Abstract
Objective
To determine safety and efficacy of subretinal gene therapy in the RPE65 form of Leber congenital amaurosis using recombinant adeno-associated virus 2 (rAAV2) carrying human RPE65 gene.
Design
Open-label, dose-escalation Phase I study of 15 patients (11-30 years) evaluated after subretinal injection of rAAV2-hRPE65 to the worse-functioning eye. Five cohorts represented four dose levels and two different injection strategies.
Main Outcome Measures
Primary outcomes were systemic and ocular safety. Secondary outcomes assayed visual function with dark-adapted full-field sensitivity testing and ETDRS visual acuity. Further assays included immune responses to the vector, static visual fields, pupillometry, mobility performance and OCT.
Results
No systemic toxicity was detected; ocular adverse events were related to surgery. Visual function improved in all patients to different degrees; improvements were localized to treated areas. Cone and rod sensitivities increased significantly in study eyes but not control eyes. Minor acuity improvements were recorded in many study and control eyes. Major acuity improvements occurred in study eyes with the lowest entry acuities and parafoveal fixation loci treated with subretinal injections. Other patients with better foveal structure lost retinal thickness and acuity after subfoveal injections.
Conclusions
RPE65-LCA gene therapy is sufficiently safe and substantially efficacious to the extrafoveal retina. There is no benefit and some risk in treating the fovea. No evidence of age-dependent effects was found. Our results point to specific treatment strategies for subsequent phases.
Application to Clinical Practice
Gene therapy for inherited retinal disease has the potential to become a future part of clinical practice.
INTRODUCTION
Treatments for previously incurable hereditary retinal degenerations are emerging (1). A gene-based therapy is currently being evaluated in clinical trials for the autosomal recessive retinal disease Leber congenital amaurosis (LCA) resulting from RPE65 (retinal pigment epithelium-specific-65-kDa) deficiency. This disorder interrupts the visual-retinoid cycle (2,3); visual pigment is not available to photoreceptors through this key pathway; and vision is severely compromised. In a canine model of RPE65 deficiency, subretinal delivery of a gene-viral vector agent led to a remarkable activation of retinal and postretinal function (4,5). Mice with RPE65 deficiency showed the same dramatic treatment effect (6-11). Following further proof-of-concept studies, dose-response data, and toxicity testing (12-14), human gene therapy in RPE65-LCA seemed to be the next worthy step and clinical trials began.
Early results of three contemporaneous human clinical trials were reported in 2008 and these preliminary results showed safety and modest efficacy after subretinal injections of AAV2-RPE65 (15-18); more recently, a fourth trial was initiated and early results published (19). Questions about the longevity of the safety and efficacy in gene therapy for RPE65-LCA have started to be addressed (20-23). Beyond the initial increases in visual sensitivity post-treatment, we detected a slow and progressive movement of fixation over many months from the anatomical fovea to the treated retinal region. The region of therapy had become a preferred locus for use in this eye under certain conditions, suggesting cortical adaptations to the restored vision (24).
We have now performed gene vector administration in five cohorts of patients, representing children and adults, in a dose-escalation study using a single subretinal injection in the first three cohorts and two injections in the same eye at the time of surgery in the last two cohorts. Safety and efficacy results from our initially-reported cohort (17,18) now represent a 3-year interval since treatment. These results of relatively long-term follow-up taken together with those from patients with shorter-term follow-up lead to a perspective on how best to advance this trial and other retinal gene therapy clinical trials.
METHODS
The clinical trial was performed at Scheie Eye Institute of the University of Pennsylvania (UP) and at University of Florida/Shands (UF). The subjects had a clinical diagnosis of LCA. RPE65 mutations were determined by the John and Marcia Carver Nonprofit Genetic Testing Laboratory (University of Iowa). Study eligibility and protocol; regulatory approvals and oversight; cGMP vector production, purification and titering; and surgical procedure for vector administration have been reported in earlier studies of Cohort 1 (17,18,20). Trial conduct was in a manner consistent with the ICH—E6 Good Clinical Practice guideline document, and was reviewed by the United States Food and Drug Administration (Investigational New Drug application BB-IND 12824) and the National Institutes of Health Recombinant DNA Advisory Committee (Protocol #0410-677). Approvals were obtained from the Institutional Review Boards (IRBs) and Institutional Biosafety Committees of UP and UF, the Vice Provost Research Review Committee of UP, the Western Institutional Review Board and the General Clinical Research Center of UF. A Data and Safety Monitoring Committee, appointed by the National Institutes of Health, monitored the trial. The tenets of the Declaration of Helsinki were followed. Informed consent or assent was obtained from all subjects. Brief summaries of the methods are given below and expanded methods are in the eSupplement.
VECTOR ADMINISTRATION
The eye with worse visual function was chosen for vector administration in all subjects except P10 (Table 1). Both eyes of P10 were severely affected but the eye with worse function had keratoconus and the contralateral eye was chosen for the procedure. Two methods of anesthesia were used. For the first two cohorts (n=6, ages 20-30 years), the procedure was performed with retrobulbar anesthesia. For the subsequent three cohorts (n=9) that included 6 patients under 18 years of age, general anesthesia was used. A standard 3-port 23-gauge vitrectomy was performed in Cohorts 1-3 as previously described (17); in Cohorts 4 and 5, a 25-gauge vitrectomy was performed. The vector was introduced into the subretinal space with a 39 gauge injection cannula (Synergetics Inc., O’Fallon, MO). A single injection was used in the first three cohorts while two injection sites were used in the last two cohorts (Table 1).
Table 1.
Results of Clinical Trial for RPE65-Related Leber Congenital Amaurosis
| Age at baseline (y)/ Gender |
RPE65 mutations | Follow- up (months) |
Anesthesia | Eye / # of injection sites |
Vector dose (vg) |
Total volume (μl) |
Subfoveal injection included |
Entry visual acuity (logMAR)a |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Study eyeb | Control eyeb | |||||||||
| Cohort 1 | ||||||||||
| P1 | 24/M | E417Q/E417Q | 24 | L | Left/1 | 5.96×1010 | 150 | Yes | 1.16, 1.00 | 0.96, 0.94 |
| P2 | 23/F | R44Q/R91W | 36 | L | Right/1 | 5.96×1010 | 150 | No | 1.04, 0.94 | 0.82, 0.80 |
| P3 | 21/M | Y368H/Y368H | 36 | L | Right/1 | 5.96×1010 | 150 | No | 1.24, 1.06 | 0.80, 0.70 |
|
| ||||||||||
| Cohort 2 | ||||||||||
| P4 | 30/M | G40S/H182Y | 24 | L | Right/1 | 11.92×1010 | 300 | Yes | 1.72, 1.78 | 1.64, 1.70 |
| P5 | 20/F | 97del20bp/97del20bp | 24 | L | Left/1 | 11.92×1010 | 300 | No | 1.92, 2.00c | 1.78, 1.76 |
| P6 | 22/F | L341S/L341S | 24 | L | Left/1 | 11.92×1010 | 300 | Yes | 1.48, 1.34 | 1.16, 1.26 |
|
| ||||||||||
| Cohort 3 | ||||||||||
| P7 | 15/F | IVS1+1G>T/IVS1+1G>T | 18 | G | Left/1 | 8.94×1010 | 225 | No | 1.18, 1.04 | 1.10, 1.02 |
| P8 | 16/F | V287F/V287F | 18 | G | Left/1 | 8.94×1010 | 225 | No | 0.90, 0.84 | 0.90, 0.84 |
| P9 | 11/M | IVS2-2A>T/L341S | 12 | G | Left/1 | 8.94×1010 | 225 | No | 1.02, 0.92 | 0.72, 0.62 |
|
| ||||||||||
| Cohort 4 | ||||||||||
| P10 | 24/M | R91W/R91W | 6 | G | Right/2 | 17.88×1010 | 450 | Yes | 1.50, 1.48 | 2.00c, 2.00c |
| P11 | 27/M | V353ins1bp/R91W | 6 | G | Right/2 | 17.88×1010 | 450 | No | 0.32, 0.34 | 0.28, 0.30 |
|
| ||||||||||
| Cohort 5 | ||||||||||
| P12 | 15/M | IVS2-2A>T/L341S | 6 | G | Right/2 | 17.88×1010 | 450 | No | 0.64, 0.58 | 0.50, 0.40 |
| P13 | 11/F | R91W/R91W | 3 | G | Left/2 | 17.88×1010 | 450 | Yes | 1.04, 0.94 | 0.40, 0.50 |
| P14 | 17/F | IVS1+5G>A/Y239D | 3 | G | Right/2 | 17.88×1010 | 450 | No | 1.06, 0.88 | 0.82, 0.84 |
| P15 | 18/F | Y368H/K354ins1bp | 1 | G | Right/2 | 7.95×1010 | 200d | No | 0.72, 0.62 | 0.64, 0.64 |
Abbreviations: vg, vector genomes; L, local; G, general
Best-corrected visual acuity measured from back-lit Early Treatment Diabetic Retinopathy Study charts adjusted for distance. 0.00 logMAR=20/20 Snellen acuity at 4 m.
