Abstract
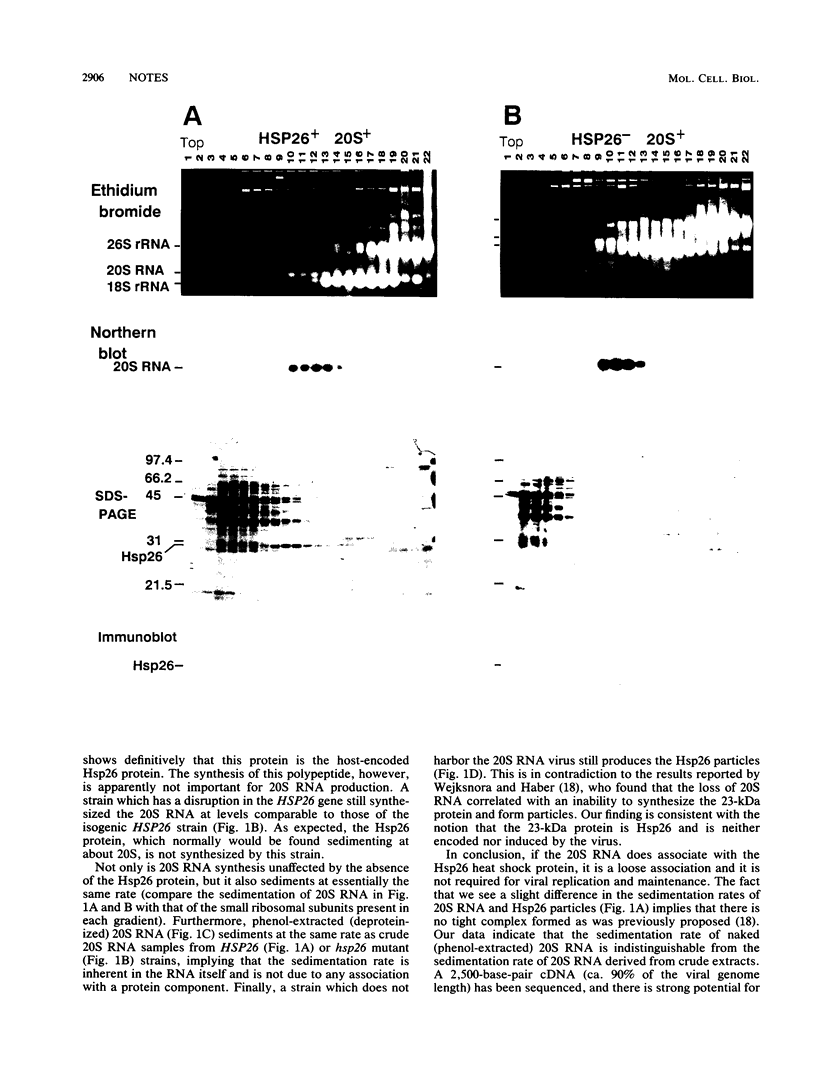
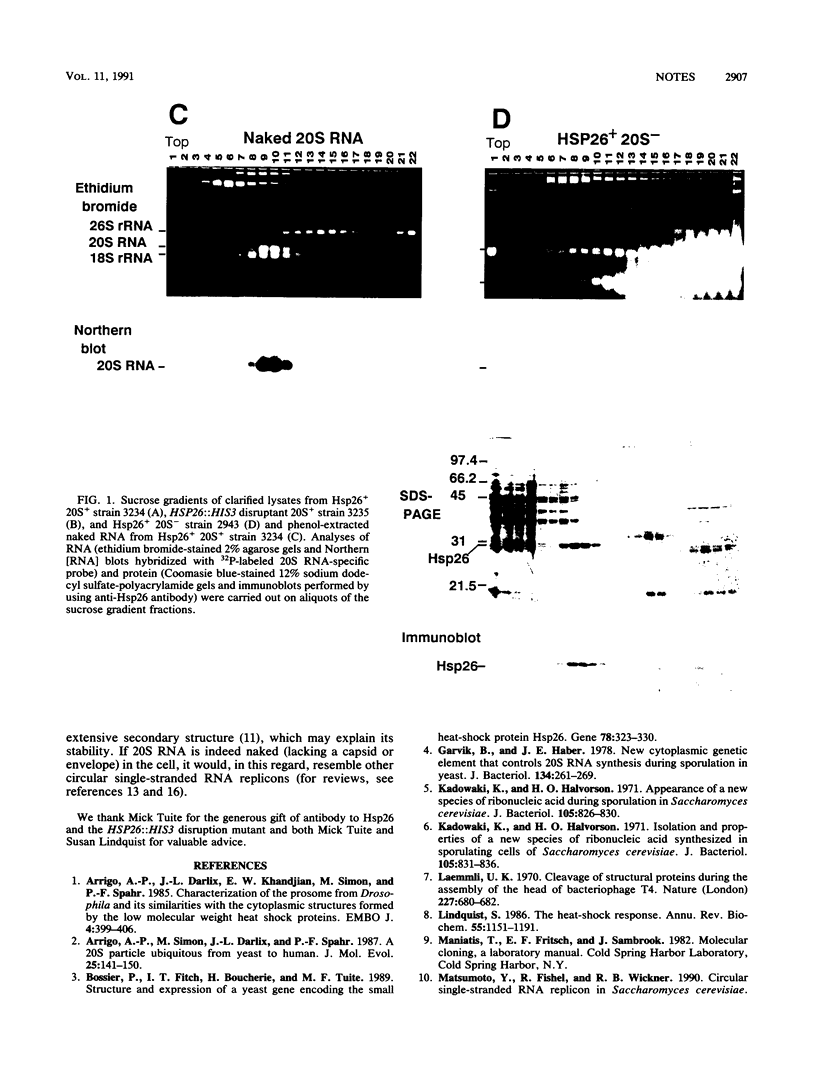
The 20S RNA of Saccharomyces cerevisiae is a single-stranded, circular RNA virus. A previous study suggested that this RNA is part of a 32S ribonucleoprotein particle, being associated with multiple copies of a 23-kilodalton protein. We show here that this protein is, in fact, the chromosome-encoded heat shock protein Hsp26. Furthermore, it is apparently not associated with 20S RNA and plays no obvious role in the life cycle of the virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Darlix J. L., Khandjian E. W., Simon M., Spahr P. F. Characterization of the prosome from Drosophila and its similarity to the cytoplasmic structures formed by the low molecular weight heat-shock proteins. EMBO J. 1985 Feb;4(2):399–406. doi: 10.1002/j.1460-2075.1985.tb03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Simon M., Darlix J. L., Spahr P. F. A 20S particle ubiquitous from yeast to human. J Mol Evol. 1987;25(2):141–150. doi: 10.1007/BF02101756. [DOI] [PubMed] [Google Scholar]
- Bossier P., Fitch I. T., Boucherie H., Tuite M. F. Structure and expression of a yeast gene encoding the small heat-shock protein Hsp26. Gene. 1989 May 30;78(2):323–330. doi: 10.1016/0378-1119(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Garvik B., Haber J. E. New cytoplasmic genetic element that controls 20S RNA synthesis during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):261–269. doi: 10.1128/jb.134.1.261-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Halvorson H. O. Appearance of a new species of ribonucleic acid during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):826–830. doi: 10.1128/jb.105.3.826-830.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Halvorson H. O. Isolation and properties of a new species of ribonucleic acid synthesized in sporulating cells of Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):831–836. doi: 10.1128/jb.105.3.831-836.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989 Mar;9(3):1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. M., Lindquist S. The intracellular location of yeast heat-shock protein 26 varies with metabolism. J Cell Biol. 1989 Feb;108(2):425–439. doi: 10.1083/jcb.108.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. L. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989 Nov;9(11):5265–5271. doi: 10.1128/mcb.9.11.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M. Hepatitis delta virus: cis and trans functions required for replication. Cell. 1990 May 4;61(3):371–373. doi: 10.1016/0092-8674(90)90516-h. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Bentley N. J., Bossier P., Fitch I. T. The structure and function of small heat shock proteins: analysis of the Saccharomyces cerevisiae Hsp26 protein. Antonie Van Leeuwenhoek. 1990 Oct;58(3):147–154. doi: 10.1007/BF00548925. [DOI] [PubMed] [Google Scholar]
- Wejksnora P. J., Haber J. E. Ribonucleoprotein particle appearing during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):246–260. doi: 10.1128/jb.134.1.246-260.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]