Abstract
Context
A 2009 Institute of Medicine report recommended protected sleep periods for medicine trainees on extended overnight shifts, a position reinforced by new Accreditation Council for Graduate Medical Education requirements.
Objective
To evaluate the feasibility and consequences of protected sleep periods during extended duty.
Design, Setting, and Participants
Randomized controlled trial conducted at the Philadelphia VA Medical Center medical service and Oncology Unit of the Hospital of the University of Pennsylvania (2009–2010). Of the 106 interns and senior medical students who consented, 3 were not scheduled on any study rotations. Among the others, 44 worked at the VA center, 16 at the university hospital, and 43 at both.
Intervention
Twelve 4-week blocks were randomly assigned to either a standard intern schedule (extended duty overnight shifts of up to 30 hours; equivalent to 1200 overnight intern shifts at each site), or a protected sleep period (protected time from 12:30 AM to 5:30 AM with handover of work cell phone; equivalent to 1200 overnight intern shifts at each site). Participants were asked to wear wrist actigraphs and complete sleep diaries.
Main Outcome Measures
Primary outcome was hours slept during the protected period on extended duty overnight shifts. Secondary outcome measures included hours slept during a 24-hour period (noon to noon) by day of call cycle and Karolinska sleepiness scale.
Results
For 98.3% of on-call nights, cell phones were signed out as designed. At the VA center, participants with protected sleep had a mean 2.86 hours (95% CI, 2.57–3.10 hours) of sleep vs 1.98 hours (95% CI, 1.68–2.28 hours) among those who did not have protected hours of sleep (P < .001). At the university hospital, participants with protected sleep had a mean 3.04 hours (95% CI, 2.77–3.45 hours) of sleep vs 2.04 hours (95% CI, 1.79–2.24) among those who did not have protected sleep (P <.001). Participants with protected sleep were significantly less likely to have call nights with no sleep: 5.8% (95% CI, 3.0%–8.5%) vs 18.6% (95% CI, 13.9%–23.2%) at the VA center (P <.001) and 5.9% (95% CI, 3.1%–8.7%) vs 14.2% (95% CI, 9.9%–18.4%) at the university hospital (P=.001). Participants felt less sleepy after on-call nights in the intervention group, with Karolinska sleepiness scale scores of 6.65 (95% CI, 6.35–6.97) vs 7.10 (95% CI, 6.85–7.33; P=.01) at the VA center and 5.91 (95% CI, 5.64–6.16) vs 6.79 (95% CI, 6.57–7.04; P <.001) at the university hospital.
Conclusions
For internal medicine services at 2 hospitals, implementation of a protected sleep period while on call resulted in an increase in overnight sleep duration and improved alertness the next morning.
Trial Registration
clinicaltrials.gov Identifier: NCT00874510.
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) adopted duty hour standards for all specialties.1 The preponderance of the evidence suggested that this did not result in any systematic improvement or worsening in patient outcomes.2–6 Six years later, the Institute of Medicine (IOM) published a report on resident work hours and work schedules to improve patient safety that recommended a protected sleep period of 5 hours during any work shift longer than 16 hours to reduce the risk of fatigue-related errors when residents are in the hospital for prolonged duty periods of up to 30 hours.7 The IOM report acknowledged that there was a paucity of data on optimizing duty hours for physicians in training but argued that the evidence on the hazards of fatigue-related performance errors in other professions likely extended to medicine.
In response, the ACGME revised the duty hour standards effective July 2011, mandating that duty periods for residents in postgraduate year 1 (interns) may not exceed 16 hours. Although residents in year 2 and beyond may be scheduled for a maximum of 24 hours of continuous duty, the ACGME strongly encourages use of alertness-management strategies, including “strategic napping, especially after 16 hours of continuous duty and between 10 p.m. and 8 a.m.”8 However, naps during prolonged duty shifts have been subject to very limited testing, leading some to question the viability of protected sleep periods for widespread implementation in health care settings.9,10
To determine whether a protected sleep period of 5 hours is feasible and effective in increasing the time slept by interns on extended duty overnight shifts, we conducted 2 randomized controlled trials in parallel: one at the Philadelphia Veterans Affairs Medical Center, the other at the Hospital of the University of Pennsylvania. We examined the effect of a protected sleep period on interns’ sleep, measures of behavioral alertness, and patient outcomes.
METHODS
Study Population
Figure 1 shows the flow of participants through the trial at each institution. Between July 2009 and June 2010, a total of 74 interns and 32 senior medical student subinterns (henceforth all referred to as interns) were randomized to serve on either an internal medicine rotation at the Philadelphia VA Medical Center, on the oncology unit of the Hospital of the University of Pennsylvania, or at both institutions, with subinterns only serving at the VA center. The unit of analysis was intern day. The rate of exclusions (eg, not wearing an actigraph) was similar between groups. All internal medicine interns were invited to participate, with no exclusions.
Figure 1.
Flow Diagram at Participating Institutions
Interns may have been randomized to either study group from one rotation to another. Subinterns represent fourth-year medical students.
Study Protocol
Institutional review board approval was obtained from the Philadelphia VA Medical Center, and all interns gave informed consent prior to participation. Interns met with study staff on the first day of each 4-week rotation for orientation to receive instructions on study measures, including wearing a wrist actigraph with accelerometer and light sensors (Actiwatch Spectrum, Philips Respironics)for continuously tracking rest-activity patterns. Data from the actigraphs were collected in 1-minute epochs and stored in the watch until downloaded at the end of each rotation. Similar actigraphy devices have been validated and applied to successfully study sleep patterns in physicians on night call.10,11
Study participants were also asked to complete a validated 3-minute psycho-motor vigilance test (PVT)12,13 on designated laptops located throughout the service each morning between 6 AM and 8 AM when they were at the hospital and every night they were on call between 11 PM and midnight. The PVT measured alertness based on reaction time to stimuli presented at random 2- to 5-second interstimulus intervals. We used response speed (reciprocal response time) and the number of lapses (response times, ≥355 ms) as our primary PVT outcome variables.14
Participants also filled out electronic sleep logs, which contained questions about patient load and sleep disruptions as well as the Karolinska sleepiness scale (KSS),15 a 9-point verbally anchored scale ranging from “very alert” (score, 1) to “very sleepy, great effort to keep awake, fighting sleep” (score, 9). Interns self-reported the proportion of time their sleep was disturbed in sleep diaries the morning postcall. In the last 3 days of the rotation, research assistants met with the interns to collect the actigraphs and distribute $25 gift cards for every week of adherence to study measures.