Baseline 1, Baseline 2
No letters seen at 0.5 m.
Intended dose was 17.88×1010vg in 450μl but only 200μl could be delivered.
OCULAR AND SYSTEMIC SAFETY PARAMETERS
Ocular safety was assessed with standard eye examinations. To quantify severity of inflammatory response, standard grading systems were used (25-27). To document fundus appearance, fundus photographs (using an infrared camera to avoid excess visible light exposure) were taken at baseline and at post-treatment visits. Systemic safety was evaluated with physical examinations at baseline and postoperative visits. Routine hematology, serum chemistry, prothrombin time (with INR), partial thromboplastin time, and urinalysis were performed at baseline and postoperatively. The schedule of study visits and list of measured parameters for each timepoint from baselines to 3 years post-operative have been published (17). In all safety and efficacy studies, the examiners were not masked to which eye was the study eye.
IMMUNOLOGY PARAMETERS
Anti-AAV2 antibody titers. Serum samples from the patients were assayed for circulating antibodies to AAV2 capsid proteins at baseline, at days 14 and 90, and at years 1, 2 and 3. Details of the assay were previously described (17). Antigen-specific response. Anti-AAV2 antigen-specific lymphocyte proliferation responses were assessed as previously described (17). AAV DNA in peripheral blood. Procedures for biodistribution of patient samples in this trial were described (17). Ex vivo and cultured Interferon-enzyme-linked immunospot assays (ELISpot). Details of blood collection and the IFN-γ ELISA were described for Cohort 1 patients (17). Further details are provided for all immunology parameters (eMethods).
EFFICACY PARAMETERS
Best-corrected visual acuity (VA) was measured using ETDRS (Early Treatment Diabetic Retinopathy Study) methodology (28) at all baseline and postoperative visits (17,20). BCVA was scored as the number of letters correctly read after adjusting for distance and expressed as logMAR. The fixation locus of each eye at each visit was determined by video imaging the retina under invisible near-infrared (NIR) light (MP1, Nidek Incorporated, Fremont, CA) while the subject was gazing at a target (18,20,29). Further details are provided (eMethods).
Full-field stimulus testing (FST) was performed using a LED-based ganzfeld stimulator (Colordome, Diagnosys LLC, Littleton, MA) as previously described (5,17,30,31). Briefly, blue and red stimuli were used for testing monocularly under two dark adapted conditions: standard dark adaptation of <2 hours and extended dark adaptation of >3 hours. The latter condition draws from our previous observations of prolonged kinetics of rod but not cone dark adaptation in RPE65-related LCA treated with gene therapy (18). Further details are provided (eMethods).
Dark-adapted static visual field testing with computerized perimetry was able to be performed in 12 of 15 patients; three patients could not distinguish where in the visual field a light stimulus originated. A modified automated perimeter (Humphrey Field Analyzer; Zeiss Meditec Inc, Dublin, CA) was used (18,20,32,33). To summarize the data, maps were constructed of loci showing significant sensitivity changes postoperatively. Further details are provided (eMethods).
The direct transient pupillary light reflex (TPLR) was elicited and recorded as previously described (5,18,34). Luminance-response functions were derived from TPLR amplitude to increasing intensities (from −6.6 to 2.3 log10 scot-cd.m−2) of green stimuli with short duration (0.1 s) presented monocularly in the dark-adapted state. TPLR luminance response functions were recorded during the short-term postoperative timepoints at 1 (P2, P3, P13, P14, and P15), 3 (P1, P4-P10, P12) or 6 (P11) months. Further details are provided (eMethods).
A mobility performance task was used to quantify the ability of the patients to move through an indoor obstacle course and determine if there was any difference in this behavior before and after treatment (n=5) or between treated and untreated eyes postoperatively (n=15). The task is a version of published methods (35-40) and further details are in eMethods.
STATISTICAL ANALYSES
Differences between baseline and postoperative values of efficacy parameters (FST blue, FST red, TPLR, and VA) were evaluated for control and study eyes with paired, two-sided t-tests. Results from multiple visits within baseline and postoperative timepoints were averaged before performing the t-tests. Repeated measures analyses of variance (ANOVA) were performed with a test of interaction comparing the magnitudes of differences between treated and control eyes. Repeated measures factors included were eyes (study versus control), and visits (baseline versus postoperative). Between subjects factors included in the ANOVA were age (younger, 11-20 years or older, 21-30 years), for the analyses of all parameters, and fixation (foveal versus extrafoveal) for the analysis of VA. All group statistics are specified as mean (SE).
OPTICAL COHERENCE TOMOGRAPHY
Cross-sectional imaging using optical coherence tomography (OCT) was used to assess retinal structure before and after administration of the agent in the treated eye; comparable data were also acquired in the untreated eye. Ultra-high speed and high resolution OCT imaging with a spectral-domain (SD) OCT instrument (RTVue-100, Optovue Inc., Fremont, CA) was used, as described (17,20). Foveal thickness measurements were performed as described and statistical comparisons made between data from different visits (17,41).
RESULTS
STUDY POPULATION, ADVERSE EVENTS AND COMPLICATIONS
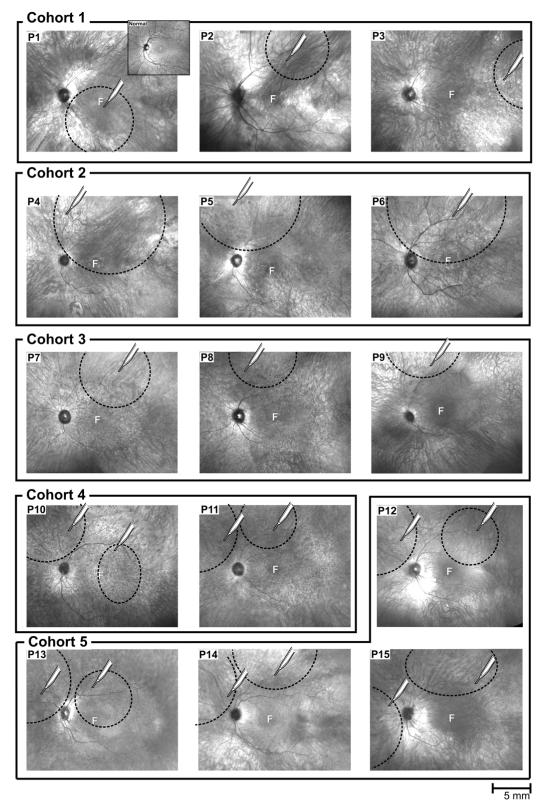
There were 15 patients (8 female and 7 male subjects) in the trial (Table 1). Other than a sibling pair (P9, P12), the patients were unrelated. P10 and P13 had the same mutant alleles (homozygous for R91W) but were not known to be related. Ninety-day and 1-year post-injection data from the three patients in Cohort 1 have been published (17,18,20,24). The first three cohorts received single subretinal injections (Figure 1). Cohorts 1 and 2 were young adult patients (ages 20-30 years) and dose-escalation occurred by doubling the initial injection volume of 150 μl of vector (Cohort 1) to 300 μl (Cohort 2). Cohort 3 patients were <18 years of age and we were advised by a UP regulatory body to reduce dosage in this first cohort of children, hence the 225 μl volume for these 3 patients. Cohorts 4 and 5 had two injections of 225 μl each (total 450 μl; Figure 1), first in young adults (ages 24 and 27 years) and then in ≤18 year-old patients. P15 had two injections but less total volume was injected. Infrared views of all 15 study eyes are shown with superimposed locations of the injection site and estimated boundaries of retinal detachments caused by the subretinal injections. The treated eyes are all portrayed as left eyes for comparison (Figure 1); the actual eye treated is tabulated (Table 1). The postoperative course was similar in 13 of 15 patients with absorption of the subretinal fluid within 48 hours and no evidence of intraocular inflammation. P7 and P11 were exceptions with a second retinal detachment and choroidal effusions, respectively (see below). By 30-60 days postoperatively, all eyes were quiet and have remained so. Systemic safety parameters including physical examinations and blood (hematology, serum chemistry, coagulation) and urine testing showed no clinically significant abnormalities after gene transfer in all patients.
Figure 1.
Fundus images with near-infrared illumination and sites of retinal detachments from subretinal injections of vector-gene in the 15 patients (P1-P15) with RPE65-LCA. Dotted circles on the images of individual patients represent the estimated areas of retinal detachment from drawings at time of surgery. The tip of each white ‘syringe’ indicates the retinotomy site that produced the detachment. All images are depicted as left eyes for comparability. F indicates fovea.
