Abstract
Formation of nitric oxide and its derivative reactive nitrogen species during endotoxemia has been implicated in the pathogenesis of the associated cardiovascular dysfunction. This stress can promote nitrosative post-translational modifications of proteins that may alter their activity and contribute to dysregulation. We utilised the ascorbate-dependent biotin-switch method to assay protein S-nitrosylation and immunoblotted for tyrosine nitration to monitor changes in nitrosative protein oxidation during endotoxemia. Hearts from lipopolysaccharide (LPS)-treated rats showed no apparent variation in global protein S-nitrosylation, but this may be due to the poor sensitivity of the biotin-switch method. To sensitise our monitoring of protein S-nitrosylation we exposed isolated hearts to the efficient trans-nitrosylating agent nitrosocysteine (which generated a robust biotin-switch signal) and then identified a number of target proteins using mass spectrometry. We were then able to probe for these target proteins in affinity-capture preparations of S-nitrosylated proteins prepared from vehicle- or LPS-treated animals. Unexpectedly this showed a time-dependent loss in S-nitrosylation during sepsis, which we hypothesised, may be due to concomitant superoxide formation that may lower nitric oxide but simultaneously generate the tyrosine-nitrating agent peroxynitrite. Indeed, this was confirmed by immunoblotting for global tyrosine nitration, which increased time-dependently and temporally correlated with a decrease in mean arterial pressure. We assessed if tyrosine nitration was causative in lowering blood pressure using the putative peroxynitrite scavenger FeTPPS. However, FeTPPS was ineffective in reducing global protein nitration and actually exacerbated LPS-induced hypotension.
Keywords: sepsis, lipopolysaccharide, nitrosative, S-nitrosylation, nitration, blood pressure
Introduction
Sepsis and endotoxemia is associated with systemic inflammation that injures and compromises the function of tissues, including those of the cardiovascular system [1]. Given the prevalence, high risk of mortality and financial healthcare burden associated with sepsis [2], it is important to define its pathogenesis at the molecular level. Cardiovascular dysfunction during sepsis includes hypotension, decreased contractility and decreased myocardial compliance. Alterations in circulating factors including prostanoids, cytokines and nitric oxide (NO) are thought to contribute to this aetiology [3; 4]. NO and its related derivatives may have causative roles in compromising cardiovascular performance during sepsis [5].
NO is a major regulator of cardiovascular function; controlling blood pressure [6], muscle tone as well as platelet aggregation [7; 8]. However, during inflammation NO production is drastically enhanced by NO synthase (NOS) stimulation, as well as by increased inducible NOS (iNOS) expression and activity triggered by circulating endotoxins and cytokines [9]. Enhanced nitric oxide production can deplete substrates needed for its biosynthesis resulting in uncoupling and superoxide formation [10; 11]. Inflammatory cells such as neutrophils and macrophages may also respond to endotoxins by generating reactive oxygen species (ROS) as well as reactive nitrogen species (RNS) [12]. Simultaneous formation of both superoxide and nitric oxide can lead to the generation of peroxynitrite (ONOO−), a RNS that can oxidise protein thiols and nitrate tyrosine residues [13]. Increased tyrosine nitration, which may occur during times of nitroxidative stress such as sepsis [14], can be monitored using pan-specific antibodies to this modification [15]. A causative role for nitroxidative species, and so perhaps the nitroxidative protein modifications, in the pathogenesis of sepsis is supported by scavengers of OONO− and other oxidants attenuating dysfunction [16; 17; 18; 19].
Covalent adduction of NO to protein cysteinyl thiols (termed S-nitrosylation) has emerged as an important regulator of cell signalling [20; 21]. Protein S-nitrosylation can be induced by small nitrosothiol containing compounds such as nitrosoglutathione (GSNO), nitrosocysteine (CysNO) [22; 23], and specific reactive nitrogen species such as the nitrosonium ion (NO+) and dinitrogen trioxide (N2O3) [24]. S-nitrosylation regulates the activity of many proteins including caspases, kinases, Hsp90 and the ryanodine receptor [25; 26]. Whilst the role of S-nitrosylation in sepsis is poorly understood, mice null for S-nitrosoglutathione reductase have enhanced global S-nitrosylation and an increased susceptibility to endotoxin mediated mortality [27]. Furthermore plasma nitrosothiol concentration increase during sepsis [28].
Here we assessed changes in myocardial nitrosative (S-nitrosylation and tyrosine nitration) protein modifications in a lipopolysaccharide (LPS) murine model of sepsis. We found no evidence for increased global protein S-nitrosylation, which may have been anticipated as outlined above. In fact, a more sensitive approach to measuring S-nitrosylation of specific proteins showed sepsis actually decreased this modification below controls. Concomitantly, however there was an increase in global protein tyrosine nitration which correlated temporally with systemic hypotension in LPS-treated mice.
Materials and methods
All procedures were performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986 in UK.