Monthly Schedule and Randomization
To ensure that improvements in intern efficiency did not bias results, the intern year was divided into twelve 4-week blocks. Within each of 6 pairs of 4-week blocks, 1 block was randomized to an intervention schedule or the standard schedule. Participants underwent the assigned condition for all 4 weeks they were on rotation, with data collection planned from Monday of the first week through Friday of the final week (25 days). Interns’ schedules were assigned independently of the study, and randomization allocations of each 4-week period were concealed from participants at the time of consent. Due to the nature of the intervention, blinding of staff administering the intervention could not be maintained, even though the statistical analysts and investigators were kept blinded until the data were thoroughly analyzed. As part of the consent process, interns were informed that the purpose of the study is to examine whether a protected sleep program can significantly increase the amount of sleep interns attain while on overnight extended duty shifts.
In the standard schedule months, the Internal Medicine Service at the VA center had 4 teams, each with 1 resident and 2 interns or 1 intern and 1 subintern. Each pair of interns was on-call with a night-float resident every fourth night. On-call interns admitted patients throughout the night, were responsible for cross-coverage until the primary team returned around 7 AM, and generally worked until approximately 1 PM the next day (a 30-hour duty period). The oncology service was staffed using a similar model, but there were no medical student substitutions for interns.
On the intervention months, the protected time was between 12:30 AM and 5:30 AM. Interns were required to hand over hospital-provided work cell phones to the covering night float resident and encouraged to sleep in a bed in a dark quiet room. If urgent assistance was required, night float residents were instructed to wake the interns. Given the higher acuity for the oncology service, an extra resident was scheduled to assist with cross-coverage and admissions from 11 PM to 7 AM. On both services, the regularly scheduled night-float resident received a $500 a week participation payment. The extra covering residents at the university hospital who agreed to 1 less week of elective time to make this intervention feasible were given a participation payment of $1000 a week.
Outcome Measures
The primary outcome was sleep time during the 5-hour protected sleep period as measured by actigraph wrist activity monitors and supplemented by self-reported electronic sleep diaries. We assessed how the primary outcome varied in accordance with self-reported numbers of patients admitted on call and number of patients the intern was primarily responsible for as of the postcall morning.
Secondary outcomes were sleep time during the entire overnight extended duty shift, mean sleep amount within the 4-day call cycle, percentage of on-call nights without sleep, length of time awake before sleep onset during or after overnight call shifts, response speed and number of lapses on the PVT in the morning postcall (interns were asked to complete this between 6 AM and 8 AM), subjective reports of sleepiness (KSS score), and whether sleep during on-call nights was disturbed by at least 1 of the following: cell phone or pager, resident or attending, physical discomfort, nurse, or worry. To address feasibility, night float residents were called each morning by study staff to report time of work cell-phone hand-off and retrieval.
We also examined patient outcomes related to length of stay, transfer to the medical intensive care unit, 30-day readmission rates, and mortality among patients who were newly admitted to the studied services. Patient care data came from the university hospital patient file and the VA patient treatment file. Readmissions were tracked only for patients admitted back to the study services at either institution. Patient comorbidities were identified using Comorbidity Software, version 3.6 from the Healthcare Cost and Utilization Project.16
Statistical Analyses
The primary analyses were unadjusted intent-to-treat analyses testing for differences between the intervention and control groups at each of the 2 sites, analyzing each site as a separate trial. The unit of analysis was the participant (intern) day. To account for the correlation among a participant’s multiple observations (days), all analyses used Huber-White robust standard errors with the participants as the clusters.17 Unadjusted differences in amount slept during protected times were estimated and compared with analyses adjusted for month of the year and baseline covariates such as intern age and sex. The similarity of the treatment groups with respect to covariates at baseline was analyzed by the χ2 test for categorical variables and the t test for continuous variables. We conducted t tests or χ2 tests on the differences in patient outcomes between control months and intervention months.
When actigraph data were missing, we used self-reported sleep over the missing time period if available. When participants were missing both acti-graph and self-reported data, we used multiple imputation to estimate plausible values for the missing data in a way that accounts for the uncertainty about the missing data and provides valid inferences under the assumption that the data are missing at random.18 Details are provided in supplementary materials. We performed 2 sensitivity analyses to determine robustness of results to assumptions about missing data by using only days that contained no missing data and by assuming all missing data were sleep or all missing data were no sleep, which provides bounds on the effect of the intervention.
Psychomotor vigilance test behavioral alertness outcomes (response speed and number of lapses) were analyzed between 6 AM and 8 AM on the first day postcall. Multiple imputation using a variance stabilizing transformation of the square root of the number of lapses19 was used for missing PVT outcomes (see the eAppendix, available at http://www.jama.com).
We explored differences in the effect of protected sleep periods in several subgroups: intern type (subinterns, non–subinterns); the number of patients admitted (0–1, 2–3, 4 or 5, >5), and the number of patients for whom each intern was primarily responsible (0–4, 5–6, 7–8, 9–10, >10). The homogeneity of the association (ie, interaction) between the group assignment and sleep during on-call periods across subgroups was assessed using a Wald test in a regression analysis. Within each subgroup, the difference in amount slept between the intervention and control groups was compared using a t test on the appropriate coefficient in the regression analyses.
Based on a conservative estimate of an intern’s standard deviation of on-call sleep per month, we calculated that there was 90% power to detect a 30-minute difference in the average on-call sleep between the intervention and control at each site, which is a meaningful increase for recovery benefits from sleep.20 No interim analyses were planned or conducted. Our study was not powered to detect small differences in patient outcomes, and analyses of secondary outcomes were exploratory. For example, at the VA the control readmission rate is 9.4%, and we would have 90% power to detect either an increase in the readmission rate in the intervention group to 13.6% or a decrease in the readmission rate to 5.9%. All reported P values are 2 sided and were not adjusted for multiple comparisons; the a priori level of significance for hypothesis testing was .05. All statistical analyses were conducted with SAS version 9.3 (SAS Institute Inc).