Among the postoperative adverse events were retinal detachment, choroidal effusions, ocular hypotension in the immediate postoperative period and ocular hypertension associated with the administration of topical steroids. P7, Cohort 3, had a retinal detachment in the region of the single subretinal injection, one day after it was deemed flat by ophthalmoscopy. This was surgically repaired and there has not been further complication. P11, Cohort 4, was detected to have choroidal effusions on the 3rd day after surgery. The choroidals were treated with topical cycloplegics and increased topical steroids. The lack of resolution of the choroidals at day 30 led to a 3-week course of systemic steroids. By 149 days postoperatively, the choroidals had resolved by clinical examination and ultrasound. There was no measured ocular hypotension from the first measurement (postoperative day 3) through day 238. This patient, however, developed increased intraocular pressure which was detected on day 149 and presumed to be secondary to the extended use of topical steroids. Cessation of steroids followed and topical beta blockers were added. After a period of quiescence, the choroidals re-appeared on day 190 and were treated with further cycloplegics and a short course of topical steroids. There was resolution by day 238. Ocular hypotension was documented in four patients (P4, P9, P12, P15) during the early postoperative period (days 2-5; intraocular pressures were ≤6 mm Hg with an interocular difference of 5-10 mm Hg). By postoperative day 7, pressure in the study eyes increased and interocular asymmetry decreased in all patients. Ocular hypertension was also documented after topical steroid use in the postoperative period in P8, P12 and P15. Cessation of steroids and treatment with topical beta blockers led to the eyes becoming normotensive.
IMMUNE RESPONSE ASSAYS
Humoral immune responses were monitored by measuring levels of circulating antibody to AAV2 capsid at baseline and at postoperative days 14, 90, 270 and years 1, 2 and 3, depending on the treatment cohort (Table 2). All patients exhibited titers at baseline and at all post-treatment timepoints well below the normal population mean of 2,148,715 mU/ml (n=99 random samples) except P12 with a titer of 2,789,606 mU/ml at day 90, which is ~60% above this patient’s baseline value. Ten of the 14 patients with at least day 14 data showed no increase in antibody titer greater than two-fold from baseline;, and most experienced a decline. Of the remaining four, P2 exhibited a four-fold increase at day 14, and returned to below baseline at day 90 and years 1 and 3. P5 experienced a 3-fold increase at day 14, returned to baseline at day 90 and year 1, but then spiked again at year 2. This pattern of episodic antibody spikes over multiple years with a return to baseline is not consistent with a humoral immune response to a one-time vector administration, and is more likely a result of periodic re-exposure to wild type AAV2 through natural viral infections. P6 experienced a 7-fold titer increase at day 14 that peaked at 11-fold at day 60, and has subsequently declined to just over 2-fold at year 2. P8 also showed an increase in titer at day 14 (3-fold) that peaked at day 90 (4-fold) and is diminishing at year 1 (2-fold over baseline). P6 and P8 are circumstantially the only patients with serum antibody titer behaviors potentially consistent with a response to the vector, but coincidental exposure to wild type AAV2 either directly or by reactivation of latent AAV2 through natural Adenoviral or Herpes viral infection cannot be ruled out. Overall, the patterns of patient serum antibody titers to AAV2 over time post-treatment suggest limited or no systemic immune response to subretinal AAV2 vector delivery.
Table 2.
Anti-AAV2 Serum Antibody Titers at Baseline and Post-Treatment Timepoints
| Antibody Titers (mU/mL) |
||||||
|---|---|---|---|---|---|---|
| Baseline | Day 14 | Day 90 | Year 1 | Year 2 | Year 3 | |
| Cohort 1 | ||||||
| P1 | 11,884 | 3,576 | 14,221 | 4,861 | nda | nd |
| P2b | 38,086 | 22,497 | 169,700 | 12,583 | nda | 30,831 |
| P3 | 118,861 | 38,125 | 38,251 | 59,141 | nda | 33,134 |
|
| ||||||
| Cohort 2 | ||||||
| P4 | 23,062 | 11,745 | 12,704 | 22,105 | 4,241 | … |
| P5 | 22,233 | 68,065 | 15,704 | 24,554 | 174,245 | … |
| P6b | 65,964 | 473,081 | 540,493 | 315,062 | 157,962 | … |
|
| ||||||
| Cohort 3 | ||||||
| P7 | 605,249 | 392,069 | 31,808 | 12,815 | … | … |
| P8 | 136,837 | 360,905 | 495,714 | 280,208 | … | … |
| P9 | 1,432,357 | 1,164,327 | 950,041 | 527,275 | … | … |
|
| ||||||
| Cohort 4 | ||||||
| P10 | 36,078 | 39,397 | 61,491 | … | … | … |
| P11 | 181,096 | 71,863 | 21,862 | … | … | … |
|
| ||||||
| Cohort 5 | ||||||
| P12 | 1,746,927 | 1,668,567 | 2,789,606 | … | … | … |
| P13 | 90,444 | 30,072 | 25,962 | … | … | … |
| P14 | 477,156 | 566,951 | 546,801 | … | … | … |
| P15 | 2,265 | 3,271 | … | … | … | … |
Abbreviations: Ellipses, testing not yet performed; nd, not done.
Testing at Year 2 timepoint was added to protocol after Cohort 1 had already completed this timepoint.
P2 had an extra titer performed at day 270 (21,059 mU/mL).
P6 had an extra titer performed at day 60 (707,306 mU/mL).
AAV2 capsid antigen-specific reactivity of peripheral lymphocytes (ASR) was monitored at baseline and at post-treatment days 14 and 90 and years 1, 2 and 3 (eTable 1). Only P1 and P2 of the patients with ASR data exhibited a significant increase in stimulation index (SI) at any timepoint (minimal level of significance for SI ranges from 2 to 3). For both patients, the change in SI was only marginally significant and only a small increase from baseline: P1 at year 1 had a SI of 2.02, a small increase from the baseline value of 1.62, and P2 at day 90 showed a SI of 2.10, also a small change from 1.89 at baseline. We conclude that the AAV2 capsid antigen-specific lymphocyte proliferation response to a single subretinal AAV2 treatment elicits neither a consistent nor pronounced AAV2 antigen-specific immune response.
The T cell immune response to AAV2 capsid was monitored by IFN-γ ELISPOT assays. PBMCs from subjects at baseline and at days 14 and 90 and years 1, 2 and 3 after treatment, depending on the patient, were stimulated with AAV2 peptide library pools and assayed for IFN-γ secretion. With one exception, there were no positive responses to AAV2 peptide pools at any of the timepoints tested in the subjects thus far (eTable 2). P9 exhibited a response to peptide pool 2C at day 90, but neither before nor after. This is inconsistent with T cell response to AAV2 vector, and a clear reason for this modest and transient increase at day 90 is not apparent. The study of AAV2-specfic memory T cells measured by the cultured ELISpot showed that some patients had pre-existing AAV2-specific T cells at baseline (Patients 2, 6, 9, 11-14; eTable 3). Most of the patients that were positive at baseline remained positive by the cultured ELISpot and most of the patients that were negative remained negative. Only P5 and P7, who were negative at baseline, became positive post-treatment by cultured ELISpot but not by ex-vivo ELISpot. Although pre-existing AAV2-specific memory T cells were found in some patients, AAV2 administration to the eye did not expand these resting cells as demonstrated by the negative data obtained by the ex vivo ELISpot. The data suggest that even in the presence of peripheral AAV2-specfic memory T cells, AAV2 administration to the eye is not sufficient to activate them.
Biodistribution of vector in the peripheral blood was monitored at baseline and at days 1, 3 and 14 post-treatment by quantitative PCR with spike-in assays to control for PCR inhibition in specific samples. For all patients at all timepoints, there were no vector genome copies detectable, thus confirming the lack of escape of subretinally administered AAV2 vector into the circulation.
FULL FIELD AND FOCAL PSYCHOPHYSICS, PUPILLOMETRY AND MOBILITY
Visual function was assessed before and after treatment using FST, TPLR, dark-adapted static visual field testing, mobility performance and ETDRS visual acuity. We did not assume that the vector used in each patient was bioactive but tested it. At the end of each surgery, unused residual vector was injected subretinally into rd12 (Rpe65-deficient) mice and an ERG bioassay performed to quantify vector activity (18,42,43). In all patients, the residual vector was proven active by this method (eFigure 1).
