Abstract
STUDY QUESTION
Can amino acid profiling differentiate between human oocytes with differing competence to mature to metaphase II (MII) in vitro?
SUMMARY ANSWER
Oocytes which remained arrested at the germinal vesicle (GV) stage after 24 h of in vitro maturation (IVM) displayed differences in the depletion/appearance of amino acids compared with oocytes which progressed to MII and patient age, infertile diagnosis and ovarian stimulation regime significantly affected oocyte amino acid turnover during IVM.
WHAT IS KNOWN ALREADY
Amino acid profiling has been proposed as a technique which can distinguish between human pronucleate zygotes and cleavage stage embryos with the potential to develop to the blastocyst stage and implant to produce a pregnancy and those that arrest. Most recently, the amino acid turnover by individual bovine oocytes has been shown to be predictive of oocyte developmental competence as indicated by the gamete's capacity to undergo fertilization and early cleavage divisions in vitro.
STUDY DESIGN, SIZE, DURATION
The study was conducted between March 2005 and March 2010. A total of 216 oocytes which were at the GV or metaphase I (MI) stages at the time of ICSI were donated by 67 patients.
PARTICIPANTS/MATERIALS, SETTINGS, METHODS
The research was conducted in university research laboratories affiliated to a hospital-based infertility clinic. Oocytes were cultured for 24 h and the depletion/appearance of amino acids was measured during the final 6 h of IVM. Amino acid turnover was analysed in relation to oocyte meiotic progression, patient age, disease aetiology and controlled ovarian stimulation regime.
MAIN RESULTS AND THE ROLE OF CHANCE
The depletion/appearance of key amino acids was linked to the maturation potential of human oocytes in vitro. Oocytes which arrested at the GV stage (n = 9) depleted significantly more valine and isoleucine than those which progressed to MI (n = 32) or MII (n = 107) (P < 0.05). Glutamate, glutamine, arginine and valine depletion or appearance differed in MII versus degenerating oocytes (n = 20) (P < 0.05). Glutamine, arginine, methionine, phenylalanine, total depletion and total turnover all differed in oocytes from patients aged < 35 years versus patients ≥35 years (P < 0.05). MII oocytes obtained following ovarian stimulation with recombinant FSH depleted more isoleucine (P < 0.05) and more alanine and lysine (P < 0.05) appeared than oocytes from hMG-stimulated cycles. MII oocytes from patients with a polycystic ovary (PCO) morphology (n = 33) depleted more serine (P < 0.05) than oocytes from women with normal ovaries (n = 61).
LIMITATIONS, REASONS FOR CAUTION
Immature oocytes collected at the time of ICSI were used as the model for human oocyte maturation. These oocytes have therefore failed to respond to the ovulatory hCG trigger in vivo (they are meiotically incompetent), and have limited capacity to support embryo development in vitro. The lack of cumulus cells and stress of the conditions in vitro may have influenced turnover of amino acids, and owing to the small sample sizes further studies are required to confirm these findings.
WIDER IMPLICATIONS OF THE FINDINGS
The findings provide support for the hypothesis that oocyte metabolism reflects oocyte quality. Longitudinal studies are required to link these functional metabolic indices of human oocyte quality with embryo developmental competence. Oocyte amino acid profiling may be a useful tool to quantify the impact of new assisted reproduction technologies (ART) on oocyte quality.
STUDY FUNDING/COMPETING INTERESTS
This project was funded by the UK Biology and Biotechnology Research Council (BB/C007395/1) and the Medical Research Council (G 0800250). K.E.H was in receipt of a British Fertility Society/Merck Serono studentship. H.J.L. is a shareholder in Novocellus Ltd, a company which seeks to devise a non-invasive biochemical test of embryo health.
Keywords: oocyte quality, developmental competence, amino acid turnover, in vitro maturation
Introduction
Increasing evidence suggests that oocyte developmental competence is acquired as a result of the sequential accumulation of RNA, DNA, proteins and cellular organelles during the protracted growth of the oocyte and supporting ovarian follicle (Picton et al., 1998; Gosden, 2002). Oocyte meiotic maturation is accompanied by cytoplasmic maturation which is manifested by the redistribution of key cytoplasmic organelles such as the mitrochondria (Sun et al., 2001; Liu et al., 2010) and cortical granules (Liu, 2011). The ability of the oocyte to initiate calcium release mechanisms (Mann et al., 2010), cortical granule release (Liu, 2011) and the capacity to remodel the chromatin of the fertilizing sperm (McLay et al., 2002) are all aquired during the terminal stages of oocyte growth and maturation along with the capacity for the storage of gene transcripts which are necessary for zygote development prior to embryonic genome activation (Bettegowda and Smith, 2007; Su et al., 2007; Yurttas et al., 2008). Oocyte maturation and the production of a fertile gamete are also critically dependent on the provision of an adequate supply of energy substrates (for review see Harris and Picton, 2007). Oocyte quality can therefore be defined as the ability of an oocyte to complete meiotic maturation and to provide the full complement of RNA, proteins and energy needed to support early embryogenesis prior to embryonic genome activation (Sirard et al., 2006). The genetic, cytoplasmic and metabolic legacy of the oocyte therefore ultimately contribute to whether the resultant embryo has the capacity to potentially produce a healthy pregnancy and live birth.
Many factors can impact upon oocyte quality. Genetic programing errors in the oocyte can compromise oocyte health and metabolism (Harris et al., 2010). However, despite the lethality of grossly depleted chromosome complements it is rarely possible to distinguish morphologically normal oocytes from those with predivision, aneuploid, or even polyploid karyotypes. Furthermore as women age, so the frequency of chromosomal abnormalities due to aneuploidy and non-disjunction events rises (Pellestor et al., 2003). For example, the age-related decline in oocyte quality and associated reduction in the success of ART is well documented (te Velde and Pearson, 2002; de Mouzon et al., 2010, 2012) as are the increased incidence of Down's syndrome children born to mothers of advanced age (Gaulden, 1992; Sherman et al., 2005) and the increased rate of foetal aneuploidy with increasing age (Kushnir and Frattarelli, 2009). While the changes in mitochondrial number, activity and distribution which occur during the cytoplasmic maturation of oocytes are well documented (Van Blerkom et al., 2002), it has also been shown that mitochondrial activity in MII oocytes is negatively correlated with maternal age (Wilding et al., 2001). The potential mechanisms by which advancing age affects the functionality of oocyte mitochondria have been reviewed by Eichenlaub-Ritter et al. (2011).
Both the cause of infertility and the ART treatment used may have an adverse impact upon the quality of the oocytes retrieved for assisted conception. Although patients diagnosed with polycystic ovary syndrome (PCOS) often have a large number of oocytes available for insemination, their overall quality is poor as determined by the degree of embryo fragmentation and miscarriage rate (reviewed by Qiao and Feng, 2011). Aneuploidy is not increased in oocytes (Harris et al., 2010) or embryos (Weghofer et al., 2007) from patients diagnosed with PCOS, although gene expression in granulosa cells (Kwon et al., 2010), cumulus cells (Kenigsberg et al., 2009) and oocytes (Wood et al., 2007) may be perturbed. Furthermore, the controlled ovarian stimulation (COS) regimen per se may also have an adverse impact upon oocyte cytogenetics as there is increasing evidence that high levels of exogenous FSH can induce errors in meiosis similar to those induced by advanced maternal age (Roberts et al., 2005).
Studies to measure oocyte quality are limited. Molecular analyses of oocytes derived from patients with good or poor chance of becoming pregnant following treatment for infertility have identified a small number of genes which may relate to oocyte developmental competence (Jones et al., 2008; Fragouli et al., 2010; Grondahl et al., 2010). However, studies of this nature are scarce and result in the destruction of the oocytes under investigation thereby preventing confirmation of the functional significance of markers of developmental potential. Alternatively, measurement of gene expression in the cumulus cells removed from oocytes before insemination in vitro has identified a number of cumulus genes whose expression profiles appear to correlate with the oocyte's potential to produce a good quality embryo and/or yield a pregnancy after transfer (McKenzie et al., 2004; Zhang et al., 2005; Cillo et al., 2007; Anderson et al., 2009; Gebhardt et al., 2011; Wathlet et al., 2011). However, there is little consistency in the marker genes identified across cumulus studies which reflects the heterogeneity of the infertile populations studied (Kenigsberg et al., 2009) and the variety of ART and COS protocols used (Wathlet et al., 2011), and the practice of pooling cells from multiple oocytes.
Functional assays of oocyte quality which are sensitive at the individual oocyte level will provide valuable insights into oocyte developmental competence. For example, the uptake of dyes, such as brilliant cresyl blue (BCB) have been used to select competent oocytes from abattoir-derived animal ovaries. However, the clinical safety of this staining method is questionable as higher levels of apoptosis have been recorded in oocytes exposed to BCB (Opiela et al., 2008) and double exposure to BCB has been found to be embryo toxic (Wongsrikeao et al., 2006). In contrast, non-invasive assays of oocyte metabolism have shown a link between metabolism and embryo development. These include measurement of metabolic indices such as glucose turnover (Lane and Gardner, 1996; Steeves et al., 1999; Spindler et al., 2000; Gardner et al., 2011) and pyruvate uptake (Hardy et al., 1989; Conaghan et al., 1993; Harris et al., 2010) by oocytes and embryos from a range of species, including human. Oxygen consumption by oocytes (Scott et al., 2008a,b; Tejera et al., 2011) and embryos (Lopes et al., 2007) has also been related to developmental competence. More recently, raman or near-infra red (NIR) spectroscopy (Seli et al., 2007; Scott et al., 2008 a,b; Vergouw et al., 2008) and proton nuclear magnetic resonance (NMR) imaging (Seli et al., 2008; Marhuenda-Egea et al., 2011) have been used to assess the composition of spent embryo culture medium to distinguish between embryos with high or low capacity to implant. NIR spectroscopy has also revealed that the metabolomic profile of oocytes is related to nuclear maturity and embryo development on Day 3 and 5 of culture as well as embryo viability (Nagy et al., 2009). However, recent clinical trials have failed to demonstrate any increase in success rates following embryo selection after NIR spectroscopy or NMR imaging (Hardarson et al., 2012; Vergouw et al., 2012) and most of these metabolomic techniques have yet to undergo randomized control trials to confirm their clinical utility.
