Abstract
OBJECTIVE
To determine if a single application of a vapor rub (VR) or petrolatum is superior to no treatment for nocturnal cough, congestion, and sleep difficulty caused by upper respiratory tract infection.
METHODS
Surveys were administered to parents on 2 consecutive days—on the day of presentation when no medication had been given the previous evening, and the next day when VR ointment, petrolatum ointment, or no treatment had been applied to their child’s chest and neck before bedtime according to a partially double-blinded randomization scheme.
RESULTS
There were 138 children aged 2 to 11 years who completed the trial. Within each study group, symptoms were improved on the second night. Between treatment groups, significant differences in improvement were detected for outcomes related to cough, congestion, and sleep difficulty; VR consistently scored the best, and no treatment scored the worst. Pairwise comparisons demonstrated the superiority of VR over no treatment for all outcomes except rhinorrhea and over petrolatum for cough severity, child and parent sleep difficulty, and combined symptom score. Petrolatum was not significantly better than no treatment for any outcome. Irritant adverse effects were more common among VR-treated participants.
CONCLUSIONS
In a comparison of VR, petrolatum, and no treatment, parents rated VR most favorably for symptomatic relief of their child’s nocturnal cough, congestion, and sleep difficulty caused by upper respiratory tract infection. Despite mild irritant adverse effects, VR provided symptomatic relief for children and allowed them and their parents to have a more restful night than those in the other study groups.
Keywords: cough, congestion, rhinorrhea, camphor, menthol, eucalyptus, placebo, upper respiratory infection
Upper respiratory infections (URIs) are the most common acute illnesses in the world,1 and symptoms caused by these infections are disruptive for children. The characteristic features of URIs are often adversely affected sleep for both ill children and their parents with an effect on subsequent daytime activities. In addition to attempting to improve their comfort, giving medications to children before bed is often an attempt by parents to improve their own sleep and functioning during the subsequent day.
Recent studies2,3 and guidelines4–7 have questioned the efficacy of many oral over-the-counter (OTC) treatments for URI symptoms. Clinicians and parents have been left with limited therapeutic options to administer to children with these disruptive symptoms. Alternatives to oral medications are popular topical preparations that contain menthol, camphor, and eucalyptus oils and have been used in adults and children for more than a century.8–12
Commenting on camphor-containing products, in 1994 the American Academy of Pediatrics Committee on Drugs wrote, “Since alternative agents exist for all indications for camphor therapy, other therapeutic agents that do not contain camphor should be considered.”13 The “alternative agent” for cough and cold symptoms cited by the Committee in 1994, dextromethorphan, was subsequently not recommended by that committee 3 years later in a policy statement on dextromethorphan use.4 Dextromethorphan continues not to be recommended,14 and therefore the question of whether clinicians can recommend topical preparations containing camphor, menthol, and eucalyptus oil for URI symptoms in children requires reevaluation.
With no contemporary evidence that supports or refutes the efficacy in children with URIs, this study sought to determine if a single application of a vapor rub (VR) or petrolatum is superior to no treatment for nocturnal cough, congestion, and sleep difficulty caused by URIs. We hypothesized that VR or petrolatum would be superior to no treatment for relief of nocturnal symptoms and that VR would be superior to petrolatum.
METHODS
From October 2008 through February 2010, patients were recruited from a university-affiliated pediatric practice in Hershey, Pennsylvania. Eligible patients were aged 2 to 11 years with symptoms attributed to URIs characterized by cough, congestion, and rhinorrhea that lasted 7 days or longer. Patients were excluded for signs or symptoms of a more treatable disease (eg, asthma, pneumonia, laryngotracheobronchitis, sinusitis, allergic rhinitis). They were also ineligible with a history of asthma, chronic lung disease, or a seizure disorder. Children with seizure disorders were excluded because of the reported association of camphor with seizures, particularly after ingestions of toxic amounts.13 Finally, subjects were excluded if on the night before enrollment they used OTC or prescription medication that contained VR components, pseudoephedrine, phenylephrine, dextromethorphan, guaifenesin, diphenhydramine, brompheniramine, chlorpheniramine, or honey.