Analysis of S-nitrosylation in isolated HEK cells and perfused rat hearts using the ascorbate dependent biotin-switch method
Confluent human embryonic kidney 293 (HEK293) cell were treated with phosphate buffered saline (PBS) alone or PBS containing either 300μM CysNO, 100μM hydrogen peroxide or 500μM diamide for 10 minutes. Langendorff perfused isolated rat hearts were stabilised for 30 minutes before being perfused with Krebs buffer alone or Krebs buffer containing either 10μM CysNO, 50μM S-Nitroso-N-acetyl-DL-penicillamine (SNAP) or 500μM SNAP for 20 minutes at constant flow. After perfusion hearts were immediately snap frozen in liquid nitrogen.
Hearts and treated HEK293 cells were processed using a modified version of the biotin-switch method to determine the level of protein S-nitrosylation [29]. In brief, hearts were homogenised in Tris-HCl, pH7.4 containing 100mM maleimide, 0.2mM neocuproine, 1mM diethylenetriaminepentaacetic acid (DTPA) and protease inhibitors to generate a 10% weight/volume homogenate, which was then supplemented with sodium dodecyl sulfate (SDS) to give a concentration of 2.5%. In one experiment a Krebs perfused rat heart was lysed into Tris-HCl, pH7.4 containing protease inhibitors. This sample was split in two groups, to one Tris-HCl, pH7.4 alone was added and to the other Tris-HCl, pH7.4 containing 200μM SNAP. After incubation at room temperature for 20 minutes these samples were supplemented with 100mM maleimide, 0.2mM neocuproine, 1mM DTPA and 2.5% SDS. Treated HEK293 cells were lysed directly into Tris-HCl, pH7.4 containing 100mM maleimide, 0.2mM neocuproine, 1mM DTPA and 2.5% SDS. All lysates were then incubated at 50°C for 25 minutes to block free thiols and then desalted to remove any unincorporated maleimide. Samples were then split into two groups, to one 30mM ascorbate was added and to the other both 30mM ascorbate and 0.1mM N-(6-(Biotinamido)hexyl)-3′-(2′-pyridyldithio)-propionamide (biotin-HPDP). After 1 hour of incubation in the dark at room temperature samples were desalted to remove unincorporated biotin-HPDP, followed by addition of equal volume of 2× Laemmli buffer (4% SDS, 20% glycerol, 0.004% bromphenol blue, 0.125 M Tris-HCl, pH6.8). Samples were anlaysed by resolving on SDS-PAGE gels followed by western blotting then immunostaining using streptavidin-HRP.
Affinity capture of S-nitrosylated proteins using the biotin-switch method
Homogenate from three control hearts were combined together and the homogenate for three CysNO perfused heart, to give one sample for control and one for CysNO perfused. Both sets of homogenate were processed using the ascorbate biotin-switch method described above. Biotin-HPDP modified proteins were purified using streptavidin-agarose. In brief, after biotin-switch analysis homogenates were combined with streptavidin-agarose, 1% triton and protease inhibitors followed by 3 hours incubation at 4°C on a rotary shaker. Streptavidin-agarose beads were pelleted and then resuspended in fresh buffer before being added to empty spin columns. Each spin column was washed 6 times with Tris-HCl, pH7.4 containing 1% triton. Modified proteins were eluted using buffer containing 100mM 2-mercaptoethanol. The same protocol was also followed when using hearts isolated from LPS-treated rats.
Mass spectrometry
Gel slices were treated with trypsin using published methods [30; 31], modified for use with an Investigator ProGest robotic digestion system (Genomic Solutions). Digested peptides were separated by using nanoflow liquid chromatography (Ultimate 3000™, Dionex) on a reverse-phase column (PepMap 100, 75 μm I.D., 15 cm length, Dionex) at a flow rate of 300 nl/min. The column was attached to a linear ion trap mass spectrometer (LTQ-XL, Thermofinnigan) set to full ion scan mode over the mass-charge (m/z) range 300-2000. Tandem mass spectrometry (MS/MS) was carried out on the top six ions in each MS scan using the data-dependent acquisition mode with dynamic exclusion enabled. Generated spectra were matched to database entries (UniProt Knowledgebase) using TurboSEQUEST software (Bioworks 3.4, Thermo Finnigan). Protein and peptide identifications were verified using Scaffold (version 1.0, Proteome Software Inc., Portland, OR). Peptides with above 95.0% probability of identification were accepted as specified by the Peptide Prophet algorithm [32]. Protein identifications were accepted if probability was greater than 99.0% and contained at least 2 identified peptides as assigned by the Protein Prophet algorithm [33].
Radiotelemetry
Mean arterial pressure (MAP) was assessed by remote radio-telemetry in conscious freely moving mice as described [34]. Briefly, randomly selected C57Bl/6 mice at 12 weeks of age were anesthetised with 2% isoflurane in 2l O2 per minute with pre and post-operative analgesia (buprenorphine, 0.1mg/kg). Radio-telemetry probe catheter (TA11PA-C10, O.D 0.4 mm, Data Science International Inc., St Paul, MN) was implanted into the aortic arch via the left carotid artery. Following one week recovery, mice housed in individual cages were placed above the telemetric receivers with output to a computer. Cardiovascular variables and activity were routinely monitored and recorded by scheduled sampling for 10 seconds every 5 min (Dataquest LabPRO Acquisition System version 3.01, Data Sciences International, St Pauls, MN).