RESULTS
Participation rates among interns were high at 100% for each group, with all 87 interns at the VA center and all 59 interns at the university hospital scheduled to be on these rotations consenting to participate. Characteristics of study participants were balanced between control and intervention (Table 1). Among 44 interns scheduled to rotate at the VA center only, 84% rotated once (all 32 subinterns only rotated once) and the rest rotated twice. Among 16 interns scheduled to rotate at the university hospital only, 38% rotated once and the rest rotated twice. Among 43 interns who rotated at both sites, 77% rotated 3 times or more.
Table 1.
Characteristics of Control and Intervention Groups
| No. (%) of Participants
|
||||
|---|---|---|---|---|
| Philadelphia VA Medical Center
|
Hospital of the University of Pennsylvania
|
|||
| Control | Intervention | Control | Intervention | |
| No. of interns studieda | 52 | 46 | 36 | 39 |
|
| ||||
| No. of intern-days studied | 1089 | 1127 | 1073 | 1116 |
|
| ||||
| Intern-days studied, %b | 90.8 | 93.9 | 89.4 | 93.0 |
|
| ||||
| Age, mean (SD), y | 27.5 (2.3) | 27.3 (2.3) | 28.1 (2.2) | 27.7 (2.0) |
|
| ||||
| Men | 25 (48.1) | 22 (47.8) | 18 (50.0) | 22 (56.4) |
|
| ||||
| Subinterns | 16 (30.8) | 16 (34.8) | 0 | 0 |
|
| ||||
| No. of patients admitted on-call (% intern d)c | ||||
| 0–1 | 6 (2.8) | 11 (4.7) | 26 (13.5) | 29 (15.2) |
|
| ||||
| 2–3 | 56 (26.5) | 61 (25.8) | 68 (35.2) | 65 (34.0) |
|
| ||||
| 4 | 56 (26.5) | 73 (30.9) | 43 (22.3) | 44 (23.0) |
|
| ||||
| 5 | 77 (36.5) | 76 (32.2) | 42 (21.8) | 39 (20.4) |
|
| ||||
| >5 | 16 (7.6) | 15 (6.4) | 14 (7.3) | 14 (7.3) |
|
| ||||
| No. of patients responsible for (% intern d)c | ||||
| 0–4 | 9 (4.3) | 22 (9.3) | 14 (7.3) | 18 (9.4) |
|
| ||||
| 5–6 | 42 (19.9) | 56 (23.7) | 27 (14.0) | 46 (24.1) |
|
| ||||
| 7–8 | 97 (46.0) | 103 (43.6) | 71 (36.8) | 62 (32.5) |
|
| ||||
| 9–10 | 60 (28.4) | 55 (23.3) | 74 (38.3) | 64 (33.5) |
|
| ||||
| >10 | 3 (1.4) | 0 | 7 (3.6) | 1 (0.5) |
Individual interns may have participated at more than 1 site or more than 1 rotation at each site.
Measured as percent intern-days studied out of total possible intern-days intended for data collection.
For Philadelphia VA Medical Center, this was not reported on 21.6% (control) and 15.1% (intervention) intern-days; for Hospital of the University of Pennsylvania, this was not reported on 26.1% (control) and 29.5% (intervention) intern-days. Distributions are based on intern-days when these data were reported.
For 98.3% of intern on-call nights, cell phones were signed out to residents as designed. Participants had missing actigraph data for 4.1% of the total study time on intervention and 5.9% on control at the university hospital, and 3.8% on intervention and 9.2% on control at the VA center. Self-reported sleep time was highly correlated with actigraph recorded sleep time (Pearson correlation, 0.81) and the mean duration of episodes of actigraph recorded sleep time was similar to episodes of self-reported sleep time (mean, 5.6 hours vs 5.8 hours, respectively). Both actigraph and self-reported data were missing for 2.8% of participants in the control group and 1.3% in the intervention group at the university hospital and for 3.3% in the control group and 1.5% in the intervention group at the VA center.
The mean sleep time during the protected period at the VA center was 2.86 hours (95% CI, 2.57–3.10) hours in the intervention months compared with 1.98 (95% CI, 1.68–2.28) in the control months (P <.001; Table 2). At the university hospital, the mean sleep time was 3.04 hours (95% CI, 2.77–3.45 hours) in the intervention months and 2.04 hours (95% CI, 1.79–2.24 hours) in the control group (P <.001).
Table 2.