Subjective parental assessments of their child’s symptoms on the previous night were assessed after informed consent was obtained through a modified version of our previously used and validated questions15 by using a 7-point Likert scale (Fig 1). Trained study coordinators were responsible for survey administration, and survey responses ranged from most severe symptoms (7 points) to no symptoms (1 point). Minimum symptom-severity criteria for enrollment were established. Only parents who answered at least “moderately often” for cough frequency and “moderately severe” for stuffy nose (both equivalent to 4 points on the scale) on the basis of the previous night’s symptoms were eligible.
FIGURE 1.
Survey questions to assess nocturnal URI symptoms.
After stratification for age (2–5 and 6–11 years), each child was randomly assigned in a partially double-blinded fashion to 1 of 3 treatment groups: VR ointment (Vicks VapoRub, which contains camphor [4.8%], menthol [2.6%], and eucalyptus oil [1.2%] [Procter and Gamble, Cincinnati, OH]); petrolatum ointment (Equate 100% pure white petroleum jelly [Wal-Mart Inc, Bentonville, AR]); and no treatment. For the 2 groups that received an ointment, the dose volume distributed was stratified according to age: 5 mL (2–5 years) and 10 mL (6–11 years). The randomization sequence was constructed by a statistician not affiliated with the study and was used to assign treatment groups. All study parents were instructed on routine care for children with URI, including hydration measures, saline nose spray use, and use of acetaminophen or ibuprofen as needed for comfort. Because nasal saline could affect some outcome measures, parents were asked to not use it within 1 hour of bedtime or throughout the night. They were instructed not to administer excluded OTC medications listed above to their child at any time after enrollment and to avoid giving them caffeinated beverages within 4 hours of bedtime.
To maintain investigator blinding, all study groups received an opaque paper bag that contained a glass specimen cup filled with either ointment or no treatment. Given the aromatic and characteristic smell of VR, a series of steps was required to maintain parental blinding. First, a second glass specimen cup labeled for parents was placed in each bag. Although the parent cup for the no-treatment group was empty, similar to their child’s cup, the cups for parents in the VR and petrolatum groups were both filled with VR. Second, 30 minutes before their child retired for the evening, parents opened their opaque bag and removed the cup labeled for parent use. Parents in the VR and petrolatum groups then applied the VR between their upper lip and nose before opening their child’s treatment. After this, for the VR- and petrolatum-treated groups, parents were instructed to apply the entire contents of the specimen cup labeled for their child to the upper chest and neck area of their child and massage the ointment in for 1 minute. Children were asked not to disclose to their parent whether the treatment had an odor, and the participating parent was instructed to ask other family members also not to comment on this. Lastly, parents agreed not to remove the VR from under their noses until after completing their survey the following morning.
This second survey, in which parents were asked the same questions related to the child’s illness as those answered at enrollment, was self-completed by parents within 30 minutes of waking the morning after the above-listed procedures. Questions were included to assess additional treatments, including analgesics and nasal saline. Specific questions about adverse effects such as hyperactivity, sleepiness, headache, rash, and skin redness were included as were some inquiring about symptoms associated with VR use (burning sensation of skin, nose, and eyes). Finally, parents were asked what treatment they thought their child had received. Blinded study coordinators contacted parents during that day to retrieve responses. A physician examination was not routinely performed on the second study day.
Sample-size calculations indicated that 138 subjects (46 per arm) would have 80% power to detect a 1-point Likert-scale difference between any 2 treatment groups on the basis of a Wilcoxon rank-sum test with Bonferroni-corrected α = .0167 to allow for 3 pairwise comparisons for outcomes related to cough, congestion, rhinorrhea, and sleep difficulty.
