regulatory assessment and agreed on the following actions.
Establish a more standardized and quantitative framework for extrapolation (see also BOS3 paper). The recently published EMA concept paper on extrapolation10 that acknowledges the value of M&S is a first step toward this direction.
Strengthen model and data-sharing initiatives. Regulators wish to be involved and discuss regulatory applicability of such initiatives (see also BOS1 paper).
An update to the current regulatory guidance on dose ranging/finding (see also BOS2 paper) will be debated.
M&S methodology and reporting standards and good practice documents are required (see also BOS4 paper). This will be an industry-led initiative in the first instance, though regulators wish to be involved early in the discussion with the ultimate objective to develop guidance documents.
A communication strategy will be established using existing regulatory pathways, i.e., the scientific advice procedure, the EMA qualification of novel methodologies, or the innovation task force meetings. Moreover, during the clinical trial authorization application, pediatric investigation plan and marketing authorization application sponsors can enhance communication by providing more details and justification for M&S (cf. Spiros Vamvakas' presentation). The purpose of this dialogue is on one hand to increase the awareness and agreement on product-related issues and on the other hand to reach consensus on the context of use, methods, and regulatory requirements in a broader sense. To further promote communication, additional multistakeholder workshops will be organized.
EMA will increase the regulatory M&S competence in Europe by coordinating the expertise across member states to provide a consistent approach in product-related and methodology-related discussions.
In conclusion, the challenges and opportunities with M&S have been identified, so has the action plan (Supplementary Table S1 online); it is now time to work toward bringing M&S to its full potential and open a new era in the development of medicinal products and regulatory review.
Conflict of Interest
S.R. is an employee of AstraZeneca. P.A.M. is an employee of Pfizer. The other authors declared no conflict of interest.
Table 1. Presentations plenary session.
Acknowledgments
The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
Supplementary Material
References
- European Medicines Agency-European Federation of Pharmaceutical Industries and Associations modelling and simulation workshop report . < http://www.ema.europa.eu/docs/en_GB/ document_library/Report/2012/05/WC500127118.pdf >
- Van Der Graaf P.H.CPT: Pharmacomet. Syst. Pharmacol. 1e8.2012. doi:10.1038/psp.2012.8. < . < http://www.nature.com/psp/journal/ v1/n9/pdf/psp20128a.pdf > [DOI] [PMC free article] [PubMed]
- Kowalski K.G., Ewy W., Hutmacher M.M., Miller R., &, Krishnaswami S. Model-based drug development - a new paradigm for efficient drug development. Biopharmaceut Report. 2007;15:2–22. [Google Scholar]
- Pammolli F., Magazzini L., &, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 2011;10:428–438. doi: 10.1038/nrd3405. [DOI] [PubMed] [Google Scholar]
- Arrowsmith J. Trial watch: Arrowsmith, J. Phase II failures: 2008–2010. Nat. Rev. Drug. Discov. 10 February 2011, Arrowsmith, J. Phase III and submission failures: 2007–2010. Nat. Rev. Drug. Discov. 10 May 2011; [DOI] [PubMed] [Google Scholar]
- European Medicines Agency: Road map to 2015 The European Medicines Agency's contribution to science, medicines and health. < . < http://www.ema.europa.eu/docs/en_GB/document_library/ Report/2011/01/WC500101373.pdf >
- Manolis E., &, Herold R. Pharmacometrics for regulatory decision making: status and perspective. Clin. Pharmacokinet. 2011;50:625–626. doi: 10.2165/11594340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dunne J.et al. Extrapolation of adult data and other data in pediatric drug-development programs Pediatrics 128e1242–e1249.2011 [DOI] [PubMed] [Google Scholar]
- Manolis E., &, Pons G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br. J. Clin. Pharmacol. 2009;68:493–501. doi: 10.1111/j.1365-2125.2009.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA draft Concept Paper on extrapolation of efficacy and safety in medicines development 2012 . < http://www.ema.europa.eu/docs/en_GB/ document_library/Scientific_guideline/ 2012/06/WC500129285.pdf >
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.