Abstract
Ventricular tachyarrhythmias are common in patients with congestive heart failure. The clinical presentation ranges from an asymptomatic incidental electrocardiographic finding to palpitations, syncope, and sudden cardiac death. Although implantable cardioverter defibrillators successfully prevent sudden cardiac death associated with ventricular fibrillation and ventricular tachycardia, recurrent implantable cardioverter defibrillators shocks remain a clinical management challenge. In this review, we discuss management strategies of ventricular tachycardia in congestive heart failure, including drug therapy, radiofrequency catheter ablation (RFCA), and recent RFCA advances.
Keywords: ventricular tachycardia, congestive heart failure, sudden cardiac death, radiofrequency catheter ablation, antiarrhythmic drugs

Background
Ventricular tachycardia (VT) is common in patients with heart failure (HF).1-3 It is a significant cause of mortality as sustained VT can degenerate into ventricular fibrillation (VF) and cause sudden cardiac death (SCD). Implantable cardioverter defibrillators (ICDs) have shown to prevent SCD in patients with HF and are the mainstay of both primary and secondary prevention therapies.4-6 ICDs, however, can be an adverse psychological burden on patients.7 Repeated shocks pose a significant clinical challenge due to pain and hemodynamic deterioration, and they are associated with increased mortality.8 Furthermore, ICDs do not provide absolute protection against SCD. In one study, the rate of SCD in patients with ICD devices was 5%.9
The limitations of ICDs create clinical scenarios in which patients require specific treatments to minimize the occurrence of VT/VF and recurrent ICD shocks. It is reasonable for clinicians to adopt alternate strategies to minimize VT/VF occurrence in this high-risk population so that ICDs serve solely as a backup. One such strategy is the use of antiarrhythmic drugs (AADs), which have been tested for prophylaxis and therapy against VT in multiple studies; however, the results have mostly been disappointing. Radiofrequency catheter ablation (RFCA) is another option that has shown promise in recent trials.10, 11 RFCA uses thermal energy to ablate the myocardium that serves as substrate for re-entrant VT circuits. Recent advances in three-dimensional (3D) electroanatomical mapping systems enable reconstruction of VT circuit pathways during sinus rhythm, allowing RFCA in patients with unmappable VTs.12
Despite the efficacy of RFCA in treating recurrent VTs in most patients, there remains a small group of patients for whom RFCA is unsuccessful. In such patients, coronary ethanol ablation has shown to be effective if the site of VT origination is mapped near a coronary artery or vein branch.13, 14 This review discusses the aforementioned advances in prophylaxis and treatment of VT in patients with heart failure.
Antiarrhythmic Drug Therapy
Over the past few decades, multiple risk markers for SCD have been used to design AAD trials in patients with coronary artery disease (CAD), nonischemic cardiomyopathy, and congestive heart failure (CHF). These include frequent premature ventricular contractions (PVCs), complex PVCs, ventricular couplets, nonsustained VT (NSVT), reduced left-ventricular ejection fraction (LVEF), and advanced HF. Several randomized clinical trials have assessed the efficacy of AADs for preventing SCD when used alone (Table 1).6, 15-31
Table 1.
List of major randomized clinical trials involving antiarrhythmic drugs and their effect on mortality and sudden arrhythmic death.