Between-study-group baseline and demographic features were compared by using a χ2 and Fisher’s exact test where appropriate for gender and race and Fisher’s exact tests for weight and age. Within-treatment-group comparisons for the 2 nights were conducted using paired t tests. Treatment group comparisons were conducted by analysis of covariance, which was deemed appropriate after assessment outcome variable distribution. For the primary analysis of treatment effect, the outcome of between-night change included baseline scores as a covariate. Fisher’s exact tests were used to compare adverse reaction rates between treatments.
The study was approved by Penn State College of Medicine’s Human Subjects Protection Office and was registered at www.clinicaltrials.gov before the first subject’s enrollment. Informed consent was obtained from participating parents, and verbal assent was obtained from children older than 7 years.
RESULTS
There were 144 children with URIs enrolled, and 138 (95.8%) completed the single-night study. The mean age of the children completing the study was 5.8 ± 2.8 years (Table 1). Of these participants, 51.4% were female, and 85.5% were described by their parents as white and non-Hispanic. The cohort had been coughing on average 4.3 ± 1.5 days and congested 4.2 ± 1.5 days before enrollment. Of the children who completed the study, 44 were randomly assigned to the VR study group, and 47 participants were randomly assigned to each of the other study groups. There were no demographic or baseline symptom-severity differences between groups.
TABLE 1.
Baseline Characteristics (N = 138)
| Characteristic | VR (N = 44) |
Petrolatum (N = 47) |
No Treatment (N = 47) |
|---|---|---|---|
| Age, mean ± SD, y | 5.8±2.9 | 5.8±2.7 | 5.8±2.9 |
| Gender, n (%) | |||
| Female | 21 (47.7) | 23 (48.9) | 27 (57.4) |
| Male | 23 (52.3) | 24 (51.1) | 20 (42.6) |
| Race/ethnicity, n (%) | |||
| White | 41 (93.2) | 40 (85.1) | 35 (74.5) |
| Black | 0 (0.0) | 1 (2.1) | 4 (8.5) |
| Hispanic | 3 (6.8) | 2 (4.3) | 1 (2.1) |
| Asian | 0 (0.0) | 2 (4.3) | 3 (6.4) |
| >1 race/ethnicity | 0 (0.0) | 2 (4.3) | 2 (4.3) |
| Other | 0 (0.0) | 0 (0.0) | 2 (4.3) |
| Duration of cough, mean ± SD, d | 4.3±1.6 | 4.1±1.4 | 4.4±1.5 |
| Duration of congestion, mean ± SD, d | 4.1±1.5 | 4.2±1.6 | 4.2±1.6 |
| Cough frequency score, mean ± SD | 5.6±1.3 | 5.1±1.2 | 5.3±1.2 |
| Cough severity score, mean ± SD | 5.1±1.3 | 5.0±1.4 | 5.0±1.2 |
| Congestion severity score, mean ± SD | 5.0±1.3 | 4.9±1.0 | 4.9±1.1 |
| Rhinorrhea severity score, mean ± SD | 3.6±1.8 | 3.4±1.8 | 3.4±1.6 |
| Effect on child ability to sleep score, mean ± SD | 5.1±1.6 | 5.3±1.5 | 5.2±1.5 |
| Effect on parent ability to sleep score, mean ± SD | 4.9±1.9 | 4.9±1.7 | 5.0±2.1 |
| Combined symptom score, mean ± SD | 29.1±6.4 | 28.5±5.9 | 28.8±5.7 |
No significant difference between treatment groups exists for any baseline characteristic.