Measurement of the turnover of amino acids in spent embryo culture media, by high performance liquid chromatography (HPLC) has consistently shown promise as a method to distinguish between pronucleate and cleavage-staged embryos with the potential to form blastocysts or arrest in vitro (Houghton et al., 2002; Stokes et al., 2007) and to give rise to a pregnancy following transfer (Brison et al., 2004). This evidence forms the basis of the ‘Quiet Embryo’ hypothesis proposed by Leese (2002, 2012), which states that embryos with a lower (‘quieter’) metabolism are operating more efficiently and have a greater potential to develop than their counterparts with a higher (‘noisy’) metabolism as reflected in a higher turnover of amino acids (Houghton et al., 2002), leading them to arrest. An association between the degree of DNA damage and amino acid turnover has been confirmed in bovine, porcine and human embryos (Sturmey et al., 2009) adding further support to the hypothesis that embryos with high levels of DNA damage have an increased metabolic requirement in order to conduct DNA repair (Baumann et al., 2007). Applying this philosophy, we have recently shown that individual bovine MII oocytes with the capacity to support zygote cleavage and blastocyst development in vitro following IVF display a different preference for amino acids compared with oocytes which failed to fertilize or undergo embryo cleavage (Hemmings et al., 2012). Specifically, the turnover of five amino acids (glutamine, alanine, arginine, leucine and tryptophan) during the final 6 h of IVM was able to predict with 63.5% accuracy which oocytes were competent to undergo fertilization and support cleavage to the 2–8 cell stage by Day 3 post-insemination. The positive predictive value of oocyte amino acid profiling for predicting which bovine oocytes failed to undergo fertilization and early embryo cleavage was 92%.
The aim of the present study was to measure the depletion/appearance of amino acids by individual human oocytes during the final stages of IVM to discover whether human oocytes of different maturational competence displayed differences in their utilization of amino acids. Amino acid profiles were also analysed in relation to patient age, infertile diagnosis and the nature of the gonadotrophin regimen used for COS. The information so gleaned may help identify the ‘nutritional fingerprint’ associated with human oocyte quality which can be used to improve the efficiency of ART.
Materials and Methods
Unless otherwise stated all chemicals were supplied by Sigma-Aldrich Chemical Company Limited (Poole, UK) and plastic ware by Nunc A/S (Roskilde, Denmark).
Patient recruitment and treatment
Oocytes which were at the -GV- or -MI stage of maturity at the time of insemination by ICSI (∼40 h after hCG) were donated after informed consent by 67 women undergoing treatment at the Reproductive Medicine Unit, Leeds General Infirmary, UK, under protocols approved by the local research Ethics Committees. Insemination by ICSI was the only inclusion criteria to enable oocytes to be released prior to the fertilization check. Ovarian stimulation and oocyte collection was performed as described previously (Harris et al., 2010). Oocytes were denuded in preparation for ICSI by brief exposure (∼30 s) to 80 IU/ml hyaluronidase (Synvitro Hyadase, Medicult, Surrey, UK) at 37°C.
Oocyte metabolism culture
On arrival in the research laboratory, any remaining cumulus cells were removed from each oocyte by repeat pipetting through glass narrow-bore pipettes. Denuded oocytes were incubated for 30 min in individual 10 µl droplets of modified oocyte maturation medium overlaid with liquid paraffin (Medicult, Surrey, UK), at 37°C in 5% CO2 in humidified air to allow for equilibration to the oocyte culture conditions. The modified IVM medium consisted of five-sixth Earle's Balanced Salt Solution, one-sixth Minimum Essential Medium (Alpha-modification) (α-MEM) supplemented with 0.8% human serum albumin (HSA; C.A.F.-D.C.F. cvba-scrl, Brussels, Belgium), 100 IU/ml penicillin, 100 µg/ml streptomycin, 0.01 IU/ml FSH (Gonal-f®; Merck Serono Ltd, Feltham, UK) 0.1 IU/ml hCG (Ovitrelle; Merck Serono Ltd), 0.25 mM sodium pyruvate, 5 µg/ml human transferrin, 5 ng/ml selenite, 10 ng/ml human insulin, 100 ng/ml Long R3 insulin-like growth factor-1, 62.5 µM d-α-aminobutyric acid (DABA) and 62.5 µM glutamine. This medium composition was based on the formulation used for the IVM of human oocytes (Wynn et al., 1998). Following equilibration, oocytes were assessed for stage of nuclear maturity and transferred to individual 2 µl droplets of fresh IVM medium at 37°C in 5% CO2 in air. Oocytes were always cultured within drops which had been immediately overlaid with liquid paraffin to prevent evaporation. After 16–18 h of overnight culture (∼56–62 h post-hCG) the oocytes were transferred to 1 µl microdroplets of fresh IVM media for assay of amino acid turnover. It was necessary to reduce the concentration of amino acids to one-sixth that normally present in α-MEM to enable detection of the flux of amino acids during this assay period. Nuclear maturity was recorded and gamete culture continued for a further 6–8 h at 37°C in 5% CO2 in air. Control droplets which did not contain oocytes were cultured in parallel. At the end of the amino acid assay period, at ∼64–68 h post hCG, the oocytes were assessed for nuclear maturity. Oocytes were removed and spent medium was stored at −80°C until amino acid analysis. Medium from a small number of severely degenerate oocytes (n = 20), displaying dark shrunken cytoplasm was analysed to provide proof that amino acid profiles are affected by oocyte health.
HPLC analysis
Spent oocyte culture medium drops were thawed at room temperature and diluted 1:50 or 1:25 with HPLC grade water (ELGA Lab Water, High Wycombe, UK) and analysed by HPLC using O-phthaldialdehyde (OPA) as the derivatizing agent, as detailed previously (Houghton et al., 2002; Brison et al., 2004; Stokes et al., 2007; Hemmings et al., 2012). It is not possible to detect proline and cysteine using the OPA method. Samples were separated by a 250 × 4.6 mm HyperClone 5 μ ODS (C18) column (Phenomenex, Macclesfield, UK) at a flow rate of 1.2 ml/min or by a 250 × 3.2 mm Hypersil ODS (C18) column (Phenomenex) at a flow rate of 0.9 ml/min. At the start of separation 100:0 Buffer A: Buffer B was passed through the column, and adjusted gradually to 0:100 Buffer A: Buffer B by the end of the analysis, which lasted 45 min. Buffer A consisted of 80% (v/v) sodium acetate (pH 5.9), 20% (v/v) methanol and 5–15 ml tetrahydrofuran/l and Buffer B consisted of 80% (v/v) methanol and 20% (v/v) sodium acetate (pH 5.9). Amino acids were eluted sequentially based on their different binding affinities to the column. Fluorescence intensity was monitored at an excitation wavelength of 348 nm and an emission wavelength of 450 nm. The baseline concentration of amino acids within the control droplets was calculated from the medium formulation available from the manufacturer. A chromatogram was produced for each sample and the area beneath each peak was given in arbitrary units which were converted to units of concentration. Any dilution errors were corrected by the inclusion of DABA at a known concentration in all oocyte incubation media. The net appearance or depletion of each amino acid per sample was calculated with reference to the mean of the control droplets cultured alongside. To monitor the consistency of the HPLC separation, amino acid standards were analysed every six samples.
Analyses of amino acid profile
The rate of amino acid depletion/appearance was calculated in pmoles/oocyte/hour according to published calculations (Houghton et al., 2002). Negative values indicated net disappearance of amino acids from the media and are termed as ‘depleted’ while positive values represent amino acids that were present in higher concentration at the end of the incubation and are termed ‘appeared’. The ‘total depletion’ of amino acids was calculated by summing all amino acids showing negative values whilst the ‘total appearance’ of amino acids was calculated by summing all amino acids with positive values. The turnover of amino acids has previously been defined by (Houghton et al., 2002) as the sum of net depletion and appearance. The net balance of amino acid depletion and appearance can be calculated by subtracting depletion from appearance.
Statistical analysis
Statistical analysis was performed using the Minitab 15 package (Minitab Ltd, Coventry, UK). All data were tested for normality using the Anderson-Darling test. Amino acid and gonadotrophin dose data did not conform to a normal distribution therefore were analysed by Mann–Whitney U-test or Kruskall–Wallis test with post hoc Mann–Whitney U-test according to the number of groups. Patient age data fitted a normal distribution and were therefore analysed by t-test. Relationships between age, gonadotrophin treatment and amino acid turnover were analysed by Spearman's Correlation. The distribution of GV and MI oocytes at the start of culture for male factor only and PCO/PCOS groups and oocyte progression to MII or arrest were evaluated by Chi-square analysis. Amino acid data were analysed in relation to oocyte meiotic status at the start of IVM, oocyte developmental progression in vitro, as well as patient age, infertile diagnosis and gonadotrophin preparation (FSH or hMG) used for COS. When considering the diagnosis of infertility, data were analysed according to The Rotterdam Consensus Workshop Group 2004 definition of polycystic ovaries at ultrasound (PCO) or PCOS (Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group, 2004). A value of P < 0.05 was considered significant.