| Drugs | Study | Inclusion Criteria | Endpoints | Drugs | Control | Key Results |
| Class I | CASH15 | Recent cardiac arrest not associated with MI | Total mortality Arrhythmic death | Propafenone Metoprolol Amiodarone | ICD | Sudden cardiac death mortality lowest in the ICD arm; increased mortality in the propafenone arm |
| CAST16, 17 | Post-MI ≥6 PVCs/hr LVEF ≤40% | Arrhythmic death | Flecainide Encainide Moricizine | Placebo | Arrhythmic death increased in all treatment arms | |
| IMPACT18 | Post-MI | Rate of PVCs and complex ventricular arrhythmias Mortality | Mexiletine | Placebo | Rate of PVCs and complex ventricular arrhythmias was lower in treatment arm at 4 months and a trend towards reduction was observed in treatment arm at 12 months; trend towards mortality increase in treatment arm | |
| Class II | BHAT19 | Post-MI | Total mortality Sudden cardiac death | Propranolol | Placebo | Total mortality and sudden cardiac death decreased in treatment arm |
| CAPRICORN20 | Post-MI LVEF ≤40% | Death or arrhythmias | Carvedilol | Placebo | Death or arrhythmia decreased in carvedilol arms; ventricular arrhythmias also decreased in treatment arm | |
| CIBIS-II21 | NYHA Class III-IV LVEF ≤35% | All-cause mortality | Bisoprolol | Placebo | All-cause mortality was less in treatment arm; rate of sudden cardiac death less in treatment arm | |
| MERIT-HF22 | NYHA Class II-IV LVEF ≤40% | All-cause death Sudden cardiac death | Metoprolol CR/XL | Placebo | All-cause death and sudden cardiac death lower in treatment arm | |
| Class III | ANDROMEDA23 | NYHA Class III-IV LVEF ≤35% | Death from any cause or hospitalization for HF Arrhythmic death | Dronedarone | Placebo | Increased mortality as well as arrhythmic death in treatment arm |
| BASIS24 | Post-MI PVCs | Total mortality Arrhythmic events | Amiodarone | Placebo | Total mortality and arrhythmic events lower in treatment arm | |
| CAMIAT25 | Post-MI ≥10 PVCs/hr or NSVT | Arrhythmic death Total mortality | Amiodarone | Placebo | Amiodarone reduced arrhythmic death but did not reduce total mortality | |
| CHF-STAT26 | CHF LVEF ≤40% ≥10 PVCs/hr | Total mortality | Amiodarone | Placebo | No effect in ischemic cardiomyopathy but there was a trend towards mortality reduction in nonischemic cardiomyopathy | |
| DIAMOND-MI27 | Post-MI (≤7 days) LVEF ≤35% | All-cause mortality Arrhythmic death | Dofetilide | Placebo | No reduction of all-cause mortality or arrhythmic death in treatment arm | |
| EMIAT28 | Post-MI LVEF ≤40% | Total mortality Arrhythmic death | Amiodarone | Placebo | Amiodarone reduced arrhythmic death but did not reduce total mortality | |
| GESICA29 | CHF LVEF ≤35% | Total mortality | Amiodarone | Best therapy | Amiodarone reduced total mortality; patients with NSVT had higher mortality | |
| MUSTT30 | Post-MI LVEF ≤30% NSVT | Arrhythmic death or cardiac arrest | ICD Class I or class III agents | No therapy | Improved survival in ICD group; no difference between antiarrhythmic therapy and no therapy | |
| SCD-HeFT6 | CHF LVEF ≤35% NYHA II-III | Total mortality Arrhythmic death Cost Quality of life | ICD Amiodarone | Placebo | Improved survival with ICD; no effect of amiodarone on survival | |
| SWORD31 | Post-MI LVEF <40% or Remote MI NYHA Class II-III | Total mortality | d-Sotalol | Placebo | Increased mortality in treatment arm |
MI: myocardial infarction; ICD: implantable cardioverter defibrillator; PVC: premature ventricular contraction; LVEF: left ventricular ejection fraction; NSVT: nonsustained ventricular tachycardia; CHF: congestive heart failure; NYHA Class: New York Heart Association heart failure class.
According to multiple large clinical trials, ICD therapy is indicated and is superior to AADs in patients with advanced HF (LVEF ≤35%) or recurrent VTs.4-6 In many patients with ICDs, however, adjuvant AAD therapy also is initiated to reduce ICD therapies. For example, in the device arm of the Antiarrhythmics versus Implantable Defibrillators (AVID) trial,32 about 18% of patients had to be started on adjuvant AAD therapy to reduce multiple shock occurrences and prevent recurrent ventricular arrhythmias. It is suggested that AADs prolong the tachycardia cycle, therefore making it more amenable to antitachycardia therapy. Also, by reducing the number of shocks, AADs can improve the device’s battery life.