Sleep Time, Psychomotor Vigilance Test Performance, and Sleepiness Summary
| Philadelphia VA Medical Center
|
Hospital of the University of Pennsylvania
|
|||||
|---|---|---|---|---|---|---|
| Mean (95% CI)a
|
Mean (95% CI)a
|
|||||
| Control | Intervention | P Value | Control | Intervention | P Value | |
| Protected period (12:30 AM-5:30 AM) Sleep time, hb | 1.98 (1.68–2.28) | 2.86 (2.57–3.10) | <.001 | 2.04 (1.79–2.24) | 3.04 (2.77–3.45) | <.001 |
|
| ||||||
| Sleep time distribution, h, % | ||||||
| 0 | 18.6 | 5.8 | <.001 | 14.2 | 5.9 | .001 |
|
| ||||||
| 0–1 | 13.4 | 7.2 | .02 | 11.5 | 5.9 | .02 |
|
| ||||||
| >1–2 | 22.3 | 13.7 | .009 | 24.1 | 12.6 | <.001 |
|
| ||||||
| >2–3 | 15.2 | 21.9 | .04 | 24.1 | 20.7 | .34 |
|
| ||||||
| >3–4 | 18.2 | 26.3 | .02 | 16.1 | 23.6 | .03 |
|
| ||||||
| >4–5 | 12.3 | 25.2 | <.001 | 10.0 | 31.4 | <.001 |
|
| ||||||
| Sleep time by day of call cycle, hb | ||||||
| Day 1, on-call | 2.54 (2.16–2.88) | 3.23 (2.92–3.49) | .003 | 2.45 (2.17–2.68) | 3.27 (2.97–3.70) | <.001 |
|
| ||||||
| Day 2, postcall | 9.54 (8.96–10.03) | 9.64 (9.14–10.07) | .89 | 9.97 (9.32–10.49) | 9.18 (8.70–9.67) | .14 |
|
| ||||||
| Day 3 | 7.29 (6.98–7.54) | 7.29 (6.91–7.62) | .88 | 7.17 (6.75–7.53) | 6.95 (6.66–7.20) | .54 |
|
| ||||||
| Day 4, precall | 7.38 (7.13–7.63) | 7.35 (7.01–7.63) | .75 | 7.18 (6.85–7.46) | 6.94 (6.69–7.18) | .35 |
|
| ||||||
| Days 1–4c | 6.68 (6.47–6.90) | 6.79 (6.55–7.03) | .49 | 6.72 (6.47–6.98) | 6.45 (6.23–6.67) | .12 |
|
| ||||||
| Psychomotor vigilance test performance | ||||||
| Attentional lapses, No. | 5.42 (4.11–6.69) | 4.11 (2.82–5.45) | .05 | 4.83 (3.68–5.82) | 4.68 (3.00–5.58) | .11 |
|
| ||||||
| Response speed (1/RT)d | 3.87 (3.76–4.02) | 4.06 (3.90–4.20) | .02 | 3.90 (3.80–4.03) | 3.97 (3.87–4.13) | .02 |
|
| ||||||
| Karolinksa sleepiness scale valuee | 7.10 (6.85–7.33) | 6.65 (6.35–6.97) | .01 | 6.79 (6.57–7.04) | 5.91 (5.64–6.16) | <.001 |
|
| ||||||
| Sleep disturbed, %f | 84.6 (78.3–90.9) | 49.9 (40.2–58.6) | <.001 | 95.1 (90.9–99.0) | 53.3 (42.0–60.0) | <.001 |
Abbreviation: RT, response time.
We reported the robust confidence intervals that accounted for the repeated measures using generalized estimating equations.
Measured as sum of sleep time in 24-hour period from 12 PM to 12 PM unless specified otherwise.
Calculated using only days from complete 4-day cycles.
Responses per second.
The Karolinksa sleepiness scale ranges from 1, very alert, to 9, very sleepy, great effort to keep awake, fighting sleep
Participants indicated whether their sleep was disturbed by at least 1 of the following: cell phone or pager, resident or attending, physical discomfort, nurse, worry, or other.
Of the interns at the VA center with shifts with protected sleep periods, 5.8% (95% CI, 3.0%–8.5%) had no sleep during on-call nights vs 18.6% (95% CI, 13.9%–23.2%) who did not (P <.001). At the university hospital, the difference was 5.9% (95% CI, 3.1%–8.7%) vs 14.2% (95% CI, 9.9%–18.4%; P =.001). In contrast, 25.2% (95% CI, 20.1%–30.3%) of interns during the intervention months slept 4 to 5 hours during the protected period compared with 12.3% (95% CI, 8.4%–16.2%) during control months at the VA center (P <.001) and 31.4% (95% CI, 25.8%–36.9%) vs 10.0% (95% CI, 6.3%–13.6%) at the university hospital (P <.001). Interns in both groups slept similar amounts on days of the call cycle other than the on–call day, resulting in mean sleep times across the 4 days of the call cycle of 6.79 hours (95% CI, 6.55–7.03 hours) vs 6.68 hours (95% CI, 6.47– 6.90 hours) at the VA center (P =.49) and a mean of 6.45 hours (95% CI, 6.23–6.67 hours) vs 6.72 hours (95% CI, 6.47–6.98 hours) at the university hospital (P =.12).
Interns in the intervention months at both sites were significantly less likely to report sleep disturbances if they were able to sleep during on-call nights: 49.9% (95% CI, 40.2%–58.6%) vs 84.6% (95% CI, 78.3%–90.9%) at the VA medical center reported disturbed sleep (P <.001) and 53.3% (95% CI, 42.0%–60.0%) vs 95.1% (95% CI, 90.9%–99.0%) reported disturbed sleep at the university hospital (P <.001).
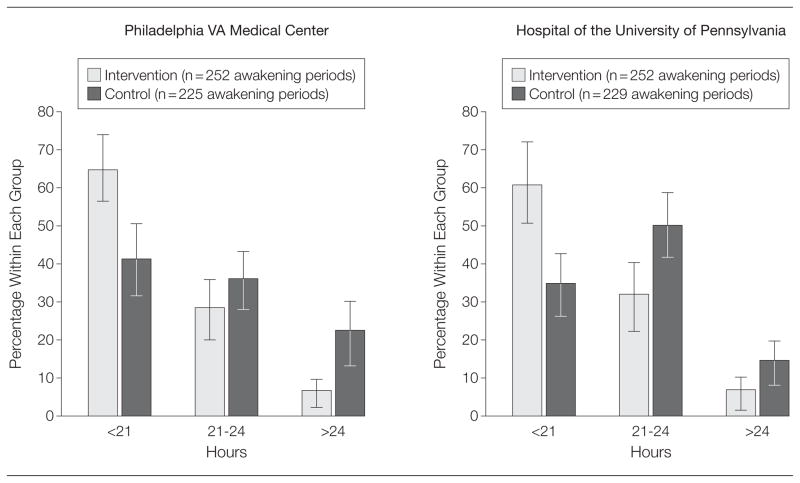
Reported sleep disturbances among interns with protected sleep periods may have been primarily attributable to factors such as stress and physical discomfort because in this group, the rate of sleep disturbance from cell phone or pagers or from being awakened by nurses was only 4.2% at the university hospital and 7.4% at the VA center, whereas 82.6% of the control group at the university hospital and 61.5% at the VA center cited these reasons for disruption of sleep. Interns during the intervention months at both sites were significantly less likely to be awake for more than 24 hours (Figure 2). Participants felt less sleepy after on-call nights in the intervention group with a mean KSS score of 6.65 (95% CI, 6.35–6.97) vs 7.10 (95% CI, 6.85–7.33) at the VA center (P =.01) and 5.91 (95% CI, 5.64–6.16) vs 6.79 (95% CI, 6.57– 7.04) at the university hospital (P <.001).