LPS treatment and heart preparation
6 week old Wistar rats were given an intraperitoneal (i.p.) injection of either saline or 10 mg/kg Escherichia coli bacterial lipopolysaccharide (LPS, E.coli 0111:B4, Sigma Aldrich, UK). Rats injected with LPS were anesthetised at 2, 4 or 8 hours followed by removal and then storage of isolated hearts in liquid nitrogen. Rats injected with saline had their hearts removed at 8 hours after the initial injection. Telemetered C57Bl/6 mice were injected i.p. with 9 mg/kg Escherichia coli bacterial lipopolysaccharide (E.coli 0111:B4) or an equivalent volume of sterile saline as described [35]. Some mice were given an i.p. injection of 100 mg/kg 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III) (FeTPPS) 1 hours before LPS treatment. During blood pressure measurement studies mice were culled 18 hours after LPS or saline treatment. For analysis of protein nitration, mice were anesthetised 6 hours after injection followed by removal and then storage of isolated hearts in liquid nitrogen.
Immunoblotting
Samples were resolved using poly-acrylamide gel electrophoresis then transferred onto PVDF membranes using Western blotting. Samples generated using the biotin-switch method were probed using streptavidin-HRP. LPS treated samples were immunostained using a 3-nitrotyrosine antibody (Millipore, clone 1A6). Affinity purified biotinylated proteins after the biotin-switch method from LPS treated rat heart homogenate was immunostained using a myosin heavy chain antibody (Abcam ab15-100).
Results
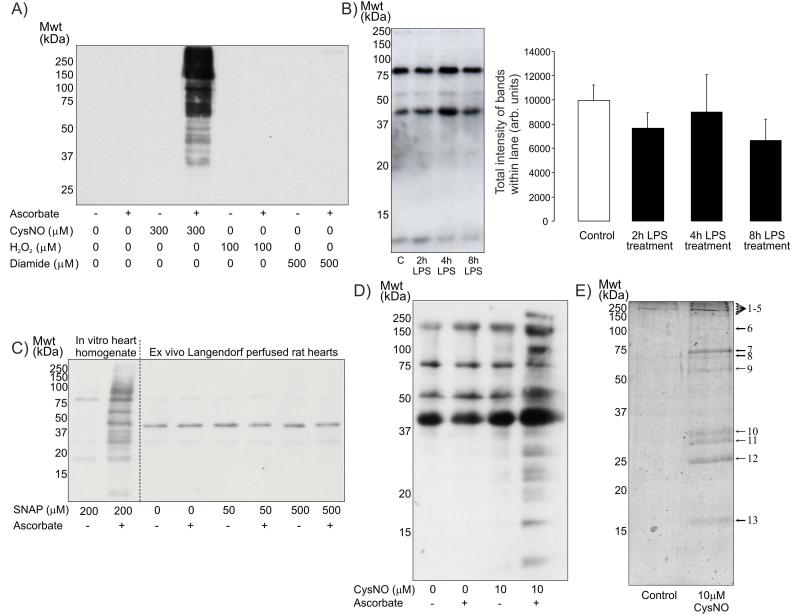
To establish if the ascorbate-dependent biotin-switch assay could specifically detect S-nitrosothiols the method was evaluated in HEK cells treated with CysNO, hydrogen peroxide (H2O2) or diamide as shown in Figure 1A. Treatment with CysNO, a known mediator of protein S-nitrosylation [36], generated a large increase in ascorbate-dependent protein labelling as anticipated. H2O2 or diamide, which preferentially induce protein sulfenic acid or disulphide bond formation respectively, generated no detectable protein labelling. This helps verify the specificity of this method for S-nitrosothiols, as well as confirm this methodology for detecting protein S-nitrosylation actually worked in our hands. We then used this method to monitor protein S-nitrosylation in hearts from saline- or LPS-treated rats; but did not find any variation in global protein S-nitrosylation as shown in Figure 1B. However, as the biotin-switch method has poor sensitivity [37], subtle changes in S-nitrosylation may have been missed.
Figure 1.