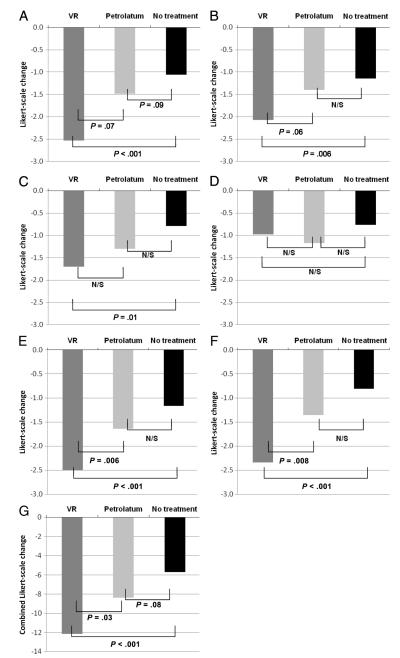
The baseline symptom scores that described the night before enrollment when no participants received treatment were compared with scores from the subsequent night when VR, petrolatum, or no treatment was given before bed. Within each treatment group, all outcomes were significantly improved on the subsequent night (P < .05 for all). When separated by treatment group, with the exception of rhinorrhea severity, significant differences were detected in the amount of improvement reported for all of the study outcomes in the planned 3-way comparison (Fig 2). Again, with the exception of rhinorrhea, all of the outcomes found VR to yield the greatest improvement, followed by petrolatum, whereas no treatment consistently showed the least amount of improvement (P < .05 for all).
FIGURE 2.
Between-night treatment effects of VR, petrolatum, and no treatment on (A) cough frequency (P = .003, 3-way treatment comparison), (B) cough severity (P = .02), (C) severity of congestion (P = .04), (D) severity of rhinorrhea (P = not significant), (E) child’s ability to sleep (P < .001), (F) parent’s ability to sleep (P < .001), and (G) combined symptom score (P < .001). N/S indicates nonsignificant.
Because differences existed for all outcomes except rhinorrhea, pairwise comparisons were then performed. For both cough severity and cough frequency, VR was significantly better than no treatment (P < .01), but only marginally better than petrolatum for frequency (P = .07) and severity (P = .06). Petrolatum was marginally better than no treatment for cough frequency (P = .09), but not better than no treatment for cough severity. For nasal congestion, the only significant pairwise comparison was VR versus no treatment (P = .01); other comparisons between individual treatments were not significant.
The most profound effects in pairwise comparisons were for the outcomes related to sleep. As rated by parents, children treated with VR were significantly more able to sleep than were children randomized to receive petrolatum (P = .006) or no treatment (P < .001). No significant difference was detected between petrolatum and no treatment. Similarly, parents of children treated with VR rated their own ability to sleep as significantly better than did parents of children randomized to petrolatum (P = .008) or no treatment (P < .001).
A combined symptom score was calculated using all study outcomes: cough frequency; cough severity; congestion; rhinorrhea; child sleep; and parent sleep. In addition to the significant 3-way treatment comparison (P < .001), VR was significantly better than petrolatum (P = .03) and no treatment (P < .001) whereas petrolatum was marginally better than no treatment (P = .08).
To help understand parents’ ability to judge their child’s nocturnal symptoms, parents quantified the number of times they checked on their child between the time the child went to sleep and the time they awoke. On the night before enrollment, parents reported checking their child a median of 4 times (interquartile range: 2–5); they checked their child a median of 3 times on the treatment night (interquartile range: 2–5). No significant difference was detected between treatment groups for either night.
Several secondary analyses were conducted. First, there was no effect of illness duration on treatment effect. Second, parents were asked about other therapies used on the treatment night both before and after the treatment bag was opened. In the hours between enrolling in the study and opening the treatment bag, there was a significant difference between treatment groups in the use of acetaminophen (P = .004), with 36% of petrolatum-treated children receiving the drug compared with 16% in the VR group and 9% in the no-treatment group. There were no significant differences between groups for any other remedy. After opening the treatment bag, 13% of children in the petrolatum group were given acetaminophen, compared with 7% in the VR-treatment and 0% in the no-treatment groups, respectively (P = .03). Among children in the no-treatment group, 9% were given a cough and cold medication by their parents, whereas no child in the other 2 study groups received such drugs (P = .03). There were no other significant differences between groups for other remedies.