Results
Kinetics of oocyte maturation
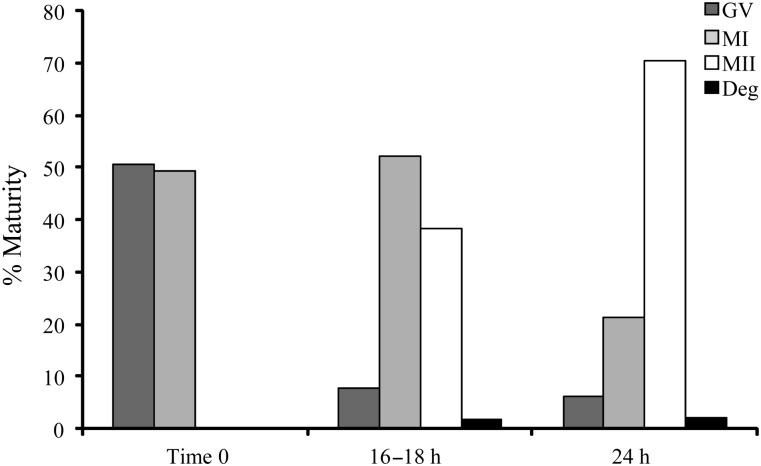
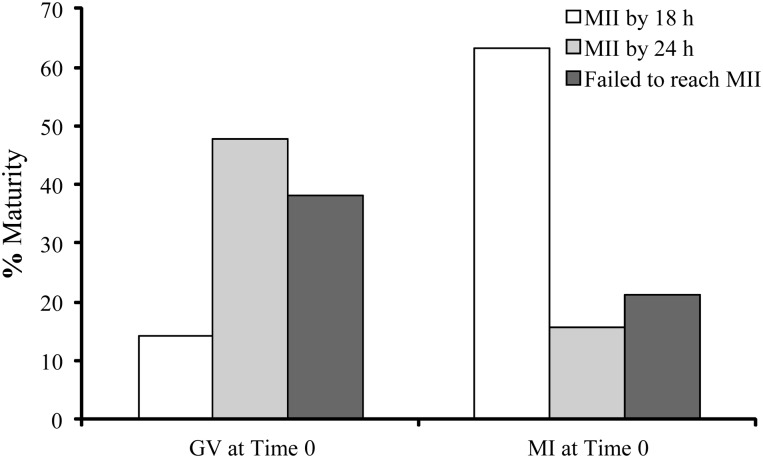
A total of 216 oocytes were donated from 67 patients with a mean age of 34.4 ± 0.5 years. Of these, 18 oocytes were not profiled, 30 were excluded due to incomplete data, 16 were degenerate upon arrival at the laboratory and 4 degenerated during the amino acid assay period. Of the viable oocytes, 51% were at the GV stage and 49% at MI on their arrival at the research laboratory. The stage of oocyte nuclear maturity was recorded after 16–18 h of IVM and again after a total of 24 h of culture following completion of the metabolism assay period. The dynamics of oocyte meiotic progression are shown in Fig. 1. After 24 h of culture only 6% of oocytes remained arrested at the GV stage, 21.4% arrested at MI, 70.3% had progressed to MII and 2.2% had degenerated. Of the oocytes which were at GV stage upon their arrival to the laboratory only 14% had progressed to MII within 18 h, the majority (48%) attained MII between 18 and 24 h and 38% either arrested or degenerated. Of the oocytes which were at MI upon arrival to the laboratory, 63% had progressed to MII within 18 h, 16% progressed to MII between 18 and 24 h culture and 21% had either arrested or degenerated as presented in Fig. 2. Oocyte maturity at the onset of IVM and at the start of the amino acid assay period are displayed in Table I. No significant differences (P > 0.05) were detected between the distribution of GV and MI oocytes at the start of culture across the different infertile diagnoses, nor were there any significant differences in the proportion of MI or MII oocytes at the start of the amino acid assay across the different infertile diagnoses.
Figure 1.
Meiotic progression of human oocytes over the in vitro maturation (IVM) culture period. Data from all stages of oocyte nuclear maturity as well as degenerate (Deg) oocytes are shown.
Figure 2.
Rate of meiotic progression of oocytes which were at either germinal vesicle (GV) or metaphase I (MI) stage at the start of the culture period.
Table I.
Patient demographics and oocyte maturity.
| Aetiology | Number of patients | Number of oocytes analysed | Stage of maturity at start of IVM | Stage of maturity at start of AAP of oocytes which progressed to MII | Age (years) | Age range (years) | Average daily FSH dose(IU) |
|---|---|---|---|---|---|---|---|
| Male Factor/Tubal | 28 | 83 | 43 GV 40 MI | 27 MI 34 MII | 35.9 ± 1.1 | 29–41 | 281 ± 21 |
| PCO/PCOS | 12 | 49 | 20 GV 29 MI | 13 MI 20 MII | 32.9 ± 1.5 | 25–40 | 214 ± 26 |
| Endometriosis | 5 | 9 | 5 GV 4 MI | 4 MI 4 MII | 35.0 ± 0.9 | 33–38 | 231 ± 59 |
| Unexplained | 4 | 7 | 2 GV 5 MI | 3 MI 2 MII | 37.5 ± 1.7 | 33–41 | 400 ± 50 |
| Total | 49 | 148 | 70 GV 78 MI | 47 MI 60 MII | 35.2 ± 0.5 | 25–41 | 271 ± 17 |
No significant difference in the proportion of germinal vesicle (GV) and metaphase I (MI) stage oocytes at the start of in vitro maturation (IVM) between male factor and polycystic ovary (PCO)/polycystic ovary syndrome (PCOS) group. No significant difference in the proportion of MI and metaphase II (MII) stage oocytes at the start of the amino acid assay between male factor and PCO/PCOS group (Chi-squared test). PCO/PCOS group contained (n = 9 patients: 40 oocytes) with PCO diagnosis and (n = 3 patients: 9 oocytes) with PCOS diagnosis.
Amino acid profile of oocytes of different developmental competence
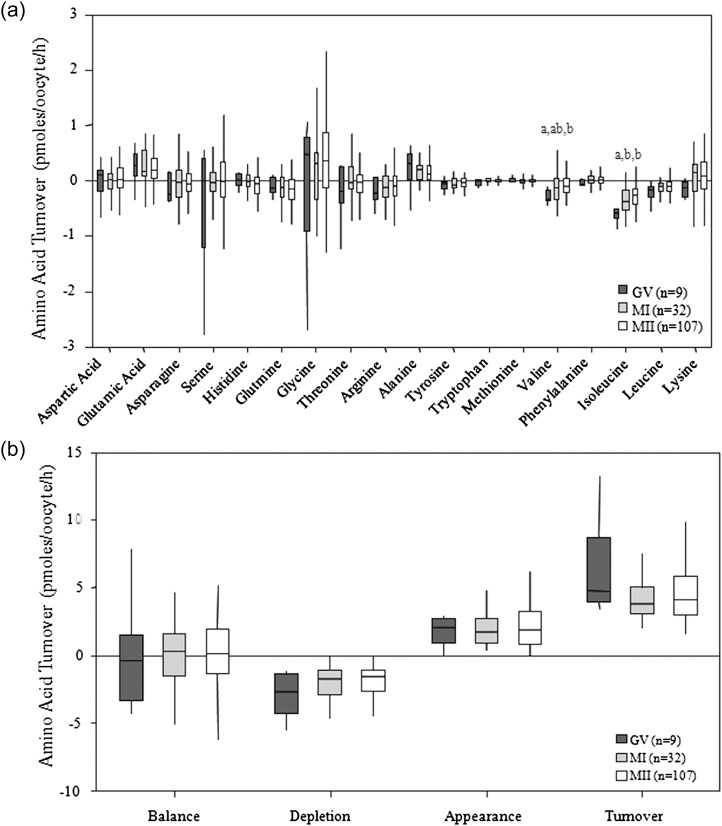
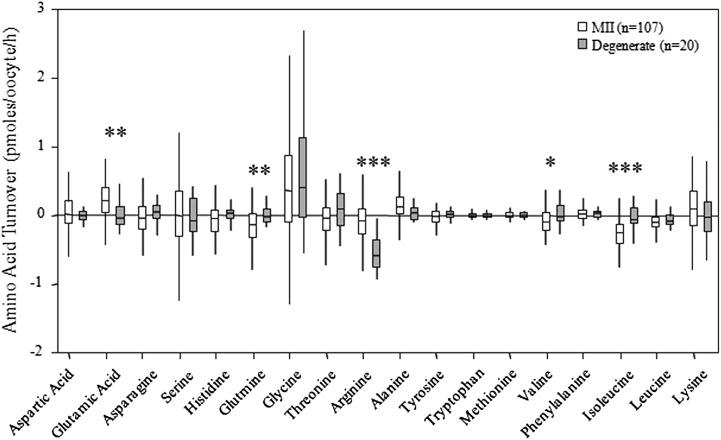
Oocyte meiotic status on entry into IVM had no significant impact (P > 0.05) on MII oocyte amino acid turnover measured after 24 h of IVM. Oocyte data were therefore pooled and amino acid profiles were analysed according to oocyte meiotic progression after 24 h of culture. Oocytes that arrested at GV depleted more valine than those that matured to MII (P < 0.01) and they also depleted more isoleucine than those that either arrested at MI (P < 0.05) or progressed to MII (P < 0.01) as presented in Fig. 3a. There were no significant differences in the net balance, depletion, appearance or turnover of amino acids between GV and MI arrested oocytes and those that progressed to MII as presented in Fig. 3b. A comparison of the average amino acid profiles obtained from degenerated oocytes compared with oocytes that matured to MII is displayed in Fig. 4. Glutamic acid (P < 0.01), glutamine (P < 0.01), arginine (P < 0.001), valine (P < 0.05) and isoleucine (P < 0.001) depletion or appearance were all significantly different between degenerate and morphologically normal MII-stage oocytes.
Figure 3.
Average amino acid profiles of human oocytes following 24 h IVM. (a) Average amino acid depletion/appearance of oocytes which arrested at GV, MI or progressed to MII following 24 h of IVM. (b) Average total net balance, depletion, appearance and turnover of amino acids by oocytes which arrested at GV, MI or progressed to MII following 24 h of IVM. Different letters denote a significant difference between groups (Kruskall–Wallis with post hoc Mann–Whitney U-test P < 0.05).
Figure 4.
Average amino acid depletion/appearance profiles of degenerated and MII human oocytes following 24 h of IVM (Mann–Whitney U-test *P < 0.05, **P < 0.01, ***P < 0.001).