A) Western blot of samples prepared using the biotin-switch method probed with streptavidin-HRP. Treatment of HEK cells for 20 minutes with 300μM CysNO generated a large increase in ascorbate-dependent protein labelling following biotin-switch analysis. In contrast, cells exposed to 100μM H2O2 or 500μM diamide does not increase protein S-nitrosylation. B) Ascorbate-dependent biotin-switch analysis of hearts from rats treated for various durations with LPS displayed no apparent variation in global protein S-nitrosylation when probed with streptavidin-HRP. C) The treatment of heart homogenate with 200μM of the NO donor SNAP in vitro increased detectable protein S-nitrosylation as measured using biotin-switch analysis. However, perfusion of isolated rat hearts with SNAP at various concentrations failed to induce protein S-nitrosylation. D) Perfusion of isolated rat hearts with 10μM CysNO increased ascorbate-dependent biotin labelling (i.e. S-nitrosylation) of multiple proteins. E) Colloidal Coomassie blue stained SDS-PAGE gel of samples prepared using streptavidin-agarose affinity capture of biotin-switch labelled proteins from control rat hearts or those perfused with CysNO.
We reasoned that the sensitivity of detecting protein S-nitrosylation may be improved by first identifying proteins in the heart that were targets of this modification. We might then use sensitive antibodies to these S-nitrosylation targets and assess whether they are present in streptavidin-agarose affinity-captured proteins in hearts from saline- or LPS-treated animals that had undergone the biotin-switch labelling protocol.
The ability of NO donors SNAP or CysNO to induce detectable S-nitrosylation in the Langendorff perfused rat heart were compared. SNAP only generated protein S-nitrosylation when directly incubated with heart homogenate in vitro and not when perfused through the coronaries vessels of isolated rat hearts (Figure 1C). In contrast perfusion with CysNO induced significant S-nitrosylation as visualised by the biotin-switch method (Figure 1D). These S-nitrosylated target proteins were affinity captured with streptavidin-agarose and resolved by SDS-PAGE and visualised with colloidal Coomassie Blue (Figure 1E), which confirmed multiple unique proteins in the samples from CysNO-treated hearts compared to controls. The unique bands were excised from the SDS-PAGE gel and identified using tandem mass spectrometry. This led to the identification of several potential targets for S-nitrosylation as listed in Table 1.
| Band number |
Protein name | Protein molecular weight (Da) |
Protein identification probability |
|---|---|---|---|
| 3 | Myosin heavy chain 6 cardiac muscle alpha isoform |
223,677.5 | 100.00% |
| 4 | Hexokinase-1 | 108,286.6 | 99.80% |
| 4 | Myomesin-1 (190 kDa titin- associated protein) |
162,435.3 | 99.00% |
| 4 | Myomesin-2 (165 kDa titin- associated protein) |
164,776.4 | 100.00% |
| 4 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) |
109,637 | 100.00% |
| 5 | Alpha-actinin-2 | 103,764.2 | 99.90% |
| 5 | Myosin-binding protein C, cardiac- type (Cardiac MyBP-C) |
140,588.5 | 99.90% |
| 7 | Cardiac troponin I | 23,895.4 | 99.00% |
| 8 | Succinyl-CoA:3-ketoacid-coenzyme A transferase 1 |
55,972.2 | 99.90% |
| 8 | Sorting and assembly machinery component 50 homolog |
51,959.5 | 99.90% |
| 8 | ATP synthase subunit alpha heart isoform, mitochondrial precursor |
59,703.3 | 100.00% |
| 9 | Voltage-dependent anion-selective channel protein 2 |
31,602.5 | 100.00% |
| 10 | Adenylate kinase isoenzyme 2, mitochondrial |
26,479.9 | 99.90% |
| 11 | Myosin light chain 1, slow-twitch muscle B/ventricular isoform |
22,138.7 | 100.00% |
| 12 | Myoglobin | 17,139.5 | 100.00% |
| 12 | Troponin C | 18,385.3 | 99.90% |
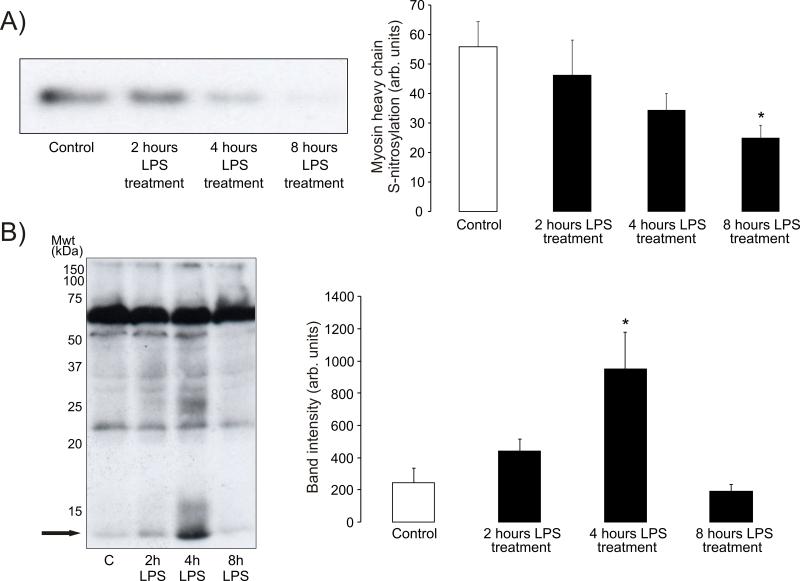
We then used a sensitive antibody to one of these newly identified S-nitrosylation targets, namely myosin heavy chain, to screen for its abundance in streptavidin-agarose affinity captures prepared from hearts from mice treated with LPS. This showed that myosin heavy chain becomes de-nitrosylated in a time-dependent manner following LPS-treatment (31±12% decrease over 8 hours) as seen in Figure 2A. This loss of protein S-nitrosylation was unexpected as we had anticipated increased S-nitrosylation due LPS-induced nitrosative stress as discussed above. Consequently, we hypothesised that the loss in S-nitrosylation may be explained by a loss in S-nitrosylating derivatives due to the biochemical conversion of NO to OONO−. Accordingly, we analysed 3-nitrotyrosine (a biomarker of OONO−) content of hearts from control or LPS-treated animals using Western immunoblotting. This analysis (Figure 2B) showed LPS treatment enhanced tyrosine nitration at 2 hours and more prominently (~4-fold increase above control) at 4 hours, before returning to basal control level by 8 hours. Protein tyrosine nitration following LPS-treatment was particularly apparent in a 13kDa protein, and was also prominent in other anti-nitrotyrosine immunoblots analysing related samples from LPS-treated animals (Figure 3B).