AADs are of particular importance in the management of electrical storms. Prompt hospitalization to reverse the precipitating factors and acute administration of AADs is indicated in these cases to ensure survival.33 However, in patients who present with electrical storms while on AADs, acute intravenous AAD therapy will likely fail. In these cases, the patient will require emergent catheter ablation.34, 35 Table 2 summarizes the major clinical trials concerning the use of adjuvant AAD therapy in patients with an ICD.36-42
Table 2.
List of major randomized clinical trials involving adjuvant antiarrhythmic drug therapy in patients with an ICD.
| Study | Inclusion Criteria | Endpoints | Drugs | Control | Key Results |
| Pacifico et al.36 | History of VTs ICD with shocks | All-cause death or all-cause ICD shock Mean frequency of shocks |
Sotalol | Placebo | Sotalol decreased all-cause death or all-cause ICD shocks; sotalol also decreased mean frequency of shocks |
| Kuhlkamp et al.37 | Sustained VT or VF Inducible VT or VF | Recurrence of VT or VF Total mortality |
Sotalol Sotalol/ICD |
Placebo ICD only |
Patients with inducible VT/VF after treatment with sotolol received ICD; sotalol decreased incidence of VT/VF recurrence; total mortality was unchanged across all arms |
| Seidl et al.38 | Indication for ICD | Appropriate ICD therapy by ATP or shock Actuarial survival rate |
Metoprolol | Sotalol | Appropriate ICD therapy was lower in metoprolol group; actuarial survival rate was not significantly different |
| Kettering et al.39 | Sustained VT or VF ICD | Recurrent VT or VF requiring ICD therapy Event-free survival Total mortality |
Metoprolol | Sotalol | The rate of VT/VF recurrence, event- free survival, and total mortality between the treatment arms was not statistically significant |
| Singer et al.40 | ICD Inducible VT At least one shock in prior year |
Frequency of appropriate ICD shocks and ATP | Azimilide | Placebo | Frequency of ICD shocks and ATP was reduced in the treatment arm |
| SHIELD41 | ICD VT or VF |
All-cause shock and ATP All-cause shock Appropriate ICD therapy |
Azimilide | Placebo | All-cause shock and ATP was reduced in the treatment arm; all-cause shock trend toward reduction in treatment arm; appropriate ICD therapy was reduced in treatment arm |
| OPTIC42 | ICDVT or VF LVEF ≤40% |
All-cause ICD shock Rate of drug discontinuation |
Amiodarone + Beta-blocker | Beta-blocker Sotalol |
All-cause ICD shock was lower in amiodarone + beta-blocker group compared to sotolol alone and beta- blocker alone; rate of drug discontinuation was highest for sotolol followed by amiodarone and lowest for beta-blocker |
VT: ventricular tachycardia; VF: ventricular fibrillation; ICD: implantable cardioverter defibrillator; ATP: antitachycardia pacing; LVEF: left ventricular ejection fraction.
There are multiple drawbacks to using AADs either as standalone or adjuvant therapy. First, most AADs are poorly tolerated by patients. In the Optimal Pharmacological Therapy in Cardioverter Defibrillator Patients (OPTIC) trial, the rate of drug discontinuation at 1 year was 23.5% for patients taking sotalol and 18.2% for those taking amiodarone. Drug toxicity is another concern in patients taking AADs. For example, long-term use of amiodarone is associated with significant pulmonary and thyroid toxicity.43 AADs also can increase mortality through a net proarrhythmic effect.16, 17 Futhermore, they also may interfere with ICD function by altering the defibrillation and pacing thresholds.44 Based on these drawbacks, AADs do not seem to be an effective long-term option and therefore should be used with extreme caution, especially in patients with significant structural heart disease.