Adverse effects were more common in the VR group (Table 2). Twenty (46%) participants in the VR group reported at least 1 adverse event, with nearly all of them being expected, mild irritant adverse effects.16,17 Parents of 28% of participants in the VR group reported a burning sensation of the skin, whereas 16% and 14% reported a burning sensation of the eyes and nose, respectively. No child in the other 2 study groups had these adverse effects (P < .001 for all comparisons). Importantly, within the group of 44 VR-treated children there was no significant differences in any study outcome, including sleep, for the 15 children who reported a burning sensation of the skin, eyes, and/or nose compared with the 29 in that study group who did not report these symptoms, which supports the notion that these were of mild severity. All other adverse effects were rare and did not differ between the 3 study groups.
TABLE 2.
Parent-Reported Adverse Effects (N = 138)
| VR (N = 44), n (%) |
Petrolatum (N = 47), n (%) |
No Treatment (N = 47), n (%) |
P | |
|---|---|---|---|---|
| Skin rash | 2 (5) | 0 (0) | 0 (0) | .10 |
| Skin redness | 2 (5) | 0 (0) | 0 (0) | .10 |
| Burning sensation of the skin | 12 (28) | 0 (0) | 0 (0) | <.001 |
| Burning sensation of the nose | 6 (14) | 0 (0) | 0 (0) | <.001 |
| Burning sensation of the eyes | 7 (16) | 0 (0) | 0 (0) | <.001 |
| Hyperactivity | 1 (2) | 0 (0) | 0 (0) | .32 |
| Sleepiness | 1 (2) | 3 (6) | 1 (2) | .62 |
| Headache | 1 (2) | 2 (4) | 0 (0) | .53 |
As expected, all 47 (100%) parents who applied no treatment correctly guessed their child’s study group when surveyed the next morning. Among VR-treating parents, 38 of 44 (86%) guessed correctly, as did 42 of 47 (89%) parents of petrolatum-treated children.
DISCUSSION
The results of this study indicate that an old, commonly used remedy is effective at providing symptomatic relief from nocturnal cold symptoms. Combinations of aromatic oils in a petrolatum base have been used for generations, but this study demonstrates that this therapy is indeed effective. As rated by their parents, children with URIs who were treated with VR had more nocturnal relief from cough, congestion, and sleep difficulty than did children treated with a placebo ointment, petrolatum, or with no treatment.
The benefits of aromatic compounds that produce a cooling sensation such as menthol seem to stem from effects on the TRPM8 cation channel. This channel is activated by menthol and thermal stimuli in the cool to cold range, which indicates that the cool sensation elicited by compounds such as menthol acts on a thermally sensitive receptor.18,19 Most likely through this mechanism, menthol has been shown to improve the nasal sensation of airflow in congested adults with URIs20 and healthy school-aged children.21 Compounds that contain menthol and/or camphor have also been shown to reduce respiratory rate and restlessness in children with acute bronchitis,22,23 reduce evoked cough in healthy adults,24 and improve mucociliary clearance in adults with chronic bronchitis.25
The improved ability of children to sleep with VR is noteworthy. A child’s inability to sleep during a URI can be disruptive to their daytime functioning as well as the functioning of other family members. The significant benefit seen in ability to sleep for those in the VR group is an important finding for families especially given the positive effect that parents reported for their own sleep after VR application to their child. Although improved parent sleep is obviously related to child’s sleep, the mechanism by which sleep is improved for children with URIs treated with VR is unclear. It is probable that some, if not all of this effect is because of relief of other symptoms. Whether an additional mechanism of aromatics is contributing is unclear. Nonetheless, the benefit on child sleep is clear in this study and indicates that the relatively common irritant adverse effects were generally transient.