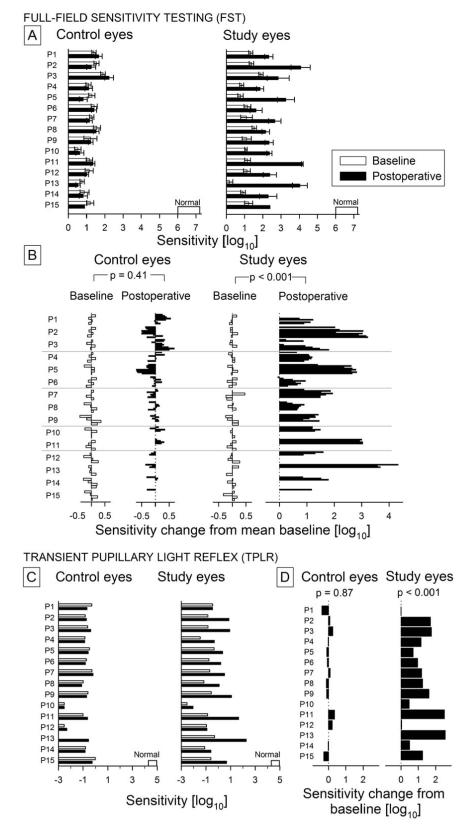
Full field sensitivity
Visual function was measured psychophysically using FST in dark-adapted eyes with blue and red flashes (Figure 2A, eFigure 2, Table 3). At baseline, the mean (SE) FST sensitivity with blue flashes in all RPE65-LCA eyes (study and control) was 1.17(0.087) log10, a value that was substantially reduced compared to 6.61 (0.10) log10 in normal eyes. At baseline, there were no significant differences (p=0.16) between control and study eyes of trial patients. Intervisit test-retest variability of the FST measure at baseline in trial patients was similar to that published previously (30,31). FST sensitivity to red flashes in all RPE65-LCA eyes at baseline was 0.72 log10 (0.11), which is greatly reduced compared to the normal value of 4.37 (0.08) log10 measured under dark-adapted conditions, or the normal value of 2.48 (0.09) log10 measured at the cone plateau (eFigure 2, Table 3). Chromatic differences defined the photoreceptor type mediating FST responses. In all patients, red FST flashes were detected by cones whereas blue FST flashes could be detected by rods (P1, P3, P5, P7, P8, P9, P10), or cones (P11, P12, P15), or rods and cones (P2, P4, P6, P13, P14) at baseline (eFigure 2).
Figure 2.
Visual function in all clinical trial participants analyzed with full-field stimulus testing (FST) and the transient pupillary light reflex (TPLR). A, FST sensitivity (mean±SD) to blue stimuli measured under dark-adapted conditions in each eye of each subject at four baseline visits (white bars) and all postoperative visits to date (black bars). Normal range for the FST is shown along the horizontal axis. B, Changes in FST sensitivity from mean baseline value in control and study eyes. White bars depict the intervisit variability at 4 baseline visits. Black bars represent all available postoperative timepoints to date ordered in groups for each patient. p values refer to two-sided paired t-test statistics between indicated groups. C, TPLR sensitivity to green flashes under dark-adapted conditions in each eye of each subject measured at 1, 3 or 6 months postoperatively (black bars) compared to baseline (white bars). Normal range for the TPLR is shown along the horizontal axis. D, Postoperative changes in TPLR sensitivity from baseline in control and study eyes. p value refers to two-sided paired t-test statistics performed between postoperative and baseline timepoints.
Table 3.
Measures of Ocular Function at Baseline and Post-operatively
| Control Eyes |
Study Eyes |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) |
Mean (SE) |
|||||||
| Measure | Baseline | Post-operative | Differencea | P Valueb | Baseline | Post-operative | Differencea | P Valueb |
| FST sensitivity (blue)c, log10 | 1.22 (0.087) | 1.17 (0.11) | −0.051 (0.057) | 0.41 | 1.12 (0.099) | 2.72 (0.21) | 1.59 (0.25) | <0.001 |
| FST sensitivity (red)d, log10 | 0.75 (0.10) | 0.74 (0.12) | −0.02 (0.055) | 0.83 | 0.70 (0.12) | 1.14 (0.12) | 0.45 (0.10) | <0.001 |
| TPLR sensitivitye, log10 | −0.90 (0.20) | −0.91 (0.18) | −0.01 (0.05) | 0.87 | −0.89 (0.14) | 0.28 (0.27) | 1.17 (0.20) | <0.001 |
| VAf, logMAR | 0.96 (0.13) | 0.91 (0.13) | −0.05 (0.02) | 0.016 | 1.09 (0.11) | 0.97 (0.11) | −0.12 (0.05) | 0.024 |
Abbreviations: FST, full-field stimulus test; TPLR, transient pupillary light reflex; VA, visual acuity
Post-operative minus baseline.
P values by two sided paired t-test.
Normal value, 6.61 (0.10) log10.
Normal values, 4.37 (0.08) log10 when rod-mediated at dark-adapted conditions and 2.48 (0.09) log10 when cone-mediated during cone-plateau period.
Normal value, 4.74 (0.06) log10.
Normal value, 0.00 logMAR corresponding to 20/20 Snellen acuity at 4 m distance.
In the postoperative period, FST sensitivities to blue flashes showed highly significant differences compared to baseline in study eyes but not in control eyes (Figure 2A, Table 3, p<0.001, repeated measures ANOVA test of interaction). The postoperative improvement in study eyes was 1.59 (0.25) log10. Chromatic differences supported mediation of blue FST flashes by rods postoperatively in all study eyes except for P6 (eFigure 2).
FST sensitivities to red flashes showed highly significant differences compared to baseline in study eyes but not in control eyes (p=0.008, repeated measures ANOVA test of interaction). The postoperative improvement in study eyes was 0.45 (0.10) log10 (Table 3). Chromatic differences supported mediation of red FST flashes by cones postoperatively in all study eyes (eFigure 2). The ages of the clinical trial participants did not have a significant effect on blue FST (P=.25, overall; P=.53, magnitude) or on red FST (P=.10, overall; P=.63, magnitude).
Pupillometry
The transmission of information from retina to the brainstem was quantified objectively with the TPLR (Figure 2C,D). Measurements were made under fully dark-adapted conditions and luminance-response functions were available in 27 of 30 eyes. For P10 at baseline, the control eye showed a sub-criterion contraction whereas the study eye showed no contraction to maximal stimulation; his sensitivities were assigned to the reciprocal of the maximum stimulus luminance for statistical purposes. For the control eye of P13, TPLR was not recorded due to time constraints. At baseline, RPE65-LCA eyes required on average 5.6 log10 unit higher luminance of a full-field green flash in order to produce a criterion pupillary contraction compared to normal eyes (RPE65-LCA= −0.88 (0.15) log10 versus normal= 4.74 (0.064) log10). The magnitude of this defect was similar to the 5.5 log10 difference observed with blue FST between RPE65-LCA and normal eyes. At baseline, there were no significant differences (p=0.81) between the control and study eyes of trial patients (Figure 2C).
In the postoperative period (1-6 months), TPLR sensitivities showed highly significant differences compared to baseline in study eyes but not in control eyes (Figure 2C, Table 3, p<0.001, repeated measures ANOVA test of interaction). The magnitude of the postoperative TPLR sensitivity improvement in study eyes was 1.17 (0.20) log10 (Figure 2D, Table 3) which corresponded to an intermediate value between the blue and red FST improvements observed. The ages of the clinical trial participants did not have a significant effect on the TPLR (P=.67, overall; P=.56, magnitude of the treatment effect).
Static visual fields
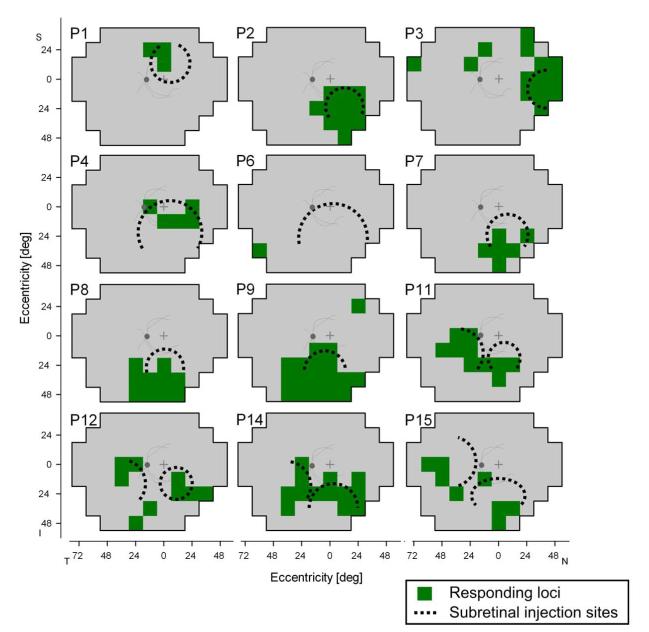
The significantly increased light sensitivity postoperatively in treated eyes using FST and TPLR prompted us to ask whether we could localize the increases in the visual field and how any localization of function was related to the sites of subretinal injection (Figure 3). Visual field maps of sensitivity change from baseline in the study eyes showed good correspondence between the loci where there were significantly increased responses to stimuli and the estimated region where the retinal detachment with subretinal injection of agent occurred. Patients in Cohorts 4 and 5, who had a second subretinal injection site in the nasal retina, showed loci with significant responding in the temporal visual field. In summary, 11 of the 12 patients with visual field maps showed correspondence between most detected loci and the area of injection; these include P1-P4, P7-P9, P11, P12, P14 and P15. It is of interest that some of the detected loci in the patients, however, were outside the estimated injection area. The basis for this effect remains uncertain. It is to be noted that most of these unexpectedly responsive loci were in the far periphery. The results of P6 indicated response at a single peripheral locus but no evidence of a response in the subretinal injection area. This temporal inferior peripheral field locus was consistently detected and it is likely to be the source of the FST and TPLR responses in this patient (Figure 2), who described this location of perception and its appearance postoperatively.