Impact of patient age on oocyte amino acid turnover
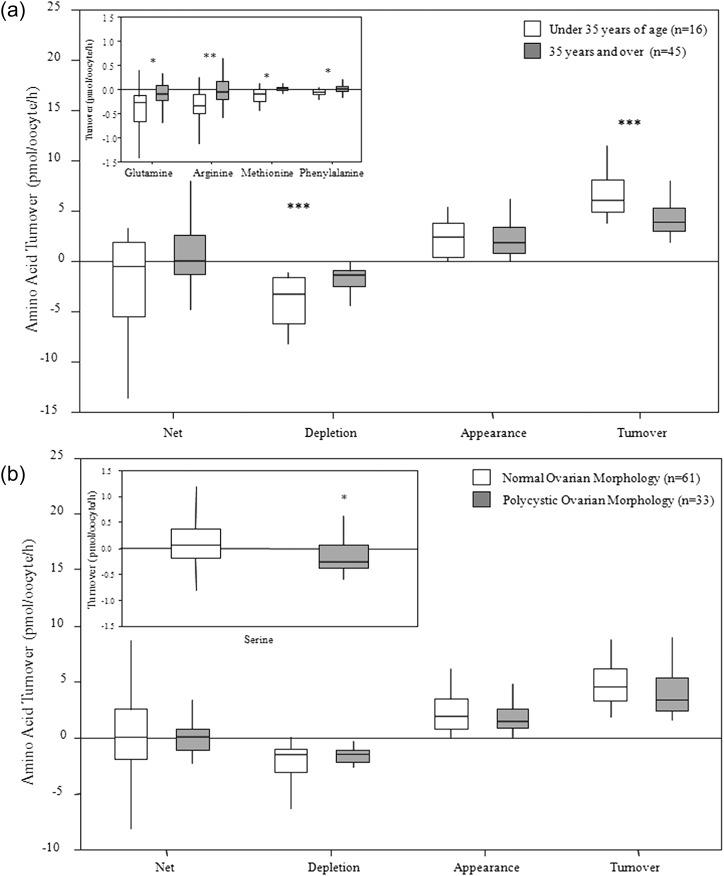
MII oocytes from patients with normal ovarian morphology aged under 35 years depleted significantly more glutamine (P < 0.05), arginine (P < 0.01), methionine (P < 0.05), phenylalanine (P < 0.05), and had a greater depletion of total amino acids (P < 0.01) and a higher amino acid turnover (P < 0.001) than oocytes from older patients (≥35 years of age) as presented in Fig. 5a. However, if the data from all MII oocytes from patients across all infertile diagnoses were pooled and analysed in relation to patient age using Spearman's Correlation, there were no significant correlations between patient age and any individual amino acid or between overall amino acid balance, depletion, appearance and turnover. There was a strong positive correlation between patient age and FSH dose (Spearman's rs 0.542; P < 0.001).
Figure 5.
Amino acid turnover by human MII oocytes. (a) Amino acid turnover of MII oocytes from women with normal ovarian morphology aged < 35 or ≥35 years. Inset shows glutamine, arginine, methionine and phenylalanine turnover to be significantly different between age groups. (b) Amino acid turnover of MII oocytes from women with normal ovarian morphology versus women with polycystic ovary morphology. Inset shows serine turnover was significantly different between women with different ovarian morphology (Mann–Whitney U-test *P < 0.05 **P < 0.01 ***P < 0.001).
Impact of patient's infertile diagnosis on oocyte amino acid turnover
To ascertain whether oocyte amino acid turnover was affected by patient's diagnosis of infertility, amino acid profile data were analysed according to the cause of infertility. The distribution of the different diagnoses of infertility for the oocytes analysed in the present study are given in Table I. Owing to the patient inclusion criteria for the study, male factor infertility with no ovarian pathology, made up approximately half of the recruited patients. One-third of recruited patients had PCO morphology and a small number of patients presented with PCOS. The data from 33 MII oocytes retrieved from patients with a diagnosis of PCO or PCOS were compared with 61 MII oocytes retrieved from patients with no ovarian pathology. Data from patients diagnosed with endometriosis or unexplained infertility were excluded from further analysis because patient numbers were too small, and in several cases, the diagnosis of endometriosis was accompanied by a diagnosis of PCO. There were no significant differences (P > 0.05) in the balance, depletion, appearance and turnover of amino acids between MII oocytes from women with normal ovarian morphology compared with women with PCO/PCOS as presented in Fig. 5b. However, MII oocytes retrieved from patients with PCO/PCOS depleted significantly more serine (P < 0.05; inset to Fig. 5b). There was no difference in either the mean age of each subpopulation (PCO/PCOS; 32.9 ± 1.5 years versus normal; 35.9 ± 1.1 years) or the amount of gonadotrophin administered (PCO/PCOS; 214 ± 26 IU per day versus normal; 281 ± 21 IU per day). There was a trend for patients with normal ovarian morphology to be treated with recombinant FSH (rFSH) and for PCO patients to be treated with hMG preparations, although this was not planned in clinical practice. For the MII oocytes from normal women, 56% of the patients underwent ovarian stimulation using rFSH and 44% received hMG. Of the PCO patients 45% received rFSH and 55% received hMG.
Impact of gonadotrophin stimulation protocol on oocyte amino acid turnover
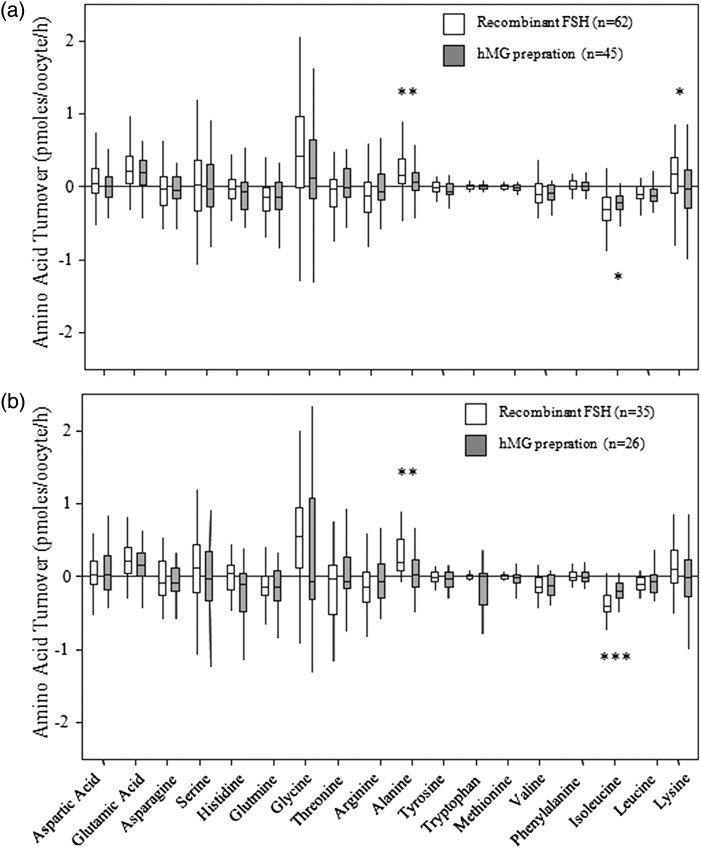
Data from all oocytes which progressed to MII were pooled according to whether patients received rFSH; (n = 23 patients; 62 MII oocytes) or hMG preparations; (n = 17 patients; 45 MII oocytes). The results show that MII oocytes from all cycles stimulated with rFSH depleted more isoleucine (P < 0.05) and more alanine (P < 0.01) and lysine (P < 0.05) appeared in the medium than oocytes from hMG stimulated cycles (Fig. 6a). There were no significant differences in the balance, depletion, appearance and turnover of amino acids from all MII oocytes retrieved from rFSH- versus hMG-stimulated cycles (data not shown). When only the subset of MII oocytes from patients with normal ovarian morphology were analysed (Fig. 6b) oocytes from rFSH stimulated cycles depleted significantly more isoleucine (P < 0.001) and released significantly more alanine (P < 0.01) into the medium than MII oocytes from hMG-stimulated cycles. There were no significant differences in the balance, depletion, appearance and turnover of amino acids from MII oocytes retrieved from patients with normal ovarian morphology according to gonadotrophin treatment (data not shown). There were also no significant differences in the number of oocytes which progressed to MII or arrested in rFSH versus hMG preparations. However, there were significant negative correlations between FSH dosage and both asparagine depletion (Spearman's rs 0.230; P < 0.05) and glutamine depletion (Spearman's rs 0.240; P < 0.05). There were no significant correlations between FSH dose and amino acid balance, depletion, appearance and turnover.
Figure 6.
Impact of gonadotrophin preparation on amino acid turnover by MII oocytes. (a) MII oocytes from all patients irrespective of diagnosis of infertility stimulated with recombinant FSH (rFSH) or hMG. (b) MII oocytes from women with normal ovarian morphology stimulated with rFSH or hMG (Mann–Whitney U-test *P < 0.05 **P < 0.01 ***P < 0.001).
Discussion
This study provides the first quantification of the depletion/appearance of amino acids by individual human oocytes which have undergone 24 h of culture to support meiotic maturation. The results show that human oocytes which fail to progress beyond the GV stage of maturation have an increased requirement for valine and isoleucine compared with oocytes which were competent to reach MII. The data also demonstrated, that amino acid depletion/appearance by human oocytes is a function of patient age, and gonadotrophin preparation and dose used for COS. Oocytes retrieved from patients under the age of 35 years with no ovarian pathology displayed differences in the profiles of glutamine, arginine, methionine, phenylalanine and, total depletion and turnover of amino acids compared with oocytes retrieved from patients aged 35 years and over. Previous investigations of human embryos, summarized in Table II, have indicated a number of amino acids to be related to embryo developmental potential (reviewed in Sturmey et al., 2008). Consistent with the present oocyte data, glutamine, arginine and methionine as well as alanine and asparagine depletion/appearance by embryos on Day 2–3 post-insemination differed significantly between embryos with the capacity to develop and those that arrested (Houghton et al., 2002), while glutamate, glutamine, glycine, arginine, alanine and lysine turnover differed between cryopreserved embryos which arrested or developed to the blastocyst stage (Stokes et al., 2007). A relationship between asparagine, glycine and leucine depletion/appearance by pronucleate stage embryos and pregnancy was also demonstrated (Brison et al., 2004). Although amino acid turnover has not previously been measured for human oocytes during IVM, we have previously shown that bovine oocytes which were matured in vitro but failed to cleave following IVF depleted significantly more glutamine, arginine, leucine and more alanine and tryptophan appeared into the culture medium than oocytes which had the potential to undergo at least one round of cleavage (Hemmings et al., 2012) and the difference for glutamine depletion and alanine appearance also existed between oocytes which remained uncleaved compared with those which progressed to the blastocyst stage.
Table II.