Figure 2.
Total Continuous Time Awake Among Participants
Hours awake include the last wake-up before an on-call shift to the first sleep during or after an on-call shift. The unit of this analysis is the awakening period that starts before the precall and ends during or after the postcall. The measurement is duration in hours of these awakening periods. Error bars indicate 95% CIs.
The results were qualitatively similar when we conducted sensitivity analyses in which we handled missing data in different ways and in models that adjusted for the day of the call cycle and the interactions between intervention month and day of call cycle, age, sex, day of the week, previous rotation, and month. For interns who had multiple rotations, the effect of the protected sleep period did not vary based on their previous (control or intervention) experience (eTable 1, available at http:www.jama.com).
In the VA center, the rate of nonad-herence to PVT testing was 21.3% in intervention and 30.5% in control groups. In the university hospital, the nonadherence rate was 37.5% in intervention and 36.5% in control groups. Participants performed the PVT on average at 7:54 AM (10.1% before 6 AM, 61.5% between 6 AM and 8 AM, 28.4% after 8 AM). At both study sites, the mean PVT response speed was faster in the intervention group than in the control group: 4.06 s−1 (95% CI, 3.90–4.20 s−1) vs 3.87 s−1 (95% CI, 3.76–4.02 s−1) for the VA center (P =0.02) and 3.97 s−1 (95% CI, 3.87–4.13 s−1) vs 3.90 s−1 (95% CI, 3.80–4.03 s−1) for the university hospital (P =.02). The intervention group participants had fewer attentional lapses, albeit statistically significant only at the VA medical center, which had a mean score of 4.11 s−1 (95% CI, 2.82–5.45 s−1) vs 5.42 s−1 (95% CI, 4.11, 6.69 s−1; P =.048). The mean score was 4.68 s−1 (95% CI, 3.00–5.58 s−1) vs 4.83 s−1 (95% CI, 3.68–5.82 s−1) for the university hospital (P =.11; Table 2).
We identified a total of 2086 patients (2419 new admissions) admitted to the VA center medical service and 571 patients (713 new admissions) admitted to the oncology unit of the university hospital. There was no systematic pattern of differences between intervention and control in any of the outcome measures including length of stay. The university hospital had a mean of 6.6 days (95% CI, 6.1–7.2 days) for the control group and 6.5 days (95% CI, 6.0–7.1 days) in the intervention group (P =.81); the VA center had a mean of 4.7 days (95% CI, 4.5–4.9 days) for the control group and 4.5 days (95% CI, 4.3–4.7 days) for the intervention group (P =.32). At the university hospital the medical intensive care unit transfer rates were a mean 4.0% (95% CI, 2.0%–6.1%) for the control group vs 4.9% (95% CI, 2.7%–7.2%) for the intervention group (P =.55); at the VA center, 5.5% (95% CI, 4.2%–6.8%) for the control group vs 5.0% (95% CI, 3.7%–6.2%) for the intervention group (P =.57).
The in-hospital deaths (ie, total inhospital death rate) for the hospital university was a mean 2.6% (95% CI, 0.9%–4.2%) for the control group vs 2.7% (1.1%–4.4%) for the intervention group (P =.89). The in-hospital deaths in the VA center was a mean 1.3% (95% CI, 0.7%–2.0%) for the control group vs 1.2% (95% CI, 0.6%–1.9%) for the intervention group (P =.82). The mean time to death for the university hospital was 15.7 days (95% CI, 3.7–27.7 days) for the control group vs 18.3 days (95% CI, 3.9–32.7 days) for the intervention group (P =.76) and for the VA center was a mean 15.1 days (95% CI, 7.7–22.5 days) for the control group vs 17.7 days (95% CI, 9.2–26.3 days) for the intervention group (P =.62). The mean 30-day readmission rates for the university hospital was 22.1% (95% CI, 17.7%–26.4%) for the control group vs 26.9% (95% CI, 22.4%–31.5%) for the intervention group (P =.13); for the VA center, the mean rate was 9.4% (95% CI, 7.8%–11.1%) for the control group vs 10.6% (95% CI, 8.8%–12.3%) for the intervention group (P =.35; Table 3).
Table 3.
Patient Characteristics and Outcomes by Group
| Philadelphia VA Medical Center
|
Hospital of the University of Pennsylvania
|
|||||