Figure 3.
Dark-adapted visual field maps in study eyes to localize regions of improved sensitivity after treatment. All maps are depicted as left eyes for comparability and with an overlaid schematic of retinal features (optic nerve and posterior pole vessels) for reference to fundus images (Figure 1). Loci in the visual field that consistently showed ≥8 dB of sensitivity change during the postoperative period are highlighted (green). Estimated boundaries of blebs resulting from subretinal injections are depicted (dotted circles, see Figure 1) on the visual field maps to ask whether there is any correspondence between locations of injection and the responding loci. I indicates inferior visual field; N nasal field; P, patient; S, superior field; T, temporal field.
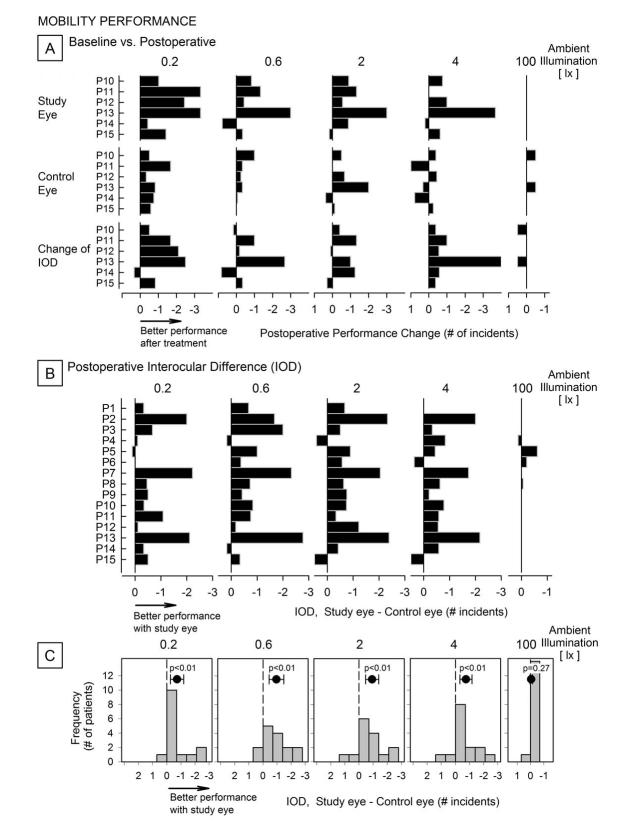
Mobility testing
We also asked whether these localized changes from baseline had any impact on the ability of the subjects to negotiate an obstacle course. In 5 patients, representing Cohorts 4 and 5, we performed the study both before and after treatment (Figure 4A). A comparison of mobility performance for study eyes relative to postoperative values (first row) indicates overall a better performance after treatment for ambient illuminations between 0.2 to 4 lux. For the 100 lux illumination, however, patients were able to navigate the course practically without errors both at baseline and postoperatively and regardless of which eye was used. For the control eyes (second row) there were less pronounced differences in performance for the lower illumination levels, which suggests a learning effect. We also determined if the difference in performance between eyes (interocular difference, IOD) changed after treatment (third row). The results in P11 and P13 indicate greater IOD after treatment, with better performance of the treated eye relative to the control eye, at the lower illumination levels. P12 performed better with the treated eye only at the lowest illumination level; P10, P14 and P15 did not show notable effects.
Figure 4.
Mobility performance of clinical trial participants as measured by the number of navigation incidents experienced while traveling an indoor course of fixed length, for five ambient illumination levels. A, Change from baseline performance for the study (first row) and control (second row) eyes of patients in Cohorts 4 and 5, as a function of ambient illumination. At lower ambient illuminations, mobility performance was better with the study eye after treatment; at the highest illumination, patients were able to navigate almost without errors with either eye before and after treatment. There where changes in interocular differences with treatment (third row) with most cases showing a difference in performance of the study eyes relative to the control eyes. Values are averages of 4 repetitions performed for each illumination level except for 100 lx, at which two runs were performed. B, Postoperative performance difference between eyes (study minus control) for each patient. Most patients tended to show better relative mobility performance when using the study eye for lower illumination levels; there was no such effect at 100 lx. C, Difference in performance grouped by illumination. As a group, patients show less incidents when navigating with the study eye postoperatively. Symbols at the top of each panel indicate the group mean interocular difference (study minus control); surrounding brackets are 95% confidence levels. P-values (t-test) indicate significance of the departure of IOD from zero for each ambient illumination level.
All study participants were assessed by the difference in performance between eyes, averaged across all post-treatment visits (Figure 4B). Results indicate a consistently lower number of incidents while navigating with the treated eye, with varying degrees of performance gain across participants. The mobility performance IOD results for all participants are summarized (Figure 4C). Mean differences between study and control eyes were significantly different from zero for the four lower illumination levels indicating that patients as a group navigated more efficiently when using their treated eyes under such conditions.
VISUAL ACUITY, FIXATION AND FOVEAL OPTICAL COHERENCE TOMOGRAPHY
At baseline, VA in control eyes was 0.96 (SE, 0.13) logMAR (corresponding to a mean Snellen acuity of 20/182; range 20/39 to worse than 20/2000) as compared to study eyes of 1.09 (0.11) logMAR (=20/246; range 20/43 to 20/1824) (Table 1, Table 3). Postoperatively, the mean VA increased to 0.91 (0.13) logMAR in control eyes and to 0.97 (0.11) logMAR in study eyes, and both changes were significant (Table 3). In contrast to other psychophysical and to pupillometric measurements, repeated measures ANOVA showed no indication (p=0.16) of a difference in the magnitude of the postoperative acuity change between study and control eyes of −0.12 (0.05) and −0.05 (0.02) logMAR, respectively. Furthermore, in the great majority (28 of 30) of the eyes, mean postoperative acuity change did not reach or surpass the 0.30 logMAR (3-line) halving of visual angle limit which is a commonly accepted criterion for clinical significance (44). It was noted that the second baseline VA was better than the first baseline measurement in 10 study eyes (range: −0.18 logMAR better to 0.08 logMAR worse) and in 9 control eyes (range: −0.10 logMAR better to 0.10 logMAR worse). Regression to the mean and learning curve effects could have potentially contributed to this tendency. Thus, an alternative analysis was performed using only the second baseline VA. This led to a decrease in the logMAR improvement postoperatively in both the study eyes (mean (SE) = −0.09 (0.05), p=0.099) and the control eyes (mean (SE) = −0.04 (0.02), p=0.077).
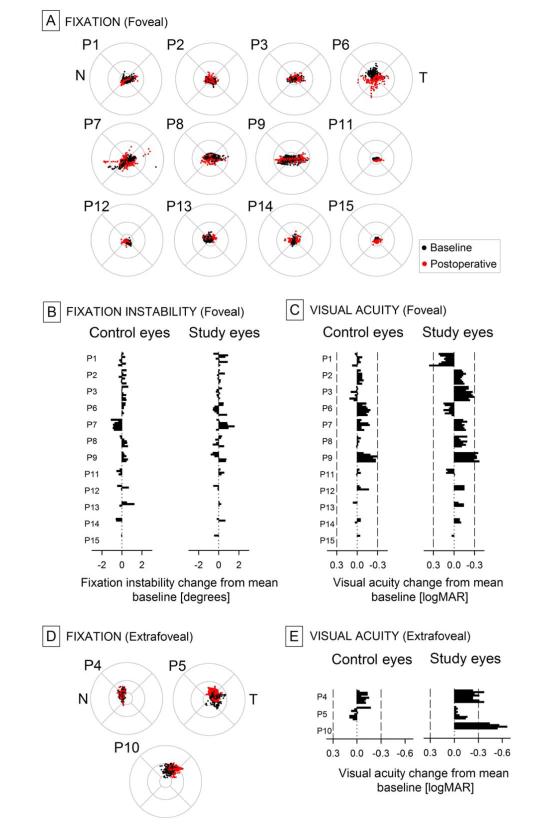
In order to understand better any possible VA changes resulting from gene therapy, fixation properties were analyzed in each eye at each visit. At baseline, in 12 of 15 study eyes mean fixation location corresponded to the anatomical foveal depression (Figure 5A) and not unexpectedly these eyes had the highest VA (Table 1). Instability of fixation (including high frequency nystagmus and lower frequency wandering eye movements) around the mean was 1.97 (0.21) degrees and correlated (r2=0.42) inversely with VA as previously published in this patient population (29). Control eyes showed less fixation instability with a mean of 1.54 (0.14) degrees (data not shown) consistent with the better acuities recorded; the difference was borderline significant (p=0.052). Postoperatively, fixation remained foveal in both study and control eyes in this subset of 12 patients (the study eye of P2 developed a secondary fixation locus which was detectable with dimmer stimuli, reference 24), and there were no significant changes to fixation instability (Figure 5B).