Figure 2.
A) Immunoblot of affinity captured S-nitrosylated proteins from LPS-treated rat hearts probed with a myosin heavy chain antibody. Clearly LPS induced a time-dependent decrease in S-nitrosylation of this protein. B) Immunoblot of heart tissue from LPS-treated rats probed with a nitrotyrosine antibody shows an increase in protein nitration at 2 hours and more so at 4 hours, before returning to basal at 8 hours. The arrow highlights a prominent ~13 kDa cardiac protein that is nitrated after LPS-treatment.
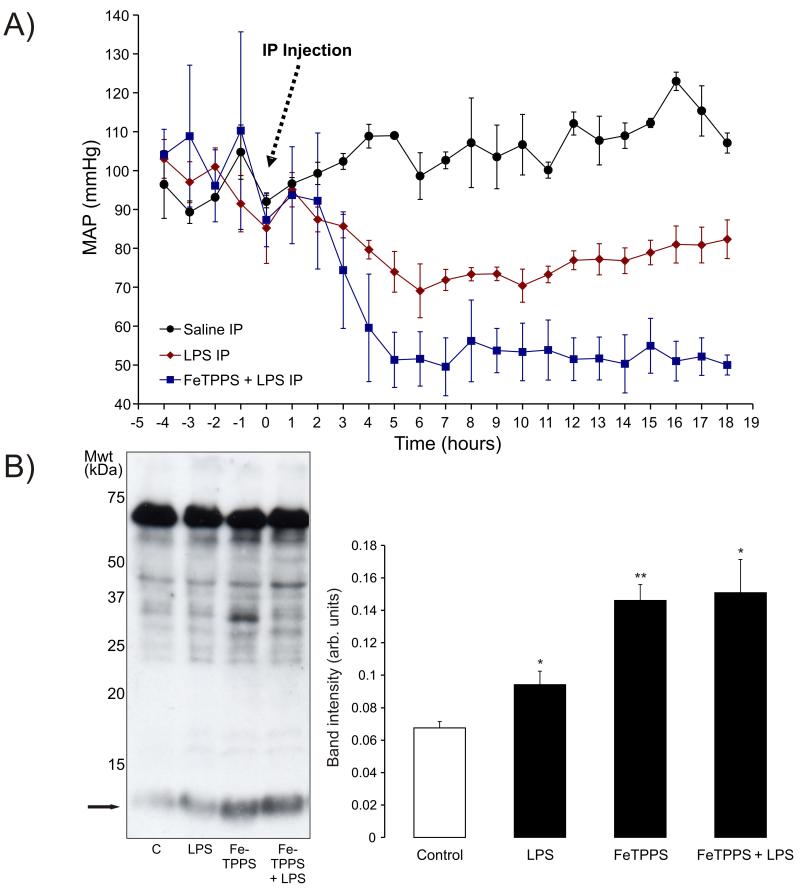
Figure 3.
A) Intraperitoneal injection of telemetered mice with LPS reduced mean arterial blood pressure (hypotension) when compared to saline-treated controls. The hypotension reached a minimum 6 hours after LPS was injected. Treatment of mice with FeTPPS before LPS injection potentiated the hypotension caused by LPS alone. B) Treatment of mice with LPS for 6 hours increased tyrosine nitration that again was especially prominent in a ~13 kDa protein. Administration of FeTPPS alone or in combination with LPS enhanced the amount of protein tyrosine nitration compared to LPS alone.