|---|---|---|---|---|---|---|
| Mean (95% CI)
|
Mean (95% CI)
|
|||||
| Control | Intervention | P Value | Control | Intervention | P Value | |
| No. of patients | 1035 | 1051 | 280 | 291 | ||
|
| ||||||
| No. of total admissions | 1198 | 1221 | 349 | 364 | ||
|
| ||||||
| Age | 66.3 (65.6 to 67.0) | 66.3 (65.6 to 67.1) | .90 | 56.0 (54.5 to 57.5) | 56.1 (54.7 to 57.6) | .89 |
|
| ||||||
| Men, No. (%) | 1145 (95.6) | 1169 (95.7) | .84 | 189 (54.2) | 199 (54.7) | .89 |
|
| ||||||
| Comorbidities | 2.74 (2.66 to 2.83) | 2.80 (2.72 to 2.89) | .31 | 2.63 (2.47 to 2.79) | 2.56 (2.40 to 2.72) | .52 |
|
| ||||||
| Length of stay, d | ||||||
| Study floors | 4.7 (4.5 to 4.9) | 4.5 (4.3 to 4.7) | .32 | 6.6 (6.1 to 7.2) | 6.5 (6.0 to 7.1) | .81 |
|
| ||||||
| Hospital, total | 6.7 (6.3 to 7.2) | 6.3 (5.9 to 6.7) | .19 | 12.0 (8.5 to 15.5) | 10.7 (8.9 to 12.6) | .53 |
|
| ||||||
| Transfers/readmissions | ||||||
| Transferred to MICU, % (95% CI) | 5.5 (4.2 to 6.8) | 5.0 (3.7 to 6.2) | .57 | 4.0 (2.0 to 6.1) | 4.9 (2.7 to 7.2) | .55 |
|
| ||||||
| 30 d readmission rate, % (95% CI) | 9.4 (7.8 to 11.1) | 10.6 (8.8 to 12.3) | .35 | 22.1 (17.7 to 26.4) | 26.9 (22.4 to 31.5) | .13 |
|
| ||||||
| Days to readmission | 12.9 (11.3 to 14.4) | 12.4 (10.9 to 13.9) | .67 | 18.6 (17.0 to 20.2) | 19.7 (18.5 to 20.9) | .28 |
|
| ||||||
| Deaths | ||||||
| Died on floors of study, No. (%) | 5 (0.4) | 5 (0.4) | .98 | 2 (0.6) | 3 (0.8) | >.99 |
|
| ||||||
| Time to death, d | 12.2 (4.3 to 20.1) | 23.4 (−0.9 to 47.7) | .26 | 22.0 (−54.2 to 98.2) | 19.7 (−54.2 to 93.5) | .92 |
|
| ||||||
| Died in MICU, No. (%) | 11 (0.9) | 9 (0.7) | .62 | 6 (1.7) | 6 (1.6) | .94 |
|
| ||||||
| Time to death, d | 16.5 (5.5 to 27.4) | 15.2 (4.5 to 25.9) | .86 | 15.5 (−3.7 to 34.7) | 19.8 (0.7 to 39.0) | .69 |
|
| ||||||
| Total in-hospital death rate, % (95% CI) | 1.3 (0.7 to 2.0) | 1.2 (0.6 to 1.9) | .82 | 2.6 (0.9 to 4.2) | 2.7 (1.1 to 4.4) | .89 |
|
| ||||||
| Time to death, d | 15.1 (7.7 to 22.5) | 17.7 (9.2 to 26.3) | .62 | 15.7 (3.7 to 27.7) | 18.3 (3.9 to 32.7) | .76 |
Abbreviation: MICU, medical intensive care unit.
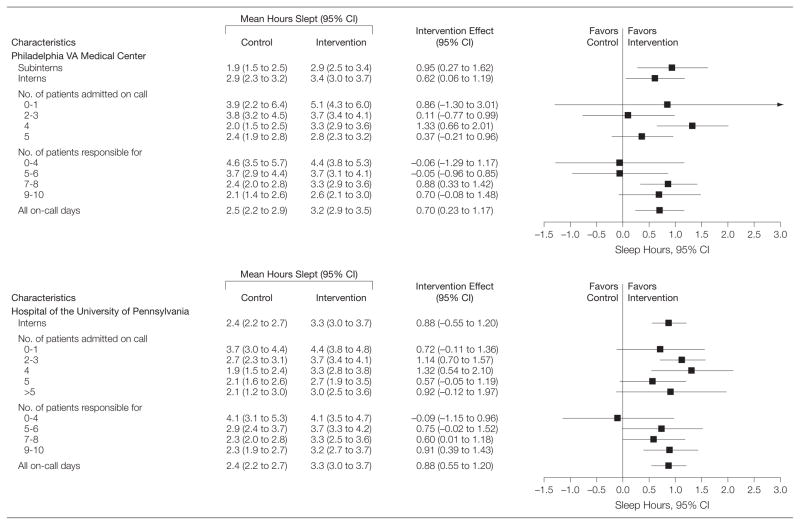
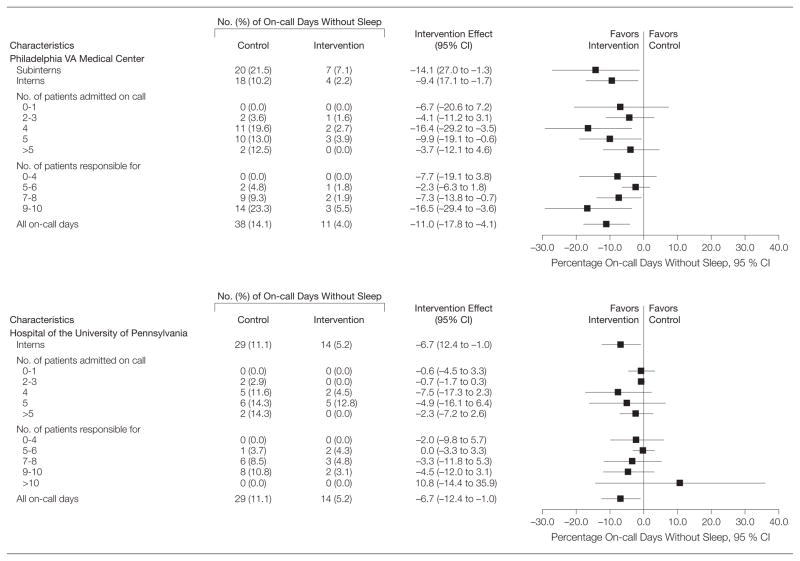
Observed differences in mean time slept during the extended duty shifts demonstrate significant increases in amount slept in most intervention subgroups relative to the standard shift (Figure 3). The mean differences in hours slept between the intervention and control groups was largest when a moderate number of patients were admitted on call (4 at the VA center and 2–4 at the university hospital). The mean differences in amount slept grew larger with the number of patients for whom interns were primarily responsible at the university hospital (test for homogeneity of association, P = .001); the relationship was not significant at the VA center (P = .24). Similar patterns were observed in terms of the percentage of on-call days without any sleep (Figure 4).
Figure 3.
Effects of Intervention on Hours Slept During On-call
Hours slept during on-call shifts are measured as the sum of sleep time in hours during a 24-hour period from midnight to midnight. We report the robust CIs that accounted for the repeated measures using generalized estimating equations. The figure shows the mean effect of the intervention on sleep for each of the subgroups for which a positive number indicates that participants slept more during intervention months than during the control months. The CIs for number of patients admitted and for the number of patients for whom the interns and medical students (subinterns; fourth-year students) were responsible were calculated using the method suggested by Agresti and Caffo21 of adding 4 pseudo observations to the intervention and control groups, half with no sleep and half with sleep. The Agresti and Caffo study show that the 95% CIs resulting from inverting large sample Wald tests often have lower coverage probability than the intended 95% and that adding the 4 pseudo observations before calculating the CIs generally brings the coverage probability close to 95%.