Although the investigators were blinded to all 3 treatment groups, one-third of the cohort, those in the no-treatment arm, were unblinded. Also, although every attempt was made to blind parents to whether their child was treated with VR or petrolatum, adherence to the protocol details could not be guaranteed. In addition, parents of participating children who experienced the sensation of skin, nose, or eye burning were likely to realize which treatment their child received even if they adhered to the protocol as instructed. Next, it is notable that children in the petrolatum group were given acetaminophen more commonly by their parents. Explanations could relate to differences in the illnesses for this treatment group or to differences in parenting practice, but symptom severity for the outcomes of interest was not different at baseline. Related to rescue treatment, 9% of parents in the no-treatment group admitted to administering a cough and cold medication overnight despite explicit instructions not to, which indicates that some parents have a strong desire to do something for URI symptoms and are not content to simply observe their children or provide “supportive care.”
An additional study limitation is that each child had a physician visit between the 2 study nights, which may provide some of the explanation for the improvement in all of the groups, including the no-treatment group, because parents may have been reassured that their child had a URI instead of a more severe illness. Alternatively, some of the improvement in all groups can also be attributed to the natural history of URIs, which generally improve with time and supportive care. The subjective survey used for this study may also be considered as a limitation, but clinicians and parents often make decisions on the basis of subjective assessment of symptom severity, which has been argued previously.3,26,27
Some clinicians may express caution about using a product that contains camphor because of the association between toxic ingestions and adverse events such as seizures.28–32 Historical concerns over the toxicity of camphor centered on the ingestion of camphor as a liquid preparation, camphorated oil,13,33 and a recent report of seizures was associated with ingestion of illegally sold camphor.28 In contrast with oral ingestion, dermal exposure to camphor, as occurs when following the directions for use of VR, results in low systemic exposure.34 Serious adverse events such as seizures associated with dermal exposure are generally limited to young infants in whom VR use is not recommended.30,35 Minimizing safety concerns, the Food and Drug Administration approved camphor as an effective antitussive, but limited the concentration in preparations to 11%.36 The concentration in VR ointment is 4.8%. Although recommended only for topical use, at this concentration it is estimated that an ingestion of 20 mL of VR is required to produce toxic effects and 40 mL to potentially cause a fatality in a child younger than 6.29
CONCLUSIONS
Parents desire effective therapies for their children with cough and cold symptoms, and clinicians want to offer evidence-based therapies. Although limited data support many commonly available and OTC remedies for cough cold symptoms, the current data indicate that VR helps to fill the therapeutic void. Despite mild irritant adverse effects, VR provided symptomatic relief for children with URIs and allowed them and their parents to have a more restful night.
WHAT’S KNOWN ON THIS SUBJECT
There are few evidence-based therapies for children with cold symptoms. Topical aromatic compounds are widely used to treat cold symptoms without contemporary evidence to support this practice.
WHAT THIS STUDY ADDS
A vapor rub combination of camphor, menthol, and eucalyptus oils provided nocturnal symptom relief for children with cold symptoms. Children treated with vapor rub had improved sleep, as did their parents, when compared with children given placebo or no treatment.
ACKNOWLEDGMENTS
This study was supported by an unrestricted research grant from Procter and Gamble to Penn State University and by National Institutes of Health General Clinical Research Center grant M01RR10732 and GCRC construction grant C06RR016499 awarded to the Pennsylvania State University College of Medicine.
Dr King had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ABBREVIATIONS
- URI
upper respiratory infection
- OTC
over-the-counter
- VR
vapor rub
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT00743990).