Radiofrequency Catheter Ablation
RFCA is a potentially curative standalone therapy in patients with idiopathic VT due to its high success rate. However, its use in patients with structural heart disease is less straightforward. In these patients, RFCA often is used as an adjunctive therapy to ICDs in order to prevent or reduce the number of ICD shocks. This strategy has become attractive since AADs are not highly effective and are poorly tolerated.
The initial success of RFCA was limited to patients with stable VTs who could tolerate RFCA during VT induction in the laboratory from a hemodynamic standpoint. The introduction of electroanatomical mapping systems (EMS) has allowed RFCA, using substrate mapping, in patients with hemodynamically nontolerated or noninducible VTs.45 EMS allows the creation of a 3D ventricular voltage map during sinus or paced rhythm. The map displays, in the 3D geometry of the left ventricle, color-coded amplitudes of the local bipolar electrical signals. Scars from previous myocardial infarction of other nonischemic infiltrative processes can be readily identified by the low amplitude of the local electrical signals (typically less than 1.5 mV).45 This map displays low-voltage areas of scarring as well as regions with late potentials within the scars. Late potentials correspond to areas of slow conduction, in which (during sinus rhythm) activations reach these sites after the QRS. These sites serve as potential reentry circuits, and ablating them can effectively interrupt VT induction (Figure 1).46 The development of irrigated electrodes that cool the electrode-tissue ablation interface allows radiofrequency delivery to deeper endocardial tissue, further facilitating RFCA of large VT circuits.47 Catheter stability and precision of radiofrequency delivery is further improved with the use of Sensei® X Robotic Catheter System (Hansen Medical, Inc., Mountain View, CA).48 Multiple clinical trials have demonstrated RFCA to be an effective therapy for VTs — either prophylactically at the time of ICD implantation or in patients with frequent ICD interventions.
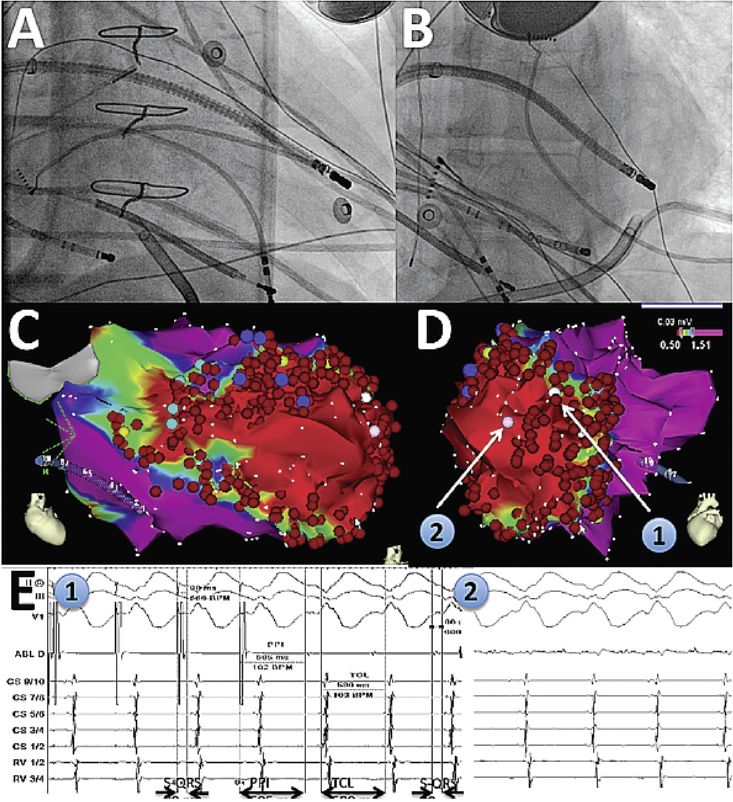
Figure 1.