Measurement of mean arterial pressure in telemetered mice treated with LPS showed a maximal decrease around 6 hours followed by slow time-dependent recovery towards basal (Figure 3A). The development of this hypotension after LPS treatment temporally correlated with the increase in protein tyrosine nitration shown in Figure 2B. In an attempt to prevent LPS-induced tyrosine nitration an intraperitoneal injection of the OONO− scavenger FeTPPS was administered 1 hour before LPS treatment. However, this potentially protective intervention actually had the reverse effect and enhanced protein nitration ~2-fold above control and also potentiated the LPS-induced hypotension. Treatment of mice with just the scavenger FeTPPS alone also significantly enhanced protein nitration ~2-fold above control.
Discussion
Sepsis induces a nitroxidative stress [14], which may be causal in the accompanying cardiovascular dysfunction. A logical potential consequence of nitroxidative stress is the post-translational modification of redox active proteins. Nitrosative species may target redox active protein thiols or tyrosine residues to induce S-nitrosylation or 3-nitrotyrosine [38]. Accordingly, we monitored these nitroxidative protein modifications in a rodent model of sepsis.
Evidence continues to accumulate supporting protein S-nitrosylation as a regulatory mechanism, with aberrant changes in this modification being causatively implicated in disease progression [21]. We monitored changes in protein S-nitrosylation using the biotin-switch method but did not find an increase in this modification, although we were confident the method was working as cells or isolated hearts exposed to CysNO generated a large signal using this labelling protocol. When the biotin-switch assay has been compared to the tri-iodide chemiluminescence measurement of protein S-nitrosylation, it was found to be relatively insensitive. High levels of S-nitrosylation (in the nanomole per milligram protein range) were required to generate an S-nitrosylation signal using the biotin-switch method, and physiological activators of NO synthesis did not increase the signal [37]. Thus the lack of a change in global nitrosylation can be explained by the poor sensitivity of the ascorbate-dependent biotin-switch method. A further confounding factor is that the biotin-switch assay like most proteomic approaches is hampered by the large variation in the relative abundance of protein [38]. Whilst the ability of proteomic methods to assess the full complement of proteins in the experimental system has great benefits, a major problem can be that stoichiometric changes in low abundance proteins are often masked against a background signal resulting from non-stoichiometric modifications of high abundance proteins. Thus low abundance proteins may change their S-nitrosylation status during sepsis but the change in amplitude of this signal is smaller than the basal signals generated from abundant proteins.
This problem caused by high abundance proteins generating a background greater than signals induced by stoichiometric S-nitrosylation of a low abundance protein can be overcome by identifying the proteins in the system susceptible to modification and then individually assessing them. Indeed, this is what we did, identifying cardiac proteins susceptible to S-nitrosylation using CysNO treatment to induce a robust, detectable signal well above background. These proteins were then affinity captured and identified using LC-MS. Armed with this knowledge we then assessed the abundance of these proteins in affinity preparations of cardiac S-nitrosylated proteins from control of LPS-treated animals. In this way we found a loss in myosin heavy chain S-nitrosylation during sepsis; not the increase we had anticipated based on the nitroxidative stress associated with sepsis [5]. Loss of myosin heavy chain S-nitrosylation during the onset of endotoxemia may itself have a functional effect, potentially contributing to contractile dysfunction during sepsis. This possibility is supported by recent work showing S-nitrosylation of skeletal and cardiac myosin caused a dose-dependent and oxygen-dependent reduction in actin filaments velocity over myosin, which double contractile force [39]. Future studies identifying and then mutating the site of S-nitrosylation to mimic or block its formation may help clarify the importance of this precise modification. Other targets of S-nitrosylation would also have to be examined on a case-by-case basis, again requiring further work.
Comparing protein S-nitrosylation induced by SNAP and CysNO highlights the classic form of NO itself is a poor S-nitrosylating agent, and is consistent with reports of other groups [40]. The poor membrane permeability of SNAP prevents direct transnitrosylation NO donation to intracellular target protein thiols. Instead SNAP, a relatively unstable donor, rapidly breaks down extracellularly and releases NO which then diffuses across the membrane. This form of free authentic NO is a poor S-nitrosylating agent and instead is scavenged by heme containing proteins such as soluble guanylate cyclase [41; 42]. In contrast, CysNO is more stable and readily crosses the membrane through the L-amino acid transporter [40], serving as a powerful intracellular nitrosylating agents by direct transfer of NO to a target protein thiol. Clearly a change in cellular protein S-nitrosylation status may not directly reflect a change in free classical NO, but rather a change in low molecular weight nitrosothiols (CysNO, GSNO) or reactive nitrosylating species such as dinitrogen trioxide (N2O3) or nitrosonium ion (NO+).
We speculated that the depletion in S-nitrosylation following LPS-treatment (identified using the myosin heavy chain screening approach) may be due to a loss in S-nitrosylating species of NO due to biochemical conversation to OONO− [43]. This could be potentiated by superoxide generation under septic conditions, which can occur when NOS enzymes uncouple due to substrate depletion [10; 11]. Endotoxins may also enhance gp91phox containing NADPH oxidase activity to further elevate superoxide. We compared tyrosine nitration, a biomarker of OONO− [13], in hearts from control or LPS-animals. LPS-treatment of rats increased global protein tyrosine nitration which was maximal after 4 hours and returned to basal by 8 hours. In mice maximal hypotension occurred 6 hours after LPS treatment and thus coincided with maximal protein tyrosine nitration.