FINANCIAL DISCLOSURE: Dr Paul has been a paid consultant for the Consumer Products Healthcare Association, McNeil Consumer Health Inc, the Procter and Gamble Company, Reckitt Benckiser Healthcare International Ltd, and Novartis Consumer Health Inc; the other authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112(suppl 6A):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 2.Paul IM, Yoder KE, Crowell KR, et al. Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics. 2004;114(1) doi: 10.1542/peds.114.1.e85. Available at: www.pediatrics.org/cgi/content/full/114/1/e85. [DOI] [PubMed] [Google Scholar]
- 3.Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM., Jr Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161(12):1140–1146. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 4.Use of codeine- and dextromethorphan-containing cough remedies in children. American Academy of Pediatrics. Committee on Drugs. Pediatrics. 1997;99(6):918–920. doi: 10.1542/peds.99.6.918. [DOI] [PubMed] [Google Scholar]
- 5.Department of Child and Adolescent Health . Cough and Cold Remedies for the Treatment of Acute Respiratory Infections in Young Children. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- 6.Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):260S–283S. doi: 10.1378/chest.129.1_suppl.260S. [DOI] [PubMed] [Google Scholar]
- 7.Shields MD, Bush A, Everard ML, McKenzie S, Primhak R. BTS guidelines: recommendations for the assessment and management of cough in children. Thorax. 2008;63(suppl 3):iii1–iii15. doi: 10.1136/thx.2007.077370. [DOI] [PubMed] [Google Scholar]
- 8.Todd GB. Notes on the eucalyptus oils presently used in medicine with a short history of the oils used during the last 10 years. Glasgow Med J. 1896;46:346. [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg A. Menthol, a replacement for cocaine as a local anesthetic in the nose and pharynx [in German] Berl Klin Wchnschr. 1885;22:449. [Google Scholar]
- 10.Fox N. Effect of camphor, eucalyptol, and menthol on the vascular state of the mucous membrane. Arch Otolaryngol. 1927;6(2):112–122. [Google Scholar]
- 11.Perrin M. A strong camphor ointment introduced in the nostrils of a young infant can have the same disadvantages as menthol [in French] Province Méd. 1912;23:145. [Google Scholar]
- 12.Dawson KP, Abbott GD, Allan J. Acute respiratory infection in childhood: a study of parental prescribing patterns and advice sources. N Z Med J. 1983;96(734):481–482. [PubMed] [Google Scholar]
- 13.Camphor revisited: focus on toxicity. Committee on Drugs. American Academy of Pediatrics. Pediatrics. 1994;94(1):127–128. [PubMed] [Google Scholar]
- 14.Letter to Joel Schiffenbauer. Food and Drug Administration; [Accessed October 11, 2010]. Sep 6, 2007. pp. 325–328. Available at: www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4323b1-02-FDA.pdf. [Google Scholar]
- 15.Haver K, Hardy SC, Weber TM, Zurakowski D, Hartnick CJ. Validation of a pediatric cough questionnaire. Presented at: American Thoracic Society 2006 International Conference; San Diego, CA. May 19–24, 2006; Abstract 374. [Google Scholar]
- 16.Green BG. Sensory characteristics of camphor. J Invest Dermatol. 1990;94(5):662–666. doi: 10.1111/1523-1747.ep12876242. [DOI] [PubMed] [Google Scholar]
- 17.Green BG. The sensory effects of l-menthol on human skin. Somatosens Mot Res. 1992;9(3):235–244. doi: 10.3109/08990229209144774. [DOI] [PubMed] [Google Scholar]