Figure 4.
Percentage of On-call Days Without Sleep
Hours slept during on-call shifts are measured as the sum of sleep time in hours during a 24-hour period from midnight to midnight. We report the robust CIs that accounted for the repeated measures using generalized estimating equations. The proportion of on-call days without sleep for which a reduction in this proportion is seen as beneficial. For calculating CIs, see the Figure 3 legend. Subinterns represent fourth-year medical students.
COMMENT
In the past decade, the ACGME has twice modified duty hour standards for physicians in training, with these changes motivated by an interest in reducing fatigue among house staff, reducing the rate of medical errors, and improving quality of care. The most recent duty hour standards, implemented in July 2011, were motivated by a 2009 congressionally mandated IOM report7(p1):
Based on its review of the scientific evidence, the committee recognized that it should focus on increasing opportunities for sleep during resident training to prevent acute and chronic sleep deprivation and to minimize fatigue-related errors, rather than on simply reducing total duty hours. It recommends a protected sleep period of 5 hours during any work shift beyond 16 hours duration.
To our knowledge, this study provides the first evidence that a mandatory program of protected time for sleep on extended duty shifts is feasible with high rates of adherence and that it can produce a significant increase in mean hours slept, a significant decrease in the proportion of interns with no sleep on extended duty overnight shifts, and an increase in behavioral alertness on mornings after overnight shifts. The reduction in the rate of prolonged wake-fulness is particularly important, as continuous wakefulness of periods of more than 21 hours is a major predictor of performance errors.22,23 Also, to our knowledge only 2 previous studies have evaluated the feasibility of deploying a pager-free sleep period at night during extended duty shifts.9,10 In both studies, the protected sleep period was not mandatory, resulting in relatively low adherence to the protected sleep period schedule. For example, in 1 study only 22% of interns signed out their own patients to the cross-covering resident during protected periods.10 In contrast, adherence to the protected period was 98.3% in our intervention. In addition, the frequency of disruptions was much lower in the intervention group for both services. Making protected sleep periods a standard part of the internship schedule as opposed to a research protocol likely provided a level of social norming that would have been difficult to achieve as part of a research protocol.
The protected sleep period also afforded residents approximately twice as many sleep durations of 3 hours or more as the control condition—a duration associated with greater recovery of performance and alertness.24 The same was seen at the other end of the spectrum. Interns who received protected sleep periods were less likely to have on-call nights with no sleep. Although it is not clear how large these benefits need to be to offset the cost and effort of implementing a 5-hour protected sleep period, studies have found significant benefits for alertness and performance with even modest increases in nap sleep duration, and we were able to confirm this for both psychomotor speed and for subjectively assessed sleepiness.22,24,25 The mean postsleep period PVT response speed for the intervention condition was 4 seconds−1 or more, which is close to daytime PVT performance of non–sleep-deprived healthy adults.12 The mean postsleep period PVT response speed for the control condition was lower than 4 seconds−1 and comparable with mean performance of participants who have been up all day (ie, at 1 AM or after 17 hours awake).12
These findings are consistent with other evidence that obtaining more sleep during prolonged duty can reduce fatigue.22,23,26 Although the protected sleep periods interns undertook during the intervention were associated with reduced sleepiness and better vigilance performance, we had no evidence that they were associated with either positive or negative changes in patient outcomes, which was limited by insufficient power to reliably detect such changes. Moreover, clinical errors by sleep-deprived interns27 were not assessed in our study, although we hypothesize that protected sleep could help mitigate such errors given that sleep improves learning and memory.28–31 There is reason to believe that protected sleep during prolonged duty could help mitigate some of the personal injury risks experienced by interns either while working or during commutes home.32,33 It is worth noting that over the 4-day call cycle, interns averaged less than 7 hours of daily sleep in both intervention and control groups. Seven hours of sleep or less on a chronic basis have been shown to lead to escalating declines in psycho-motor vigilance with potential adverse effects for errors and safety.34
Limitations of this study include its generalizability to interns at other internal medicine residency programs as well as to interns in different specialties, in which protected sleep periods might be structured differently. Furthermore, this study was completed before implementation of the 2011 ACGME rules, which no longer allow interns to work shifts longer than 16 hours and thus relate more directly to residents for whom strategic napping is currently recommended. The 2 different programs we studied achieved similar increases in the mean amount of sleep, although the program at the VA center was personnel neutral and the program at university hospital involved an extra night resident. The requirement of an additional night resident at the university hospital made this intervention less likely to be feasible for wider implementation. A further limitation is that we did not measure other important outcomes such as data entry errors for medications, and because these studies were powered based on hours slept, we have inadequate power to find small differences in patient outcomes. However, the point estimates did not suggest any meaningful difference in patient outcomes between the groups. We were also unable to blind interns or study staff due to the nature of the intervention; thus, interns were aware of the study hypothesis.
This study indicates that protected sleep periods during prolonged duty are feasible, likely to increase the amount of uninterrupted sleep interns obtain during extended duty overnight shifts, reduce the number of 24-hour periods awake, and improve behavioral alertness in the morning following on-call nights. Although there is evidence that obtaining sleep (relative to no sleep) during prolonged duty helps reduce fatigue and that the amount of fatigue reduction increases with the amount of sleep, from this study we do not have evidence that this is also associated with improvements in patient outcomes. A rigorous comparative effectiveness analysis of protected sleep times vs 16-hour shifts in improving intern alertness and cognitive function and patient outcomes could have a significant effect on policy. To the extent that protected sleep periods are feasible and improve alertness, they may provide a reasonable alternative to mandated shorter shifts.
Acknowledgments
Funding/Support: This work was funded by educational grant 08–429 from the Veterans Affairs Health Services Research and Development Service. Dr Dinges was also supported in part by grant NR004281 from the National Institutes of Health.
Role of the Sponsor: The sponsors/funders have had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Dinges served as an unpaid committee member on the 2009 IOM report, and he currently is an unpaid advisor to the American College of Surgeons Committee to Enhance Peak Performance in Surgery through Recognition and Mitigation of the Impact of Fatigue, and the Royal College of Canada Patient Safety Expert Working Group for the National Steering Committee on Resident Duty Hours. Dr Volpp reported having served as an unpaid member of the ACGME Committee on Innovations from 2005–2009. No other financial disclosures were reported.