Figure 5.
Retinal location and instability of fixation in RPE65-LCA eyes at baseline and postoperatively, and its relation to changes in visual acuity. A, D, Fixation clouds of all study eyes during a 10 sec epoch recorded while gazing to a 1 deg diameter stationary target adjusted to be visible to each eye. Foveally fixating eyes shown in A and extrafoveally fixating eyes shown in D. Circular patterns show the standard grid centered on the anatomical fovea extending to radii of 1.65, 5 and 10 degrees. All panels are shown in equivalent left retina representation. N and T refer to nasal and temporal retina, respectively. B, Fixation instability values are shown as change from mean baseline values at all postoperative visits in control and study eyes with foveal fixation. VAs are shown as change from mean baseline at all postoperative visits in control and study eyes with foveal (C) or extrafoveal (E) fixation. Limits for 0.30 logMAR (15 letter or 3 line) gain or loss shown with vertical dashed lines.
Three study eyes (P4, P5, and P10) with the worst baseline acuities (Table 1) fixated parafoveally at mean eccentricities of 2.1, 5.1 and 3.4 degrees, respectively, from the anatomical foveal depression (Figure 5D). Their fixation instability was 2.3, 3.7, and 2.2 degrees, respectively. Control eyes of these three patients also fixated eccentrically; mean locus of fixation was 2.0 and 2.3 degrees eccentric for P4 and P5, respectively, whereas it could not be quantified in P10. Postoperatively, fixation remained extrafoveal for all three patients (Figure 5D).
Next, VA changes were considered in the context of the type of fixation. There was a larger VA improvement in patients with extrafoveal fixation (−0.30 (0.14) logMAR, Figure 5E) compared to those with foveal fixation (−0.09 (0.04) logMAR, Figure 5C). A repeated measures ANOVA demonstrated a statistically significant difference (p=0.036, test of interaction) between the magnitude of postoperative VA improvement in the patients with the two types of fixation. In subgroup analyses, acuity change in foveal-fixating study eyes, or extrafoveal-fixating study and control eyes did not achieve statistical significance (p=0.07, 0.17, 0.56, respectively); counterintuitively, foveal-fixating control eyes showed a significant (p=0.02) improvement of VA. Of note were the large VA improvements (P4=−0.29 and P10=−0.55 logMAR) in two of the extrafoveal-fixating eyes where the central retina including the fixation was involved in the subretinal injection, as compared to the lack of any such large improvement in the extrafoveal-fixating eye without a central detachment (P5=−0.06 logMAR), or in two foveal-fixating eyes with a foveal detachment (P1=0.20, P13=−0.11 logMAR). Until there are physiologically-based hypotheses for improvements in untreated control eyes or in eyes without foveal detachment, parsimony would suggest that small VA improvements in foveal-fixating eyes may have been influenced by the high expectations and motivation in patients taking part in an open-label study as well as a possible learning effect (45). Clinically significant unilateral VA improvements in extrafoveal-fixating eyes, on the other hand, appear consistent with independent data (18,24) showing improvements in extrafoveal cone sensitivity following gene therapy.
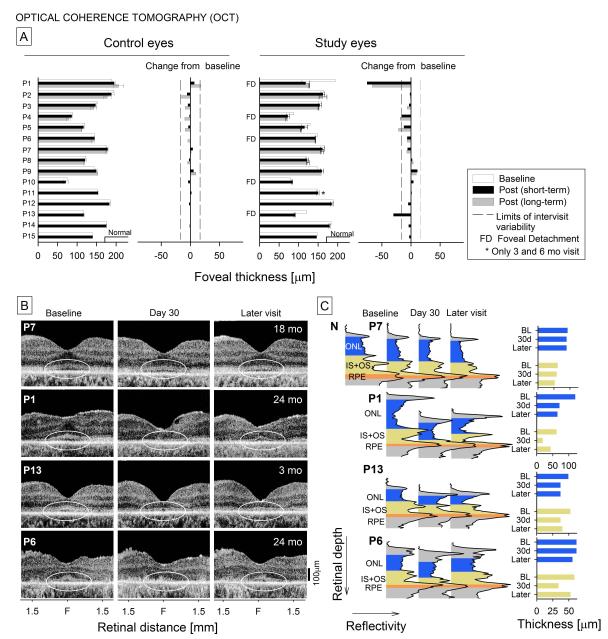
Retinal laminar architecture across the fovea was quantified in all patients. Foveal thickness in both eyes of the 15 patients pre- and post-operatively are summarized (Figure 6A). For control eyes, changes from baseline are within published intervisit variability for a retinal degeneration population (17,41). For study eyes, there are two notable examples of foveal thinning in the short-term: P1 and P13. Long-term follow-up in P1 showed that foveal thinning was still present. In both P1 and P13, the fovea was detached in the subretinal injection (Figure 1). P4, another patient with foveal detachment showed less pronounced but still significant thinning long-term but not short-term. P4’s control eye also showed long-term thinning but not to the degree as in the study eye. As a counter example, P5 who did not have foveal detachment showed similar results as P4. The other patients with a foveal detachment, P6 and P10, showed no such effects. All other study eyes without foveal detachment did not have thinning within the time period studied.
Figure 6.
Foveal structure and quantitation of thickness using OCT scans in control and study eyes. A, Foveal thickness measurements in control and study eyes at baseline, and at short-term and long-term postoperative timepoints. Changes from baseline are displayed adjacent to the foveal thickness measurements. FD, the eyes that had foveal detachments as part of their subretinal injection procedure. B, OCT scans along the horizontal meridian in four representative patients – one without foveal detachment (P7) and three with foveas detached at the time of the procedure (P1, P13, P6). Ellipses denote the central retinal region of interest that shows changes in the IS/OS lamination in 3 of the 4 study eyes at early timepoints but with some resolution at later times. C, Longitudinal reflectivity profiles (LRPs) through the fovea in the patients compared with a normal LRP (upper row, left). The LRPs are color coded and labeled for ONL, IS+OS and RPE to illustrate the postoperative changes. ONL and IS+OS measurements are shown to the right of the LRPs.
Representative horizontal OCT cross-sections and longitudinal reflectivity profiles through the fovea (highlighted and labeled for outer retinal laminae and with histograms of layer thickness) are shown for four study eyes at baseline and at early and later postoperative times (Figure 6B,C). P7, a patient without foveal detachment, shows no remarkable changes at 30 days and 18 months postoperatively. P1, P13 and P6 had foveal detachments and all showed disturbance of the IS/OS laminar architecture at 30 days postoperatively but recovery at later visits. Unlike P6, however, P1 and P13 also showed loss of ONL at 30 days and later timepoints. How do these structural findings relate to visual acuity? The only patient with clinically significant loss of visual acuity was P1 at last visit (Figure 5) and this patient had the most prominent foveal abnormalities by OCT.
COMMENT
This five-cohort 15-patient RPE65-LCA retinal gene therapy clinical trial can be summarized as follows: there were no detectable systemic safety concerns; certain ocular adverse events occurred and these were attributable to the surgical procedure (46); and improved visual function (by FST) was present in all patients to different degrees. Like the other concurrent early phase RPE65-LCA clinical trials (15,16,21,22), our trial of relatively small numbers of patients has had limitations. Study design did not involve randomization of eyes and there was an inherent imbalance between eyes in variables related to outcome since study eyes by definition had worse vision. The statistical approach was thus confounded by such issues. Examiners were not masked to the study eye versus control eye (study eyes showed residual conjunctival injection for weeks postoperatively). Later phase trials in this disease and in other rare genetic retinal degenerations may be able to confront these issues. Despite limitations of the ongoing RPE65-LCA clinical trials, the fact remains that the longstanding concept that genetic retinal degenerations are incurable and vision cannot be improved has been revised. The advantages of beginning the era of treating rare inherited retinal diseases with RPE65-LCA are discussed in the eComment.
Some key questions to consider in planning future phases of gene therapy clinical trials in RPE65-LCA are as follows: Where in the retina should we treat and where should we not treat? Is there sufficient evidence for “age-dependent effects” (21) of this gene therapy to recruit younger and younger RPE65-LCA patients in future cohorts? Is cone-based vision improved by the currently used vectors?
Where in the retina should treatment be directed and where to avoid?
All 15 patients in this trial and a further 15 patients reported by two other trials (15,21) received subretinal injections to one eye. Where have the injections been delivered? Sixteen of 30 (53%) procedures detached the macula and fovea. All but two of these 16 patients were reported (15) to have some measure of efficacy from the treatment. The motivation to include the fovea in these macular injections would be two-fold: 1) to determine if there was any safety issue such as a deleterious effect to the foveal cones, which are known to be abnormally reduced at all ages in RPE65-LCA (29); and 2) to ask whether this treatment affects the visual cycle at the fovea and leads to efficacy such as improved visual acuity, which is reduced in nearly all of the patients (8,29 33).