- 18.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 19.Patel T, Ishiuji Y, Yosipovitch G. Menthol: a refreshing look at this ancient compound. J Am Acad Dermatol. 2007;57(5):873–878. doi: 10.1016/j.jaad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Eccles R, Jawad MS, Morris S. The effects of oral administration of (–)-menthol on nasal resistance to airflow and nasal sensation of airflow in subjects suffering from nasal congestion associated with the common cold. J Pharm Pharmacol. 1990;42(9):652–654. doi: 10.1111/j.2042-7158.1990.tb06625.x. [DOI] [PubMed] [Google Scholar]
- 21.Kenia P, Houghton T, Beardsmore C. Does inhaling menthol affect nasal patency or cough? Pediatr Pulmonol. 2008;43(6):532–537. doi: 10.1002/ppul.20797. [DOI] [PubMed] [Google Scholar]
- 22.Berger H, Jarosch E, Madreiter H. Effect of Vaporub and petrolatum on frequency and amplitude of breathing in children with acute bronchitis. J Int Med Res. 1978;6(6):483–486. doi: 10.1177/030006057800600612. [DOI] [PubMed] [Google Scholar]
- 23.Berger H, Madreiter H, Jarosch E. Effect of Vaporub on the restlessness of children with acute bronchitis. J Int Med Res. 1978;6(6):491–493. doi: 10.1177/030006057800600614. [DOI] [PubMed] [Google Scholar]
- 24.Morice AH, Marshall AE, Higgins KS, Grattan TJ. Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax. 1994;49(10):1024–1026. doi: 10.1136/thx.49.10.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasani A, Pavia D, Toms N, Dilworth P, Agnew JE. Effect of aromatics on lung mucociliary clearance in patients with chronic airways obstruction. J Altern Complement Med. 2003;9(2):243–249. doi: 10.1089/10755530360623356. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JA, Novack AH, Almquist JR, Rogers JE. Efficacy of cough suppressants in children. J Pediatr. 1993;122(5 pt 1):799–802. doi: 10.1016/s0022-3476(06)80031-4. [DOI] [PubMed] [Google Scholar]
- 27.Hutton N, Wilson MH, Mellits ED, et al. Effectiveness of an antihistamine-decongestant combination for young children with the common cold: a randomized, controlled clinical trial. J Pediatr. 1991;118(1):125–130. doi: 10.1016/s0022-3476(05)81865-7. [DOI] [PubMed] [Google Scholar]
- 28.Khine H, Weiss D, Graber N, Hoffman RS, Esteban-Cruciani N, Avner JR. A cluster of children with seizures caused by camphor poisoning. Pediatrics. 2009;123(5):1269–1272. doi: 10.1542/peds.2008-2097. [DOI] [PubMed] [Google Scholar]
- 29.Love JN, Sammon M, Smereck J. Are one or two dangerous? Camphor exposure in toddlers. J Emerg Med. 2004;27(1):49–54. doi: 10.1016/j.jemermed.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Shannon M, McElroy EA, Liebelt EL. Toxic seizures in children: case scenarios and treatment strategies. Pediatr Emerg Care. 2003;19(3):206–210. doi: 10.1097/01.pec.0000081249.98249.51. [DOI] [PubMed] [Google Scholar]
- 31.Gibson DE, Moore GP, Pfaff JA. Camphor ingestion. Am J Emerg Med. 1989;7(1):41–43. doi: 10.1016/0735-6757(89)90083-1. [DOI] [PubMed] [Google Scholar]
- 32.Siegel E, Wason S. Camphor toxicity. Pediatr Clin North Am. 1986;33(2):375–379. doi: 10.1016/s0031-3955(16)35008-8. [DOI] [PubMed] [Google Scholar]
- 33.American Academy of Pediatrics. Committee on Drugs Camphor: who needs it? Pediatrics. 1978;62(3):404–406. [PubMed] [Google Scholar]
- 34.Martin D, Valdez J, Boren J, Mayersohn M. Dermal absorption of camphor, menthol, and methyl salicylate in humans. J Clin Pharmacol. 2004;44(10):1151–1157. doi: 10.1177/0091270004268409. [DOI] [PubMed] [Google Scholar]
- 35.Uc A, Bishop WP, Sanders KD. Camphor hepatotoxicity. South Med J. 2000;93(6):596–598. [PubMed] [Google Scholar]
- 36.Food and Drug Administration Proposed rules: external analgesic drug products for over-the-counter human use; tentative final monograph. Federal Register. 1983;48 [Google Scholar]