Online-Only Material: The Author Video Interview, eAppendix, and eTable are available at http://www.jama.com.
Disclaimer: These contents do not represent the views of the Department of Veterans Affairs or the US government.
Author Contributions: Dr Volpp had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shea, Small, Novak, Mollicone, Dinges, Volpp.
Acquisition of data: Norton, Ecker, Novak, Bellini, Dine, Mollicone, Dinges.
Analysis and interpretation of data: Shea, Small, Basner, Zhu, Novak, Bellini, Dinges, Volpp.
Drafting of the manuscript: Shea, Small, Basner, Novak, Volpp.
Critical revision of the manuscript for important intellectual content: Small, Basner, Zhu, Norton, Ecker, Novak, Bellini, Dine, Mollicone, Volpp.
Statistical analysis: Small, Basner, Zhu.
Obtained funding: Shea, Dinges, Volpp.
Administrative, technical, or material support: Norton, Ecker, Novak, Bellini, Mollicone, Volpp.
Study supervision: Shea, Norton, Bellini, Dinges, Volpp.
Additional Contributions: We thank Ilene Rosen, MD, MSCE, University of Pennsylvania, Perelman School of Medicine, and Karen Warburton, MD, Philadelphia VA Medical Center, for their assistance in conducting the study. Neither received any compensation from the supporters of this research grant for their role in this study.
References
- 1. [Accessed November 1, 2012.];Common program requirements for duty hours: Accreditation Council for Graduate Medical Education. http://www.ama-assn.org/resources/doc/rfs/dutyhours.pdf. February 11, 2003.
- 2.Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):975–983. doi: 10.1001/jama.298.9.975. [DOI] [PubMed] [Google Scholar]
- 3.Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among patients in VA hospitals in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):984–992. doi: 10.1001/jama.298.9.984. [DOI] [PubMed] [Google Scholar]
- 4.Shetty KD, Bhattacharya J. Changes in hospital mortality associated with residency work-hour regulations. Ann Intern Med. 2007;147(2):73–80. doi: 10.7326/0003-4819-147-2-200707170-00161. [DOI] [PubMed] [Google Scholar]
- 5.Rosen AK, Loveland SA, Romano PS, et al. Effects of resident duty hour reform on surgical and procedural patient safety indicators among hospitalized Veterans Health Administration and Medicare patients. Med Care. 2009;47(7):723–731. doi: 10.1097/MLR.0b013e31819a588f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpp KG, Rosen AK, Rosenbaum PR, et al. Did duty hour reform lead to better outcomes among the highest risk patients? J Gen Intern Med. 2009;24(10):1149–1155. doi: 10.1007/s11606-009-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulmer C, Wolman D, Johns M, editors. Resident Duty Hours: Enhancing Sleep, Supervision and Safety. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 8.Nasca TJ, Day SH, Amis ES, Jr ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010;363(2):e3. doi: 10.1056/NEJMsb1005800. [DOI] [PubMed] [Google Scholar]
- 9.Richardson GS, Wyatt JK, Sullivan JP, et al. Objective assessment of sleep and alertness in medical house staff and the impact of protected time for sleep. Sleep. 1996;19(9):718–726. [PubMed] [Google Scholar]
- 10.Arora V, Dunphy C, Chang VY, Ahmad F, Humphrey HJ, Meltzer D. The effects of on-duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144(11):792–798. doi: 10.7326/0003-4819-144-11-200606060-00005. [DOI] [PubMed] [Google Scholar]
- 11.Malmberg B, Kecklund G, Karlson B, Persson R, Flisberg P, Ørbaek P. Sleep and recovery in physicians on night call: a longitudinal field study. BMC Health Serv Res. 2010;10:239. doi: 10.1186/1472-6963-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69(11–12):949–959. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basner M, Rubinstein J. Fitness for duty: a 3-minute version of the Psychomotor Vigilance Test predicts fatigue-related declines in luggage-screening performance. J Occup Environ Med. 2011;53(10):1146–1154. doi: 10.1097/JOM.0b013e31822b8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Co-morbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL, editors. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 18.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: J Wiley & Sons; 1987. [Google Scholar]
- 19.Ramsey F, Schafer D. The Statistical Sleuth, A Course in Methods of Data Analysis. Pacific Grove, CA: Duxbury Resource Center; 2001. [Google Scholar]
- 20.Brooks A, Lack L. A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006;29(6):831–840. doi: 10.1093/sleep/29.6.831. [DOI] [PubMed] [Google Scholar]
- 21.Agresti A, Caffo B. Simple and effective confidence intervals for proportions and differences of proportions result from adding two successes and two failures. Am Stat. 2000;4:280–288. [Google Scholar]
- 22.Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep. 1987;10(4):313–329. [PubMed] [Google Scholar]
- 23.Dinges DF, Whitehouse WG, Orne EC, Orne MT. The benefits of a nap during prolonged work and wakefulness. Work Stress. 1988;2(2):139–153. [Google Scholar]
- 24.Jewett ME, Dijk DJ, Kronauer RE, Dinges DF. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. 1999;22(2):171–179. doi: 10.1093/sleep/22.2.171. [DOI] [PubMed] [Google Scholar]
- 25.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33(8):1013–1026. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosekind MR, Neri DF, Dinges DF. From laboratory to flightdeck: promoting operational alertness. Fatigue and Duty Limitations—An International Review; Proceedings of The Royal Aeronautical Society; London, England. 1997. pp. 7.1–7.14. [Google Scholar]
- 27.Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–1848. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 28.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 29.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103(3):756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427(6972):352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 31.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 32.Ayas NT, Barger LK, Cade BE, et al. Extended work duration and the risk of self-reported percutaneous injuries in interns. JAMA. 2006;296(9):1055–1062. doi: 10.1001/jama.296.9.1055. [DOI] [PubMed] [Google Scholar]
- 33.Barger LK, Cade BE, Ayas NT, et al. Harvard Work Hours, Health, and Safety Group. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352(2):125–134. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 34.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]