Summary of the amino acids associated with poor human oocyte and embryo developmental potential.
| Developmental stage | Biological characteristic | Amino acid | Comment | References |
|---|---|---|---|---|
| Oocyte | Increased depletion | ValineE, IsoleucineE | Associated with failure to reach MII | Current manuscript |
| Pronucleate | Increased depletion | AsparagineN | Associated with failure to give rise to a pregnancy | Brison et al. (2002) |
| Increased production | GlycineN | |||
| Decreased depletion | LeucineE | |||
| Cleavage D2–3 | Increased depletion | AsparagineN, methionineE | Associated with arrest prior to blastocyst formation | Houghton et al. (2002) |
| Increased depletion | GlutamineN, arginineN, total amino acids | Houghton et al. (2002) and Stokes et al. (2007) | ||
| Decreased production | GlutamateN | Stokes et al. (2007) | ||
| Increased production | GlycineN, alanineN | Houghton et al. (2002) and Stokes et al. (2007) | ||
| Increased production | LysineE | Stokes et al. (2007) | ||
| Increased turnover | Total amino acids | Stokes et al. (2007) | ||
| Decreased depletion | AsparagineN | Associated with chromosomal abnormality | Picton et al. (2010) | |
| Increased production | ValineE | |||
| Decreased depletion | GlycineN | |||
| Cleavage D3–4 | Increased production | SerineN, lysineE | Associated with chromosomal abnormality | Picton et al. (2010) |
| Decreased depletion | LeucineE | |||
| 8 cell-morula | Increased depletion | SerineN, total amino acids | Associated with arrest prior to blastocyst formation | Houghton et al. (2002) and Stokes et al. (2007) |
| Increased production | GlycineN, alanineN | Houghton et al. (2002) and Stokes et al. (2007) | ||
| Decreased depletion | LeucineE | Houghton et al. (2002) | ||
| Blastocyst | Increased turnover | Total amino acids | Associated with increased DNA damage | Sturmey et al. (2009) |
E, essential amino acids; N, non-essential amino acids.
The observed differences in the turnover of valine and isoleucine between meiotically competent and incompetent human oocytes may be unique to the stages of human oocyte maturation studied as we have not previously recorded differences in these two amino acids between developmentally competent or incompetent human embryos (Houghton et al., 2002; Brison et al., 2004; Stokes et al., 2007) or bovine MII oocytes (Hemmings et al., 2012). Valine and isoleucine share a similar catabolic pathway (Voet and Voet, 1995). However, it is unclear why arrested oocytes should have a greater need to metabolize these branched chain amino acids than oocytes which have matured although it likely reflects the perturbed metabolism of these abnormal cells. Discrepancies between studies in the differences observed between amino acids may reflect: species-specific differences between oocytes; differences between oocyte and embryo amino acid metabolism; and/or the fact that the oocytes used in the present study failed to complete meiotic maturation in vivo in response to hCG stimulation. The proposition that discrete differences exist in the turnover of key amino acids between good versus poor quality human oocytes is further supported by the observation that degenerating oocytes displayed differences in the depletion/appearance of glutamate, glutamine, arginine, valine and isoleucine compared with oocytes which completed maturation to MII. Indeed a characteristic of degenerating oocytes was a much increased depletion of arginine, which may be utilized to generate nitric oxide (NO) by nitric oxide synthase (Kwon et al., 1990). The three forms of nitric oxide synthase (endothelial, neuronal and inducible) have all been identified in porcine (Chmelikova et al., 2010) and bovine oocytes (Tesfaye et al., 2006) and high levels of NO have been associated with apoptosis in bovine pre-implantation embryos (Tranguch et al., 2003). This finding reflects the previously reported observations for both human embryos and bovine oocytes, that high glutamine and arginine depletion relate to poor developmental progression (Houghton et al., 2002; Stokes et al., 2007; Hemmings et al., 2012), whereas glutamate appearance is a reflection of good developmental competence (Stokes et al., 2007). Similarly, pig oocytes presumed to be polyspermic displayed differences in their profiles of leucine, isoleucine, valine, phenylalanine, threonine, glutamine and glutamate compared with oocytes fertilized with a concentration of sperm known to reduce the level of polyspermy (Booth et al., 2005). Recently, higher concentrations of isoleucine and leucine have been observed in human follicular fluid obtained from follicles which produced an oocyte which subsequently developed and implanted to yield an ongoing pregnancy compared with those which failed to implant (Wallace et al., 2012). It is plausible that reduced availability of specific amino acids within the follicle could result in an increased depletion of these amino acids by oocytes once placed into culture.
The relationship between amino acid turnover by MII oocytes and FSH dose was examined and showed that oocytes from patients receiving lower doses of gonadotrophins depleted more asparagine and glutamine than oocytes from patients receiving higher doses. This reduced depletion may reflect an adverse impact of the high-dose regimen on oocyte metabolism. Furthermore, we also observed a strong positive correlation between patient age and FSH dose. The data indicate that oocyte metabolism, and specifically the turnover of key amino acids, may provide a functional index of the reduction in oocyte quality in women of advancing maternal age. Although the hormonal changes of ovarian ageing and the changes in ovarian follicle population dynamics are well characterized (Broekmans et al., 2009) and the close relationship between advancing maternal age and increased oocyte and embryo aneuploidy (Pellestor et al., 2003) is widely acknowledged, details of exactly how age disrupts meiotic maturation and oocyte quality remain unclear. Age-related increases in the dysfunction of the mitochondrial genome may directly affect oocyte metabolism through disruption of molecular transport across cell membranes (Schon et al., 2000). The reduced metabolic activity of aged oocytes may result from decreased mitochondrial number and activity (Iwata et al., 2011). Alternatively, life-long exposures to environmental factors, oxidative stress and/or reactive oxygen species can lead to compromised folliculogenesis, absent or permissive cell-cycle checkpoints and/or defective spindle formation with resultant pre-segregation and non-disjunction of chromosomes (Eichenlaub-Ritter et al., 2011). Indeed, altered expression of genes involved with cell-cycle regulation and chromosome alignment have been recorded in oocytes from women of advanced age (Grondahl et al., 2010) and are related to the incidence of aneuploidy in human oocytes (Fragouli et al., 2010). While we did not quantify oocyte aneuploidy in the present study we have previously reported that amino acid turnover by human cleavage-stage embryos is linked to embryo aneuploidy (Picton et al., 2010) such that asparagine, glycine and valine profiles differed significantly in Day 2–3 embryos in which all blastomeres were either chromosomally normal or abnormal, while serine, leucine and lysine differed on Day 3–4 (Picton et al., 2010). We can therefore propose that maternal age per se, and/or age-related differences in the gonadotrophin dose used for COS, may account for the observed differences in amino acid turnover with age in MII oocytes.
An important finding reported here was the observed difference in the depletion of serine by MII oocytes retrieved from women with PCO morphology compared with that from patients with normal ovarian morphology. We have previously observed serine appearance in the media from all bovine oocytes with developmental potential (Hemmings et al., 2012) suggesting that serine depletion could be a marker of dysfunctional oocyte metabolism and hence poor oocyte quality recorded from women with PCO morphology. We have also demonstrated altered carbohydrate metabolism in oocytes from women with PCOS (Harris et al., 2010). The different metabolic signatures observed in oocytes from women with PCO morphology and women with PCOS, both in terms of their amino acid turnover as reported here and their altered capacity for glucose and pyruvate metabolism (Harris et al., 2010), merit further investigation in relation to the metabolite content of the follicular fluid and metabolic capacity of the supporting follicular cells.
The increased depletion of isoleucine and appearance of alanine by MII oocytes retrieved from normal patients who had undergone COS with rFSH compared with patients receiving hMG is of interest because cumulus cell gene expression patterns have been shown to differ between patients treated with antagonist/recombinant gonadotrophin regimens compared with agonist/hMG treatment protocols (Wathlet et al., 2011), and between rFSH and hMG agonist protocols (Adriaenssens et al., 2010). Cumulus cell protein expression profiles also differed between patients with no ovarian pathology who were treated with rFSH versus highly purified hMG (HP-hMG) and even between treatment protocols within the same patient (Hamamah et al., 2006). Furthermore, the systemic hormonal profiles differ between patients stimulated with hMG versus rFSH (Andersen et al., 2006; Smitz et al., 2007) and the use of HP-hMG resulted in a smaller number of intermediate sized follicles and less frequent bi/multifollicular development compared with patients treated with rFSH (Platteau et al., 2006). An increased number of oocytes retrieved following rFSH administration has been recorded in a number of studies (Platteau et al., 2008; Lehert et al., 2010), although the number of oocytes retrieved does not accurately predict pregnancy (Arce et al., 2005). With regard to the contribution made by LH stimulation to oocyte quality, lower apoptosis levels have been observed in cumulus cells retrieved from patients supplemented with recombinant LH (rLH) from Day 8 of stimulation compared with patients who only received rFSH throughout (Ruvolo et al., 2007). Coincidentally, fewer oocytes were retrieved and lower peak estradiol levels were reached in the rLH group although a higher number of embryos were available for transfer (Ruvolo et al., 2007). Furthermore, recent clinical data show enhanced implantation and pregnancy rates in older women (>35 years) compared with younger women when stimulated with both FSH and LH (Alviggi et al., 2009). This observation supports the idea that the ratio of FSH to LH stimulation is a determinant of oocyte quality and that alteration of the LH:FSH ratio may explain some of the observed effects of ovarian ageing on oocyte quality.
In the present study, human oocytes, immature at the time of retrieval for ICSI, were used as a model to study oocyte metabolism. These oocytes had a high potential to mature to MII over 24 h of IVM so confirming our previous observations (Clyde et al., 2003; Harris et al., 2010). The oocytes used in the present study were heterogeneous with respect to stage of maturity upon arrival in the laboratory, with ∼50% at GV and MI. Following 18 h of IVM, 38% of these oocytes had reached MII stage, but this proportion increased to 70% after 24 h. It must be emphasized that the shorter culture period used to mature the GV/MI oocytes in the present experimental series is not equivalent to the clinical IVM used to treat patients (Soderstrom-Anttila et al., 2005; Bos-Mikich et al., 2011). The immature, denuded GV/MI oocytes used here have been exposed to the ovulatory hCG stimulus in vivo some 36 h prior to oocyte recovery and, in the absence of cumulus cells, they complete meiotic progression over a shortened 24 h window. This is in marked contrast to the IVM of compact, cumulus-enclosed GV oocytes which had not been exposed to hCG in vivo (Wynn et al., 1998). Notwithstanding the differences between our model oocytes and true clinical IVM, the denuded GV/MI oocytes used in the present studies represent a valuable research tool for studying human oocyte biology (Clyde et al., 2001, 2003; Harris et al., 2010; Liu et al., 2010; van den Berg et al., 2011; Zhang et al., 2011). Furthermore, a number of babies have been born following the insemination of oocytes of this origin (Yuzpe et al., 2000; Chen and Kattera, 2003; Lombardi et al., 2003; Vanhoutte et al., 2005; DeUgarte et al., 2006; Menezo et al., 2006; Otsuki, 2006; Fauque et al., 2008). However, clinical success rates for oocytes from this source are generally very poor (Reichman et al., 2010). Indeed, karyotype analysis of such oocytes by multiparameter fluorescence in-situ hybridization in our laboratory has shown these gametes to have a high rate of predivision of chromosomes (Clyde et al., 2003), although we have failed to observe a significant link between oocyte chromosomal errors and patient aetiologies, such as PCOS (Harris et al., 2010). Other groups have also demonstrated high levels of aneuploidy in human oocytes which failed to respond correctly to the in vivo stimulus to progress to MII over the normal timeframe (Magli et al., 2006). Furthermore, such oocytes may contain imprinting defects (Borghol et al., 2006).