Beginning with safety, two of our 5 patients with foveal inclusion in the macular detachment (P1,P13) showed foveal thinning at early postoperative timepoints; in P1, long-term data indicated that this process continued. A third patient (P4) had less dramatic long-term thinning of the fovea, and this degree of thinning also occurred in patients without foveal injections (P5) or in untreated eyes (P2). We postulate that the less dramatic long-term foveal thinning likely represents the natural history of foveal change in RPE65-LCA, albeit asymmetrical, whereas short-term effects are a complication of subfoveal injection. Also, in two of the patients with foveal detachments (P1,P6) and long-term follow-up, there was evidence that photoreceptor IS/OS structure was disrupted in the short-term postoperatively but did recover later. Experimental retinal detachment also shows that loss of OS is one of the earliest features to occur post-detachment but there is reversal with reattachment (47).
Of possible relevance, predictors of poor visual outcome in macula-off rhegmatogenous retinal detachment after reattachment surgery are persistent subretinal fluid and pre-operative increased thickness (48). There was no persistent subretinal fluid in this trial. P1 had the thickest fovea at baseline, compared to the four other patients with foveal detachments; P13 ranked third in thickness. We previously postulated that foveal thinning in P1 was due to proximity of the subretinal injection site (17) and there was similarity of this injection site to that in a patient who developed a macular hole in another RPE65-LCA trial (16). The site of injection in P13, however, was near the superior vessel arcade. Another explanation is that some patients with RPE65-LCA are vulnerable to surgical trauma of the fovea. Of the 5 patients with retinal detachments that included the fovea, the two patients with most foveal photoreceptors (foveal thickness) to lose were the ones that lost them – P1 and P13. The other 3 patients had more severe central disease with foveal atrophic lesions (P4, P10) or a thinned ONL layer at the fovea (P6). Other RPE65-LCA clinical trial reports have displayed but not measured OCTs in patients with foveal inclusion in the detachment (15,16,21).
We conclude that there is a risk to foveal cone cells in retinal detachments that include the fovea. Is there proven benefit, specifically increased visual acuity or slower natural history of foveal cone loss independent of functional gain? There is no consensus among the ongoing trials on this point. In the present study, group statistics for visual acuity indicated statistical improvement compared to baseline. The improvements in most eyes, however, were substantially less than the 0.3 logMAR (15 letter) limit generally accepted as clinically significant. Further, both study and control eyes showed improvement; and there was no statistically significant difference in the magnitude of improvement between treated study eyes and untreated control eyes (21,22). Two patients (P4, P10) showed an increase of >0.3 logMAR postoperatively. These two patients had the two lowest acuities at baseline in our study, had foveal inclusion in the detachments, and improved by maintaining extrafoveal fixation loci in the treated areas. Another trial reported no improvement in acuity (15) whereas group statistics in a third trial showed significant changes in study eyes (see Suppl. Table 5 in reference 21); individual results suggest improvements in many control eyes but group statistics were not provided (21,22). Using the 0.3 logMAR criterion as a clinically significant change in visual acuity, there was improvement in 4 of 8 patients with macular injections that included the fovea in this third trial (16,21,22); and 2 of 12 untreated control eyes also showed large improvements in acuity (16,21,22). Fixation was not reported. Complicating the risk-benefit issue for this trial is the report of a decrease in acuity in a 10-year-old subject’s treated eye (21).
Taking a conservative, patient safety-first approach, the lack of consensus in visual acuity results within and between the contemporaneous trials and our evidence of foveal thickness loss lead to the recommendation that the fovea should not be included in macular subretinal injections except when there is foveal atrophy (by OCT) or very reduced visual acuity and fixation is parafoveal (readily detectable on OCT). This approach also opens the door to treatment of patients at later disease stages when there is foveal atrophy but preserved extrafoveal ONL, best exemplified by P4 and P10 in the current study.
Our two-site injection protocol in Cohorts 4 and 5 was safe in adults and children to date. This is a positive step in the direction of a subretinal injection protocol with the goal of increasing even further the visual area affected by vector. Most of our single injections in this study were directed at the superior retina in order to subserve inferior visual field function. When two injections were used, a nasal retinal injection was added to enhance temporal peripheral visual function that can be detected in untreated RPE65-LCA patients even at relatively late stages (33). We propose that a next step in previously-untreated patients would be to administer the agent to three sites of injection: 1) superior retina involving the macula but not the fovea unless there is foveal atrophy and extrafoveal fixation; 2) nasal-superior retina; and 3) temporal retina. Ideally, a preoperative map of remaining ONL would help guide the injections (8,49) or at least a pre-operative visual field (using sufficiently bright stimuli) would help to determine where residual vision is detectable (33).
To date, we have elected to use the eye with worse vision as the study eye. In some cases, this non-preferred eye has been strabismic and possibly amblyopic. This strategy was motivated by safety, but has also been convenient for patients who in the postoperative period can continue to be visually active despite a protective occluder worn on the operated eye. For the initial 5 cohorts in this safety trial, that decision was appropriate. Some patients, however, have continued to use their preferred (control) eye after treatment and are only aware of visual gain in the treated eye when asked to occlude the control eye for our studies. The three-injection protocol proposed above would ideally be used in the preferred eye. Factoring in the remarkable visual improvements in our Cohort 1 who received 150 ul at a single site and the lack of toxicity to date of two-site injections totaling 450 ul, we recommend three injections of 150 ul each. A single eye treated with such a protocol will test the safety and efficacy of this approach and leave the contralateral eye for further advances in the field (50).
Is there evidence of an age dependence on the effects of gene therapy?
The RPE65-LCA phenotype includes not only a severe visual dysfunction but also a progressive retinal degeneration (8) and the relationship between severity of retinal degeneration and age can be complex. On an individual patient basis, there is certainly age dependence of severity of disease; visual field extent decreases with age when followed longitudinally over more than a decade (33). But this longitudinal progression in individual patients does not simply translate to cross-sectional studies across different patients at different ages, and this is especially true in the first three decades of life (33). Our studies of visual function and photoreceptor topography have indicated that severity of dysfunction and degeneration can be as profound in some young patients in the first decade of life as in some patients in the third decade of life (33,49). Given comparable numbers of photoreceptors remaining in the retina (49) and placement of the vector injection(s) in the region(s) of these photoreceptors, there should be an equal chance of efficacy of therapy and this would be independent of age (assuming RPE health is similar). Did we find an age dependent effect of gene therapy in our study, as reported in another trial (21)? For FST, TPLR and VA, age did not have a statistically significant effect either overall or on the magnitude of the treatment effect in our trial. As previously discussed, the most dramatic visual acuity increases were in two of our older patients (ages 24 and 30 at time of procedure) and this was consistent with no significant relationship between age and the visual acuity outcome reported in another trial (21). So, what is the basis of the previously reported conclusion of age dependence of gene therapy outcomes (21)? It seems to be a matter of emphasis. For example, the improved light sensitivity of an 8-year-old patient (CH08; Fig 2C, reference 21) is emphasized but there is equal improvement in light sensitivity in a 35-year old patient in the same trial (CH13; Fig 2C, reference 21). Mobility performance of younger patients is also emphasized, but these patients are studied uniocularly and with lower room illuminations whereas most older patients are only studied binocularly at 250 lux light levels (see reference 21, Supplementary Table 6), a condition we found does not reveal any differences in performance before and after treatment or between eyes. Considerable heterogeneity of disease severity in RPE65-LCA is a fact and when determining candidacy for this therapy there should be evaluation on an individual basis, independent of age. The extent of retinal disease in human RPE65-LCA at different ages has not been as predictable as, for example, a murine model (51). The temptation should be resisted to assume there is a common natural history among humans without measuring it. With greater understanding of the human disease, a better evidence-based formula may emerge for candidacy, in which age is one of several parameters.
Are cones affected by the vectors?
There is consensus from all current trials that some measures of visual function improve in response to gene therapy in RPE65-LCA patients (15-24). Evidence of rods and extrafoveal cones responding to the vector-gene has been presented in our previous work (18,20) and this is confirmed and extended in the present work to larger numbers of patients. Importantly, foveal cones have not been proven conclusively to show either increased vision from the current vector-gene treatments or protection from further cell loss. It is thus possible that there is a difference in targeting efficiency of foveal versus extrafoveal RPE in RPE65-LCA with the current vectors, or there is localized toxicity (directly or indirectly) to foveal cones. Foveal and extrafoveal cones, for example, do not have the same relationship with RPE apical processes (52). Alternatively or additionally, contributions of chromophore required from retinal and RPE visual cycle pathways may also differ between foveal and extrafoveal cones (2,3,53). There is also evidence of RPE65 localization in cones (54,55) suggesting a pathway that may need to be subserved by vectors capable of expressing RPE65 not only in the RPE but also in cones. In this context, using a promoter in the vector that does not limit expression to just the RPE may turn out to be beneficial.