LPS-induced tyrosine nitration could be causatively linked with the associated hypotension. Consistent with this possibility, others found direct injection of OONO− significantly lowered blood pressure in rats [44; 45]. Thus there may be specific target protein(s) that alter their function following nitration and contribute to cardiovascular dysfunction (hypotension, impaired cardiac output) during sepsis. This possibility was also supported by reports [17; 18; 46], showing the OONO− scavenger FeTPPS attenuates dysfunction during sepsis. To examine the potential causal relationship between tyrosine nitration and blood pressure lowering we also utilised FeTPPS, hypothesising that it would scavenge LPS-induced OONO− formation and so limit protein tyrosine nitration and consequently attenuate the hypotension. However, we observed precisely the opposite effect; FeTPPS dramatically enhanced LPS-induced tyrosine nitration and exacerbated the hypotension. This disparity with other reports may be explained by subtle differences in the model and how the FeTPPS was administered; we used an intraperitoneal injection whereas other used intravenous treatment. Further complexity results from OONO− potentially targeting redox active protein thiols, the oxidation of which can activate vasodilatory pathways [47; 48].
To conclude, this study demonstrates dynamic change in the myocardial nitrosative environment during early endotoxemia. Further work is required to clarify the potential role of protein S-nitrosylation or tyrosine nitration in the pathogenesis of this deleterious, life-threatening pathology.
Acknowledgements
We thank the Wellcome Trust for providing a Sir Henry Wellcome Fellowship (JRB) and the Medical Research Council for project grant support (PE).
Abbreviations
- biotin-HPDP
N-(6-(Biotinamido)hexyl)-3′-(2′-pyridyldithio)-propionamide
- CysNO
nitrosocysteine
- DTPA
diethylenetriaminepentaacetic acid
- FeTPPS
5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III)
- GSNO
nitrosoglutathione
- HEK293
human embryonic kidney 293 cells
- LPS
lipopolysaccharide
- NO
nitric oxide
- PBS
phosphate buffered saline
- SDS
sodium dodecyl sulfate
- SNAP
S-Nitroso-N-acetyl-DL-penicillamine
References
- [1].Hunter JD, Doddi M. Sepsis and the heart. Br J Anaesth. 2010;104:3–11. doi: 10.1093/bja/aep339. [DOI] [PubMed] [Google Scholar]
- [2].Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15:1918–35. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zardi EM, Zardi DM, Dobrina A, Afeltra A. Prostacyclin in sepsis: a systematic review. Prostaglandins Other Lipid Mediat. 2007;83:1–24. doi: 10.1016/j.prostaglandins.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [4].Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15:392–7. doi: 10.1097/MCC.0b013e3283307a4e. [DOI] [PubMed] [Google Scholar]
- [5].Wendel M, Heller AR. Mitochondrial function and dysfunction in sepsis. Wien Med Wochenschr. 2010;160:118–23. doi: 10.1007/s10354-010-0766-5. [DOI] [PubMed] [Google Scholar]
- [6].Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–40. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- [7].Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113(Pt 10):1671–6. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- [8].Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986;88:411–5. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- [10].Boczkowski J, Lisdero CL, Lanone S, Samb A, Carreras MC, Boveris A, Aubier M, Poderoso JJ. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. Faseb J. 1999;13:1637–46. doi: 10.1096/fasebj.13.12.1637. [DOI] [PubMed] [Google Scholar]
- [11].Pan M, Choudry HA, Epler MJ, Meng Q, Karinch A, Lin C, Souba W. Arginine transport in catabolic disease states. J Nutr. 2004;134:2826S–2829S. doi: 10.1093/jn/134.10.2826S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- [12].Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–64. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- [13].Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- [14].Alvarez S, Evelson PA. Nitric oxide and oxygen metabolism in inflammatory conditions: sepsis and exposition to polluted ambients. Front Biosci. 2007;12:964–74. doi: 10.2741/2117. [DOI] [PubMed] [Google Scholar]
- [15].Beckmann JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–8. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- [16].Liaw WJ, Chen TH, Lai ZZ, Chen SJ, Chen A, Tzao C, Wu JY, Wu CC. Effects of a membrane-permeable radical scavenger, Tempol, on intraperitoneal sepsis-induced organ injury in rats. Shock. 2005;23:88–96. doi: 10.1097/01.shk.0000145937.70085.89. [DOI] [PubMed] [Google Scholar]
- [17].Lancel S, Tissier S, Mordon S, Marechal X, Depontieu F, Scherpereel A, Chopin C, Neviere R. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J Am Coll Cardiol. 2004;43:2348–58. doi: 10.1016/j.jacc.2004.01.047. [DOI] [PubMed] [Google Scholar]
- [18].Cuzzocrea S, Mazzon E, Di Paola R, Esposito E, Macarthur H, Matuschak GM, Salvemini D. A role for nitric oxide-mediated peroxynitrite formation in a model of endotoxin-induced shock. J Pharmacol Exp Ther. 2006;319:73–81. doi: 10.1124/jpet.106.108100. [DOI] [PubMed] [Google Scholar]