Conclusion
To conclude, human oocytes capable of maturing to MII stage over a 24 h period of IVM cause depletion/appearance of amino acids from the culture medium in a manner which distinguishes them from oocytes which lack the competence to progress to MII. Furthermore, the nature of the amino acid profiles obtained from oocytes with the competence to mature to MII appears to be affected by patient age, infertile diagnosis and the nature and dose of the gonadotrophin preparation used for COS. In particular, oocytes obtained from women of advancing years have a lower depletion and turnover of total amino acids compared with younger patients. These findings suggest that the measurement of amino acid turnover by individual human oocytes may provide functional insights into the biological mechanisms associated with the acquisition of oocyte developmental competence in vivo and following assisted conception.
Authors' roles
K.E.H. conducted all aspects of the laboratory research, data generation and analysis and was responsible for manuscript preparation. D.M and S.V. were involved in patient recruitment and clinical data collection both of which were conducted under the supervision and clinical governance of A.H.B. J.E.H. coordinated clinical oocyte recoveries. D.M, S.V, J.E.H and A.H.B. also contributed to the final review of the manuscript. B.K.C and H.J.L. contributed to study conception and design as well as manuscript preparation. H.M.P. was the principle investigator and holder of both research grants and was responsible for the conception and design of the study, supervision of all aspects of the laboratory research, data generation and analysis and the preparation and final approval of the manuscript.
Funding
This project was funded by the UK Biology and Biotechnology Research Council (BB/C007395/1) and the Medical Research Council (G 0800250). K.E.H was in receipt of a British Fertility Society/Merck Serono studentship. Funding to pay the Open Access publication charges for this article was provided by the UK Biology and Biotechnology Research Council and the Medical Research Council.
Conflict of interest
H.J.L. is a shareholder in Novocellus Ltd, a company which seeks to devise a non-invasive biochemical test of embryo health.
References
- Adriaenssens T, Wathlet S, Segers I, Verheyen G, De Vos A, Van der Elst J, Coucke W, Devroey P, Smitz J. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25:1259–1270. doi: 10.1093/humrep/deq049. doi:10.1093/humrep/deq049. [DOI] [PubMed] [Google Scholar]
- Alviggi C, Humaidan P, Howles CM, Tredway D, Hillier SG. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol. 2009;7:101. doi: 10.1186/1477-7827-7-101. doi:10.1186/1477-7827-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21:3217–3227. doi: 10.1093/humrep/del284. doi:10.1093/humrep/del284. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Sciorio R, Kinnell H, Bayne RAL, Thong KJ, de Sousa PA, Pickering S. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. Reproduction. 2009;138:629–637. doi: 10.1530/REP-09-0144. doi:10.1530/REP-09-0144. [DOI] [PubMed] [Google Scholar]
- Arce JC, Andersen AN, Collins J. Resolving methodological and clinical issues in the design of efficacy trials in assisted reproductive technologies: a mini-review. Hum Reprod. 2005;20:1757–1771. doi: 10.1093/humrep/deh818. doi:10.1093/humrep/deh818. [DOI] [PubMed] [Google Scholar]
- Baumann CG, Morris DG, Sreenan JM, Leese HJ. The quiet embryo hypothesis: molecular characteristics favoring viability. Mol Reprod Dev. 2007;74:1345–1353. doi: 10.1002/mrd.20604. doi:10.1002/mrd.20604. [DOI] [PubMed] [Google Scholar]
- Bettegowda A, Smith GW. Mechanisms of maternal mRNA regulation: implications for mammalian early embryonic development. Front Biosci. 2007;12:3713–3726. doi: 10.2741/2346. doi:10.2741/2346. [DOI] [PubMed] [Google Scholar]
- Booth PJ, Humpherson PG, Watson TJ, Leese HJ. Amino acid depletion and appearance during porcine preimplantation embryo development in vitro. Reproduction. 2005;130:655–668. doi: 10.1530/rep.1.00727. doi:10.1530/rep.1.00727. [DOI] [PubMed] [Google Scholar]
- Borghol N, Lornage J, Blachere T, Garret AS, Lefevre A. Epigenetic status of the H19 locus in human oocytes following in vitro maturation. Genomics. 2006;87:417–426. doi: 10.1016/j.ygeno.2005.10.008. doi:10.1016/j.ygeno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bos-Mikich A, Ferreira M, Hoher M, Frantz G, Oliveira N, Dutra CG, Frantz N. Fertilization outcome, embryo development and birth after unstimulated IVM. J Assist Reprod Genet. 2011;28:107–110. doi: 10.1007/s10815-010-9490-8. doi:10.1007/s10815-010-9490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison DR, Houghton FD, Falconer D, Roberts SA, Hawkhead J, Humpherson PG, Lieberman BA, Leese HJ. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum Reprod. 2004;19:2319–2324. doi: 10.1093/humrep/deh409. doi:10.1093/humrep/deh409. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. doi:10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod. 2003;18:2118–2121. doi: 10.1093/humrep/deg325. doi:10.1093/humrep/deg325. [DOI] [PubMed] [Google Scholar]
- Chmelikova E, Jeseta M, Sedmikova M, Petr J, Tumova L, Kott T, Lipovova P, Jilek F. Nitric oxide synthase isoforms and the effect of their inhibition on meiotic maturation of porcine oocytes. Zygote. 2010;18:235–244. doi: 10.1017/S0967199409990268. doi:10.1017/S0967199409990268. [DOI] [PubMed] [Google Scholar]
- Cillo F, Brevini TAL, Antonini S, Paffoni A, Ragni G, Gandolfi F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction. 2007;134:645–650. doi: 10.1530/REP-07-0182. doi:10.1530/REP-07-0182. [DOI] [PubMed] [Google Scholar]
- Clyde JM, Gosden RG, Rutherford AJ, Picton HM. Demonstration of a mechanism of aneuploidy in human oocytes using Multifluor fluorescence in situ hybridization. Fertil Steril. 2001;76:837–840. doi: 10.1016/s0015-0282(01)01989-6. doi:10.1016/S0015-0282(01)01989-6. [DOI] [PubMed] [Google Scholar]
- Clyde JM, Hogg JE, Rutherford AJ, Picton HM. Karyotyping of human metaphase II oocytes by Multifluor fluorescence in situ hybridization. Fertil Steril. 2003;80:1003–1011. doi: 10.1016/s0015-0282(03)01158-0. doi:10.1016/S0015-0282(03)01158-0. [DOI] [PubMed] [Google Scholar]
- Conaghan J, Hardy K, Handyside AH, Winston RML, Leese HJ. Selection criteria for human embryo transfer—a comparison of pyruvate uptake and morphology. J Assist Reprod Genet. 1993;10:21–30. doi: 10.1007/BF01204436. doi:10.1007/BF01204436. [DOI] [PubMed] [Google Scholar]
- de Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, Kupka M, Nygren KG, Andersen AN. Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Hum Reprod. 2010;25:1851–1862. doi: 10.1093/humrep/deq124. doi:10.1093/humrep/deq124. [DOI] [PubMed] [Google Scholar]
- de Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, Kupka M, Nygren KG, Andersen AN. Assisted reproductive technology in Europe, 2007: results generated from European registers by ESHRE. Hum Reprod. 2012;27:954–966. doi: 10.1093/humrep/des023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeUgarte CM, Li M, Jordan B, Hill D, DeCherney A, Surrey M. Rescue intracytoplasmic sperm injection and preimplantation genetic diagnosis in combination can result in pregnancy. Fertil Steril. 2006;86:200–202. doi: 10.1016/j.fertnstert.2006.03.021. doi:10.1016/j.fertnstert.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Wieczorek M, Lueke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11:783–796. doi: 10.1016/j.mito.2010.08.011. doi:10.1016/j.mito.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Fauque P, Guibert J, Jouannet P, Patrat C. Successful delivery after the transfer of embryos obtained from a cohort of incompletely in vivo matured oocytes at retrieval time. Fertil Steril. 2008;89:991–991. doi: 10.1016/j.fertnstert.2007.03.095. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Bianchi V, Patrizio P, Obradors A, Huang Z, Borini A, Delhanty JDA, Wells D. Transcriptomic profiling of human oocytes: association of meiotic aneuploidy and altered oocyte gene expression. Mol Hum Reprod. 2010;16:570–582. doi: 10.1093/molehr/gaq033. doi:10.1093/molehr/gaq033. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Wale PL, Collins R, Lane M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum Reprod. 2011;26:1981–1986. doi: 10.1093/humrep/der143. doi:10.1093/humrep/der143. [DOI] [PubMed] [Google Scholar]
- Gaulden ME. Maternal age effect - the enigma of down-syndrome and other trisomic conditions. Mutat Res. 1992;296:69–88. doi: 10.1016/0165-1110(92)90033-6. doi:10.1016/0165-1110(92)90033-6. [DOI] [PubMed] [Google Scholar]
- Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril. 2011;96:47–U454. doi: 10.1016/j.fertnstert.2011.04.033. doi:10.1016/j.fertnstert.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Oogenesis as a foundation for embryogenesis. Mol Cell Endocrinol. 2002;186:149–153. doi: 10.1016/s0303-7207(01)00683-9. doi:10.1016/S0303-7207(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Grondahl ML, Andersen CY, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010;25:957–968. doi: 10.1093/humrep/deq014. doi:10.1093/humrep/deq014. [DOI] [PubMed] [Google Scholar]
- Hamamah S, Matha V, Berthenet C, Anahory T, Loup V, Dechaud H, Hedon B, Fernandez A, Lamb N. Comparative protein expression profiling in human cumulus cells in relation to oocyte fertilization and ovarian stimulation protocol. Reprod Biomed Online. 2006;13:807–814. doi: 10.1016/s1472-6483(10)61028-0. doi:10.1016/S1472-6483(10)61028-0. [DOI] [PubMed] [Google Scholar]