Supplementary Material
Acknowledgements
We thank the members of the Data and Safety Monitoring Committee: Maryann Redford, DDS, MPH (Program Director, Collaborative Clinical Research, National Eye Institute, National Institutes of Health); Stephen Gange, PhD (Chair); Karl Csaky, MD, PhD; Herbert Goldstein, PhD, PA; Donald Hood, PhD; Ruth Macklin, PhD; Jerry Mendell, MD; Daniel Salomon, MD; and Donald Zack, MD, PhD. The DSMC approved this manuscript. We are grateful for the expertise and all the kindness shown to our patients by members of the UF CTSI (GCRC) during the early postoperative period.
Funding: The clinical trial was supported by the National Eye Institute (National Institutes of Health, Department of Health and Human Services) grant U10 EY017280. SGJ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author Disclosure Statement B.J.B., W.W.H. and the University of Florida have a financial interest in the use of AAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. University of Pennsylvania, University of Florida and Cornell University hold a patent on the described gene therapy technology (United States Patent 20070077228, “Method for Treating or Retarding the Development of Blindness”).
Trial Registration: clinicaltrials.gov Identifier: NCT00481546.
REFERENCES
- 1).Jacobson SG, Cideciyan AV. Treatment possibilities for retinitis pigmentosa. N Engl J Med. 2010;363(17):1669–1671. doi: 10.1056/NEJMcibr1007685. [DOI] [PubMed] [Google Scholar]
- 2).Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29(5):398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–99. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 5).Aguirre GK, Komáromy AM, Cideciyan AV, et al. Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med. 2007;4(6):e230. doi: 10.1371/journal.pmed.0040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Lai CM, Yu MJ, Brankov M, et al. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65-/- knockout mouse eye results in limited rescue. Genet Vaccines Ther. 2004;2(1):3. doi: 10.1186/1479-0556-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Dejneka NS, Surace EM, Aleman TS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9(2):182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 8).Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc Natl Acad Sci USA. 2005;102(17):6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13(3):565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10).Bemelmans AP, Kostic C, Crippa SV, et al. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3(10):e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65-/- mice. Invest Ophthalmol Vis Sci. 2006;47(3):1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 12).Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12(6):1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Jacobson SG, Acland GM, Aguirre GD, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13(6):1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 14).Jacobson SG, Boye SL, Aleman TS, et al. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther. 2006;17(8):845–858. doi: 10.1089/hum.2006.17.845. [DOI] [PubMed] [Google Scholar]
- 15).Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 16).Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Banin E, Bandah-Rozenfeld D, Obolensky A, et al. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Hum Gene Ther. 2010;21(12):1749–1757. doi: 10.1089/hum.2010.047. [DOI] [PubMed] [Google Scholar]
- 20).Cideciyan AV, Hauswirth WW, Aleman TS, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20(9):999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18(3):643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Bainbridge JW, Smith AJ, Rubin GS, et al. Clinical trial of gene therapy for early onset severe retinal dystrophy caused by defects in RPE65. Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 4493. [Google Scholar]
- 24).Cideciyan AV, Hauswirth WW, Aleman TS, et al. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med. 2009;361(7):725–727. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I. Anterior uveitis. Am J Ophthalmol. 1959;47(5,Part 2):155–170. doi: 10.1016/s0002-9394(14)78239-x. [DOI] [PubMed] [Google Scholar]
- 26).Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 27).Ladas JG, Wheeler NC, Morhun PJ, Rimmer SO, Holland GN. Laser flare-cell photometry: methodology and clinical applications. Surv Ophthalmol. 2005;50(1):27–47. doi: 10.1016/j.survophthal.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 28).Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 29).Jacobson SG, Aleman TS, Cideciyan AV, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci USA. 2007;104(38):15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Roman AJ, Schwartz SB, Aleman TS, et al. Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp Eye Res. 2005;80(2):259–272. doi: 10.1016/j.exer.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 31).Roman AJ, Cideciyan AV, Aleman TS, Jacobson SG. Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiol Meas. 2007;28(8):N51–N56. doi: 10.1088/0967-3334/28/8/N02. [DOI] [PubMed] [Google Scholar]
- 32).Jacobson SG, Voigt WJ, Parel JM, et al. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986;93(12):1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- 33).Jacobson SG, Aleman TS, Cideciyan AV, et al. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2009;50(5):2368–2375. doi: 10.1167/iovs.08-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Aleman TS, Jacobson SG, et al. Impairment of the transient pupillary light reflex in Rpe65(-/-) mice and humans with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45(4):1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- 35).Haymes S, Guest D, Heyes A, Johnston A. Comparison of functional mobility performance with clinical vision measures in simulated retinitis pigmentosa. Optom Vis Sci. 1994;71(7):442–453. doi: 10.1097/00006324-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 36).Geruschat DR, Turano KA, Stahl JW. Traditional measures of mobility performance and retinitis pigmentosa. Optom Vis Sci. 1998;75(7):525–537. doi: 10.1097/00006324-199807000-00022. [DOI] [PubMed] [Google Scholar]
- 37).Turano KA, Broman AT, Bandeen-Roche K, et al. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004;81(5):298–307. doi: 10.1097/01.opx.0000134903.13651.8e. [DOI] [PubMed] [Google Scholar]
- 38).Hartong DT, Jorritsma FF, Neve JJ, Melis-Dankers BJ, Kooijman AC. Improved mobility and independence of night-blind people using night-vision goggles. Invest Ophthalmol Vis Sci. 2004;45(6):1725–1731. doi: 10.1167/iovs.03-1061. [DOI] [PubMed] [Google Scholar]
- 39).Leat SJ, Lovie-Kitchin JE. Measuring mobility performance: experience gained in designing a mobility course. Clin Exp Optom. 2006;89(4):215–228. doi: 10.1111/j.1444-0938.2006.00050.x. [DOI] [PubMed] [Google Scholar]
- 40).Rubin GS, Bainbridge JW, Roche, et al. Visually-guided mobility in patients treated with gene therapy for leber’s congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 1392. [Google Scholar]
- 41).Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2005;46(9):3349–3354. doi: 10.1167/iovs.04-1383. [DOI] [PubMed] [Google Scholar]
- 42).Pang JJ, Chang B, Hawes NL, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- 43).Roman AJ, Boye SL, Aleman TS, Pang JJ, et al. Electroretinographic analyses of Rpe65-mutant rd12 mice: developing an in vivo bioassay for human gene therapy trials of Leber congenital amaurosis. Mol Vis. 2007;13:1701–1710. [PubMed] [Google Scholar]
- 44).Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration : phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103(10):3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Miller JW. Preliminary results of gene therapy for retinal degeneration. N Engl J Med. 2008;358(21):2282–2284. doi: 10.1056/NEJMe0803081. [DOI] [PubMed] [Google Scholar]
- 46).Thompson JT. Advantages and limitations of small gauge vitrectomy. Surv Ophthalmol. 2011;56(2):162–172. doi: 10.1016/j.survophthal.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 47).Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retinal: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24(3):395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 48).Ricker LJ, Noordzij LJ, Goezinne F, et al. Persistent subfoveal fluid and increased preoperative foveal thickness impair visual outcome after macula-off retinal detachment repair [e-pub] Retina. 2011 doi: 10.1097/IAE.0b013e31820a6910. [DOI] [PubMed] [Google Scholar]
- 49).Jacobson SG, Cideciyan AV, Aleman TS, et al. Photoreceptor layer topography in children with Leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2008;49(10):4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Yin L, Greenberg K, Hunter JJ, et al. Intravitreal injection of AAV2 transduces macaque inner retina. Invest Ophthalmol Vis Sci. 2011;52(5):2775–2783. doi: 10.1167/iovs.10-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Caruso RC, Aleman TS, Cideciyan AV, et al. Retinal disease in Rpe65-deficient mice: comparison to human leber congenital amaurosis due to RPE65 mutations. Invest Ophthalmol Vis Sci. 2010;51(10):5304–5313. doi: 10.1167/iovs.10-5559. [DOI] [PubMed] [Google Scholar]
- 52).Anderson DH, Fisher SK. The relationship of primate foveal cones to the pigment epithelium. J Ultrastruct Res. 1979;67(1):23–32. doi: 10.1016/s0022-5320(79)80014-3. [DOI] [PubMed] [Google Scholar]
- 53).Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res. 2011;30(2):115–28. doi: 10.1016/j.preteyeres.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Znoiko SL, Crouch RK. Moiseyev G, Ma JX. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci. 2002;43(5):1604–1609. [PubMed] [Google Scholar]
- 55).Tang PH, Wheless L, Crouch RK. Regeneration of photopigment is enhanced in mouse cone photoreceptors expressing RPE65 protein. Journal of Neurosci. 2011;31(28):10403–10411. doi: 10.1523/JNEUROSCI.0182-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.