- [19].Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–7. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- [20].Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–46. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Doulias PT, Greene JL, Greco TM, Tenopoulou M, Seeholzer SH, Dunbrack RL, Ischiropoulos H. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marino SM, Gladyshev VN. Structural analysis of cysteine S-nitrosylation: a modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol. 2010;395:844–59. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Foster MW, Stamler JS. New insights into protein S-nitrosylation. Mitochondria as a model system. J Biol Chem. 2004;279:25891–7. doi: 10.1074/jbc.M313853200. [DOI] [PubMed] [Google Scholar]
- [25].Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burgoyne JR, Eaton P. Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent cross-talk to beta-adrenergic-like signaling. J Biol Chem. 2009;284:29260–8. doi: 10.1074/jbc.M109.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–28. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- [28].Tejedor C, Lorente JA, Delgado MA, Fernandez-Segoviano P, De Paula M, Tobalina R, Alonso M, Moscoso A, Soto F, Blazquez J, Esteban A. Interaction between hemoglobin and glutathione in the regulation of blood flow in normal and septic pigs. Crit Care Med. 2002;30:2493–500. doi: 10.1097/00003246-200211000-00015. [DOI] [PubMed] [Google Scholar]
- [29].Burgoyne JR, Eaton P. A rapid approach for the detection, quantification, and discovery of novel sulfenic acid or S-nitrosothiol modified proteins using a biotin-switch method. Methods Enzymol. 2010;473:281–303. doi: 10.1016/S0076-6879(10)73015-9. [DOI] [PubMed] [Google Scholar]
- [30].Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- [31].Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379:466–9. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- [32].Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- [33].Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- [34].Huetteman DA, Bogie H. Direct blood pressure monitoring in laboratory rodents via implantable radio telemetry. Methods Mol Biol. 2009;573:57–73. doi: 10.1007/978-1-60761-247-6_4. [DOI] [PubMed] [Google Scholar]
- [35].Marshall M, Anilkumar N, Layland J, Walker SJ, Kentish JC, Shah AM, Cave AC. Protein phosphatase 2A contributes to the cardiac dysfunction induced by endotoxemia. Cardiovasc Res. 2009;82:67–76. doi: 10.1093/cvr/cvp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Y, Hogg N. S-Nitrosothiols: cellular formation and transport. Free Radic Biol Med. 2005;38:831–8. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- [37].Zhang Y, Keszler A, Broniowska KA, Hogg N. Characterization and application of the biotin-switch assay for the identification of S-nitrosated proteins. Free Radic Biol Med. 2005;38:874–81. doi: 10.1016/j.freeradbiomed.2004.12.012. [DOI] [PubMed] [Google Scholar]
- [38].Eaton P. Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med. 2006;40:1889–99. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- [39].Evangelista AM, Rao VS, Filo AR, Marozkina NV, Doctor A, Jones DR, Gaston B, Guilford WH. Direct regulation of striated muscle myosins by nitric oxide and endogenous nitrosothiols. PLoS One. 2010;5:e11209. doi: 10.1371/journal.pone.0011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci U S A. 2004;101:7891–6. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu SF, Crawley DE, Rohde JA, Evans TW, Barnes PJ. Role of nitric oxide and guanosine 3′,5′-cyclic monophosphate in mediating nonadrenergic, noncholinergic relaxation in guinea-pig pulmonary arteries. Br J Pharmacol. 1992;107:861–6. doi: 10.1111/j.1476-5381.1992.tb14538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wedel B, Harteneck C, Foerster J, Friebe A, Schultz G, Koesling D. Functional domains of soluble guanylyl cyclase. J Biol Chem. 1995;270:24871–5. doi: 10.1074/jbc.270.42.24871. [DOI] [PubMed] [Google Scholar]
- [43].Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- [44].Graves JE, Lewis SJ, Kooy NW. Peroxynitrite-mediated vasorelaxation: evidence against the formation of circulating S-nitrosothiols. Am J Physiol. 1998;274:H1001–8. doi: 10.1152/ajpheart.1998.274.3.H1001. [DOI] [PubMed] [Google Scholar]
- [45].Benkusky NA, Lewis SJ, Kooy NW. Attenuation of vascular relaxation after development of tachyphylaxis to peroxynitrite in vivo. Am J Physiol. 1998;275:H501–8. doi: 10.1152/ajpheart.1998.275.2.H501. [DOI] [PubMed] [Google Scholar]
- [46].Chirino YI, Hernandez-Pando R, Pedraza-Chaverri J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 2004;4:20. doi: 10.1186/1471-2210-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schroder E, Wait R, Begum S, Kentish JC, Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006;281:21827–36. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- [48].Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–7. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]