- Hardarson T, Ahlstrom A, Rogberg L, Botros L, Hillensjo T, Westlander G, Sakkas D, Wikland M. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Reprod. 2012;27:89–96. doi: 10.1093/humrep/der373. doi:10.1093/humrep/der373. [DOI] [PubMed] [Google Scholar]
- Hardy K, Hooper MAK, Handyside AH, Rutherford AJ, Winston RML, Leese HJ. Non-invasive measurement of glucose and pyruvate uptake by individual human oocytes and preimplantation embryos. Hum Reprod. 1989;4:188–191. doi: 10.1093/oxfordjournals.humrep.a136869. [DOI] [PubMed] [Google Scholar]
- Harris SE, Picton HM. Metabolism of oocytes during growth and maturation. In: Tan SL, Chian RC, Bucket W, editors. In vitro Maturation of Human Oocytes: Basic Science to Clinical Applications. Informa Healthcare London; 2007. [Google Scholar]
- Harris SE, Maruthini D, Tang T, Balen AH, Picton HM. Metabolism and karyotype analysis of oocytes from patients with polycystic ovary syndrome. Hum Reprod. 2010;25:2305–2315. doi: 10.1093/humrep/deq181. doi:10.1093/humrep/deq181. [DOI] [PubMed] [Google Scholar]
- Hemmings KE, Leese HJ, Picton HM. Amino acid turnover by bovine oocytes provides an index of oocyte developmental competence in vitro. Biol Reprod. 2012;86:1–12. doi: 10.1095/biolreprod.111.092585. [DOI] [PubMed] [Google Scholar]
- Houghton FD, Hawkhead JA, Humpherson PG, Hogg JE, Balen AH, Rutherford AJ, Leese HJ. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod. 2002;17:999–1005. doi: 10.1093/humrep/17.4.999. doi:10.1093/humrep/17.4.999. [DOI] [PubMed] [Google Scholar]
- Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23:424–432. doi: 10.1071/RD10133. doi:10.1071/RD10133. [DOI] [PubMed] [Google Scholar]
- Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, Findlay JK, Jenkin G, Trounson AO. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod. 2008;23:1138–1144. doi: 10.1093/humrep/den085. doi:10.1093/humrep/den085. [DOI] [PubMed] [Google Scholar]
- Kenigsberg S, Bentov Y, Chalifa-Caspi V, Potashnik G, Ofir R, Birk OS. Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod. 2009;15:89–103. doi: 10.1093/molehr/gan082. doi:10.1093/molehr/gan082. [DOI] [PubMed] [Google Scholar]
- Kushnir VA, Frattarelli JL. Aneuploidy in abortuses following IVF and ICSI. J Assist Reprod Genet. 2009;26:93–97. doi: 10.1007/s10815-009-9292-z. doi:10.1007/s10815-009-9292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon NS, Nathan CF, Gilker C, Griffith OW, Matthews DE, Stuehr DJ. L-citrulline production from L-arginine by macrophage nitric oxide synthase. The ureido oxygen derives from dioxygen. J Biol Chem. 1990;265:13442–13445. [PubMed] [Google Scholar]
- Kwon H, Choi D-H, Bae J-H, Kim J-H, Kim Y-S. mRNA expression pattern of insulin-like growth factor components of granulosa cells and cumulus cells in women with and without polycystic ovary syndrome according to oocyte maturity. Fertil Steril. 2010;94:2417–2420. doi: 10.1016/j.fertnstert.2010.03.053. doi:10.1016/j.fertnstert.2010.03.053. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Selection of viable mouse blastocysts prior to transfer using a metabolic criterion. Hum Reprod. 1996;11:1975–1978. doi: 10.1093/oxfordjournals.humrep.a019527. doi:10.1093/oxfordjournals.humrep.a019527. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–849. doi: 10.1002/bies.10137. doi:10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction. 2012;143:417–427. doi: 10.1530/REP-11-0484. doi:10.1530/REP-11-0484. [DOI] [PubMed] [Google Scholar]
- Lehert P, Schertz JC, Ezcurra D. Recombinant human follicle-stimulating hormone produces more oocytes with a lower total dose per cycle in assisted reproductive technologies compared with highly purified human menopausal gonadotrophin: a meta-analysis. Reprod Biol Endocrinol. 2010;8:12. doi: 10.1186/1477-7827-8-112. doi:10.1186/1477-7827-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. The biology and dynamics of mammalian cortical granules. Reprod Biol Endocrinol. 2011;9:149. doi: 10.1186/1477-7827-9-149. doi:10.1186/1477-7827-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li Y, Gao X, Yan JH, Chen ZJ. Changes in the distribution of mitochondria before and after in vitro maturation of human oocytes and the effect of in vitro maturation on mitochondria distribution. Fertil Steril. 2010;93:1550–1555. doi: 10.1016/j.fertnstert.2009.03.050. doi:10.1016/j.fertnstert.2009.03.050. [DOI] [PubMed] [Google Scholar]
- Lombardi E, Tiveron M, Inza R, Valcarcel A, Young E, Bisioli C. Live birth and normal 1-year follow-up of a baby born after transfer of cryopreserved embryos from rescue intracytoplasmic sperm injection of 1-day-old oocytes. Fertil Steril. 2003;80:646–648. doi: 10.1016/s0015-0282(03)00996-8. doi:10.1016/S0015-0282(03)00996-8. [DOI] [PubMed] [Google Scholar]
- Lopes AS, Madsen SE, Ramsing NB, Lovendahl P, Greve T, Callesen H. Investigation of respiration of individual bovine embryos produced in vivo and in vitro and correlation with viability following transfer. Hum Reprod. 2007;22:558–566. doi: 10.1093/humrep/del404. doi:10.1093/humrep/del404. [DOI] [PubMed] [Google Scholar]
- Magli MC, Ferraretti AP, Crippa A, Lappi M, Feliciani E, Gianaroli L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil Steril. 2006;86:629–635. doi: 10.1016/j.fertnstert.2006.02.083. doi:10.1016/j.fertnstert.2006.02.083. [DOI] [PubMed] [Google Scholar]
- Mann JS, Lowther KM, Mehlmann LM. Reorganization of the endoplasmic reticulum and development of Ca(2+) release mechanisms during meiotic maturation of human oocytes. Biol Reprod. 2010;83:578–583. doi: 10.1095/biolreprod.110.085985. doi:10.1095/biolreprod.110.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhuenda-Egea FC, Gonsalvez-Alvarez R, Martinez-Sabater E, Lledo B, Ten J, Bernabeu R. Improving human embryos selection in IVF: non-invasive metabolomic and chemometric approach. Metabolomics. 2011;7:247–256. doi:10.1007/s11306-010-0245-4. [Google Scholar]
- McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, Amato P, Matzuk MM. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19:2869–2874. doi: 10.1093/humrep/deh535. doi:10.1093/humrep/deh535. [DOI] [PubMed] [Google Scholar]
- McLay DW, Carroll J, Clarke HJ. The ability to develop an activity that transfers histones onto sperm chromatin is acquired with meiotic competence during oocyte growth. Dev Biol. 2002;241:195–206. doi: 10.1006/dbio.2001.0499. doi:10.1006/dbio.2001.0499. [DOI] [PubMed] [Google Scholar]
- Menezo YJR, Nicollet B, Rollet J, Hazout A. Pregnancy and delivery after in vitro maturation of naked ICSI GV oocytes with GH and transfer of a frozen thawed blastocyst: case report. J Assist Reprod Genet. 2006;23:47–49. doi: 10.1007/s10815-005-9014-0. doi:10.1007/s10815-005-9014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy ZP, Jones-Colon S, Roos P, Botros L, Greco E, Dasig J, Behr B. Metabolomic assessment of oocyte viability. Reprod Biomed Online. 2009;18:219–225. doi: 10.1016/s1472-6483(10)60259-3. doi:10.1016/S1472-6483(10)60259-3. [DOI] [PubMed] [Google Scholar]
- Opiela J, Katska-Ksiazkiewicz L, Lipinski D, Slomski R, Bzowska A, Rynska B. Interactions among activity of glucose-6-phosphate dehydrogenase in immature oocytes, expression of apoptosis-related genes Bcl-2 and Bax, and developmental competence following IVP in cattle. Theriogenology. 2008;69:546–555. doi: 10.1016/j.theriogenology.2007.11.001. doi:10.1016/j.theriogenology.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Otsuki J. Timed IVM followed by ICSI in a patient with immature ovarian oocytes. Reprod Biomed Online. 2006;13:101–103. doi: 10.1016/s1472-6483(10)62022-6. doi:10.1016/S1472-6483(10)62022-6. [DOI] [PubMed] [Google Scholar]
- Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112:195–203. doi: 10.1007/s00439-002-0852-x. [DOI] [PubMed] [Google Scholar]
- Picton H, Briggs D, Gosden R. The molecular basis of oocyte growth and development. Mol Cell Endocrinol. 1998;145:27–37. doi: 10.1016/s0303-7207(98)00166-x. doi:10.1016/S0303-7207(98)00166-X. [DOI] [PubMed] [Google Scholar]
- Picton HM, Elder K, Houghton FD, Hawkhead JA, Rutherford AJ, Hogg JE, Leese HJ, Harris SE. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol Hum Reprod. 2010;16:557–569. doi: 10.1093/molehr/gaq040. doi:10.1093/molehr/gaq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platteau P, Andersen AN, Balen A, Devroey P, Sorensen P, Helmgaard L, Arce JC. Similar ovulation rates, but different follicular development with highly purified menotrophin compared with recombinant FSH in WHO Group II anovulatory infertility: a randomized controlled study. Hum Reprod. 2006;21:1798–1804. doi: 10.1093/humrep/del085. doi:10.1093/humrep/del085. [DOI] [PubMed] [Google Scholar]
- Platteau P, Andersen AN, Loft A, Smitz J, Danglas P, Devroey P. Highly purified HMG versus recombinant FSH for ovarian stimulation in IVF cycles. Reprod Biomed Online. 2008;17:190–198. doi: 10.1016/s1472-6483(10)60194-0. doi:10.1016/S1472-6483(10)60194-0. [DOI] [PubMed] [Google Scholar]
- Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. doi: 10.1093/humupd/dmq032. doi:10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman DE, Politch J, Ginsburg ES, Racowsky C. Extended in vitro maturation of immature oocytes from stimulated cycles: an analysis of fertilization potential, embryo development, and reproductive outcomes. J Assist Reprod Genet. 2010;27:347–356. doi: 10.1007/s10815-010-9416-5. doi:10.1007/s10815-010-9416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, Hardy K. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod. 2005;72:107–118. doi: 10.1095/biolreprod.104.032003. doi:10.1095/biolreprod.104.032003. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. doi:10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril. 2007;87:542–546. doi: 10.1016/j.fertnstert.2006.06.059. doi:10.1016/j.fertnstert.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Schon EA, Kim SH, Ferreira JC, Magalhães P, Grace M, Warburton D, Gross SJ. Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod. 2000;15:160–172. doi: 10.1093/humrep/15.suppl_2.160. doi:10.1093/humrep/15.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- Scott R, Seli E, Miller K, Sakkas D, Scott K, Burns DH. Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study. Fertil Steril. 2008a;90:77–83. doi: 10.1016/j.fertnstert.2007.11.058. doi:10.1016/j.fertnstert.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Scott L, Berntsen J, Davies D, Gundersen J, Hill J, Ramsing N. Human oocyte respiration-rate measurement - potential to improve oocyte and embryo selection? Reprod Biomed Online. 2008b;17:461–469. doi: 10.1016/s1472-6483(10)60232-5. doi:10.1016/S1472-6483(10)60232-5. [DOI] [PubMed] [Google Scholar]
- Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88:1350–1357. doi: 10.1016/j.fertnstert.2007.07.1390. doi:10.1016/j.fertnstert.2007.07.1390. [DOI] [PubMed] [Google Scholar]
- Seli E, Botros L, Sakkas D, Burns DH. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2008;90:2183–2189. doi: 10.1016/j.fertnstert.2008.07.1739. doi:10.1016/j.fertnstert.2008.07.1739. [DOI] [PubMed] [Google Scholar]
- Sherman SL, Freeman SB, Allen EG, Lamb NE. Risk factors for nondisjunction of trisomy 21. Cytogenet Genome Res. 2005;111:273–280. doi: 10.1159/000086900. doi:10.1159/000086900. [DOI] [PubMed] [Google Scholar]
- Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65:126–136. doi: 10.1016/j.theriogenology.2005.09.020. doi:10.1016/j.theriogenology.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Smitz J, Andersen AN, Devroey P, Arce JC. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod. 2007;22:676–687. doi: 10.1093/humrep/del445. doi:10.1093/humrep/del445. [DOI] [PubMed] [Google Scholar]
- Soderstrom-Anttila V, Makinen S, Tuuri T, Suikkari AM. Favourable pregnancy results with insemination of in vitro matured oocytes from unstimulated patients. Hum Reprod. 2005;20:1534–1540. doi: 10.1093/humrep/deh768. doi:10.1093/humrep/deh768. [DOI] [PubMed] [Google Scholar]
- Spindler RE, Pukazhenthi BS, Wildt DE. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol Reprod Dev. 2000;56:163–171. doi: 10.1002/(SICI)1098-2795(200006)56:2<163::AID-MRD7>3.0.CO;2-3. doi:10.1002/(SICI)1098-2795(200006)56:2<163::AID-MRD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Steeves TE, Gardner DK, Zuelke KA, Squires TS, Fry RC. In vitro development and nutrient uptake by embryos derived from oocytes of pre-pubertal and adult cows. Mol Reprod Dev. 1999;54:49–56. doi: 10.1002/(SICI)1098-2795(199909)54:1<49::AID-MRD7>3.0.CO;2-2. doi:10.1002/(SICI)1098-2795(199909)54:1<49::AID-MRD7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Stokes PJ, Hawkhead JA, Fawthrop RK, Picton HM, Sharma V, Leese HJ, Houghton FD. Metabolism of human embryos following cryopreservation: Implications for the safety and selection of embryos for transfer in clinical IVF. Hum Reprod. 2007;22:829–835. doi: 10.1093/humrep/del447. doi:10.1093/humrep/del447. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Brison DR, Leese HJ. Assessing embryo viability by measurement of amino acid turnover. Reprod Biomed Online. 2008;17:486–496. doi: 10.1016/s1472-6483(10)60234-9. doi:10.1016/S1472-6483(10)60234-9. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Hawkhead JA, Barker EA, Leese HJ. DNA damage and metabolic activity in the preimplantation embryo. Hum Reprod. 2009;24:81–91. doi: 10.1093/humrep/den346. doi:10.1093/humrep/den346. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol. 2007;302:104–117. doi: 10.1016/j.ydbio.2006.09.008. doi:10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, Day BN, Prather RS, Schatten H. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction. 2001;122:155–163. doi:10.1530/rep.0.1220155. [PubMed] [Google Scholar]
- Tejera A, Herrero J, de los Santos MJ, Garrido N, Ramsing N, Meseguer M. Oxygen consumption is a quality marker for human oocyte competence conditioned by ovarian stimulation regimens. Fertil Steril. 2011;96:618–U141. doi: 10.1016/j.fertnstert.2011.06.059. doi:10.1016/j.fertnstert.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Tesfaye D, Kadanga A, Rings F, Bauch K, Jennen D, Nganvongpanit K, Holker M, Tholen E, Ponsuksili S, Wimmers K, et al. The effect of nitric oxide inhibition and temporal expression patterns of the mRNA and protein products of nitric oxide synthase genes during in vitro development of bovine pre-implantation embryos. Reprod Domest Anim. 2006;41:501–509. doi: 10.1111/j.1439-0531.2006.00701.x. doi:10.1111/j.1439-0531.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. doi:10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Steuerwald N, Huet-Hudson YA. Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol Reprod. 2003;68:1538–1544. doi: 10.1095/biolreprod.102.009282. doi:10.1095/biolreprod.102.009282. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406. doi: 10.1093/humrep/17.2.393. doi:10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- van den Berg IM, Eleveld C, van der Hoeven M, Birnie E, Steegers EA, Galjaard RJ, Laven JS, van Doorninck JH. Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum Reprod. 2011;26:1181–1190. doi: 10.1093/humrep/der030. doi:10.1093/humrep/der030. [DOI] [PubMed] [Google Scholar]
- Vanhoutte L, De Sutter P, Van der Elst J, Dhont M. Clinical benefit of metaphase I oocytes. Reprod Biol Endocrinol. 2005;3:6. doi: 10.1186/1477-7827-3-71. doi:10.1186/1477-7827-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergouw CG, Botros LL, Roos P, Lens JW, Schats R, Hompes PGA, Burns DH, Lambalk CB. Metabolomic profiling by near-infrared spectroscopy as a tool to assess embryo viability: a novel, non-invasive method for embryo selection. Hum Reprod. 2008;23:1499–1504. doi: 10.1093/humrep/den111. doi:10.1093/humrep/den111. [DOI] [PubMed] [Google Scholar]
- Vergouw CG, Kieslinger DC, Kostelijk EH, Botros LL, Schats R, Hompes PG, Sakkas D, Lambalk CB. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012;27:2304–2311. doi: 10.1093/humrep/des175. [DOI] [PubMed] [Google Scholar]
- Voet D, Voet JG. Biochemistry. 2nd edn. New York, USA: John Wiley & Sons, Inc; 1995. [Google Scholar]
- Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril. 2012;97:1078–1084.e8. doi: 10.1016/j.fertnstert.2012.01.122. [DOI] [PubMed] [Google Scholar]
- Wathlet S, Adriaenssens T, Segers I, Verheyen G, de Velde HV, Coucke W, El RR, Devroey P, Smitz J. Cumulus cell gene expression predicts better cleavage-stage embryo or blastocyst development and pregnancy for ICSI patients. Hum Reprod. 2011;26:1035–1051. doi: 10.1093/humrep/der036. doi:10.1093/humrep/der036. [DOI] [PubMed] [Google Scholar]
- Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88:900–905. doi: 10.1016/j.fertnstert.2006.12.018. doi:10.1016/j.fertnstert.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. doi:10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- Wongsrikeao P, Otoi T, Yamasaki H, Agung B, Taniguchi M, Naoi H, Shimizu R, Nagai T. Effects of single and double exposure to brilliant cresyl blue on the selection of porcine oocytes for in vitro production of embryos. Theriogenology. 2006;66:366–372. doi: 10.1016/j.theriogenology.2005.12.001. doi:10.1016/j.theriogenology.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Wood JR, Dumesic DA, Abbott DH, Strauss JF. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. doi:10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- Wynn P, Picton HM, Krapez JA, Rutherford AJ, Balen AH, Gosden RG. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum Reprod. 1998;13:3132–3138. doi: 10.1093/humrep/13.11.3132. doi:10.1093/humrep/13.11.3132. [DOI] [PubMed] [Google Scholar]
- Yurttas P, Vitale AM, Fitzhenry RJ, Cohen-Gould L, Wu WZ, Gossen JA, Coonrod SA. Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo. Development. 2008;135:2627–2636. doi: 10.1242/dev.016329. doi:10.1242/dev.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzpe AA, Liu ZS, Fluker MR. Rescue intracytoplasmic sperm injection (ICSI) - salvaging in vitro fertilization (IVF) cycles after total or near-total fertilization failure. Fertil Steril. 2000;73:1115–1119. doi: 10.1016/s0015-0282(00)00522-7. doi:10.1016/S0015-0282(00)00522-7. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Jafari N, Barnes RB, Confino E, Milad M, Kazer RR. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril. 2005;83:1169–1179. doi: 10.1016/j.fertnstert.2004.11.030. doi:10.1016/j.fertnstert.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Liu Y, Xing Q, Zhou P, Cao YX. Cryopreservation of human failed-matured oocytes followed by in vitro maturation: vitrification is superior to the slow freezing method. Reprod Biol Endocrinol. 2011;9:6. doi: 10.1186/1477-7827-9-156. doi:10.1186/1477-7827-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]