Catheter ablation of ventricular tachycardia using a robotic system. A and B show right anterior oblique and left anterior oblique fluoroscopic views of the robotic catheter system reaching the LV apex (in particular, the lateral aspect of the apex) where the LV VT substrate was found. An epicardial sheath is also present. C and D show corresponding 3D maps of bipolar endocardial voltage amplitudes, demonstrating a large scar (red). Sites 1 and 2 correspond to the exit site of the VT circuit and its mid-diastolic location, respectively. E1 on the left shows pacing from site 1, with concealed entrainment and post-pacing interval (PPI) identical to the VT cycle length (TCL), and stimulus-to-QRS delay identical to signal-to-QRS (S-QRS). E2 on the right shows a mid-diastolic potential at site 2 during VT.
LV: left ventricular; VT: ventricular tachycardia.
Prophylactic RFCA therapy for ICD shock prevention has been assessed in two large clinical trials. The Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia (SMASH-VT) multicenter trial enrolled patients with a history of MI who were either undergoing ICD implantation or had an ICD implanted within 6 months prior to enrollment. The indication for ICD implantation included VF, hemodynamically unstable VT, or syncope with inducible VT during electrophysiological testing.10 Patients also were enrolled if they had their ICDs implanted for primary prevention and subsequently received appropriate shock therapy for a single event. In the ablation arm, there was a 65% reduction in ICD therapies (shocks and antitachycardia pacing) during the 2 years of follow up (P = 0.007). When antitachycardia pacing was excluded, the patients with ablation had a 73% (P=0.003) reduction in the risk of receiving an ICD shock therapy. A trend towards fewer deaths during follow-up was also observed in patients within the ablation arm. The second trial, Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH), enrolled patients who had an indication for ICD for secondary prevention after stable irreversible VT, coronary artery disease, previous MI, or reduced LVEF (≤50%).11 These patients received RFCA using electroanatomical substrate mapping prior to ICD implantation. The primary endpoint of this trial was time to first VT or VF recurrence. Patients were followed for a mean of 22.5 months. Time to recurrence of first VT or VF event was 18.6 months in the ablation group compared to 5.9 months in the control group. At 2 years, the estimate of survival free from VT or VF was 47% in the ablation arm and 29% in the control arm (P = 0.045). ICD shocks occurred in 32.7% of the ablation group at 2 years follow-up compared to 53.7% of the control group. The incidence of ablation-related death was 0% in both trials, and the rate of major complications was 4.7% and 3.8%, respectively. In light of these two studies, RFCA is recommended in patients with an ICD device and multiple ICD therapies.
Two large prospective trials have evaluated the use of RFCA in patients with ICD who had experienced multiple shock deliveries or incessant VTs and had failed AAD therapy.
The Cooled RF Ablation System clinical trial enrolled 146 patients who had hemodynamically stable VTs but had failed at least two AAD therapies.49 The patients were randomized to either RFCA or continuation of AADs. Of these patients, 79% either had an ICD at the time of enrollment or had one implanted prior to discharge. The mean number of VT episodes within the preceding 2 months was 25 ± 31. Acute termination of all mappable VTs was achieved in 75% of patients, while 41% of patients had acute termination of all VT types. Clinical success, defined as ≥75% reduction of VT frequency at the 2-month follow-up, was observed in 81% of the patients. One or more episodes of VT occurred in 46% of patients, with a median time to first VT episode of 24 days. The Kaplan-Meier recurrence rate of VT was 56% at 1 year. The rate of major complications related to RFCA was 8%, and the mortality rate at 1-year follow-up was 25%.
The Multicenter Thermocool VT Ablation Trial was a larger multicenter study with 231 patients. In contrast to the Cooled RF trial, this trial included patients with both mappable and unmappable VTs. Of these patients, 37% had a previous RFCA procedure, 70% had failed AAD therapy with amiodarone, and 94% had an ICD at the time of enrollment. A median of 11 VT episodes was recorded in each patient in the preceding 6 months, and 16% of patients had incessant VTs. The primary endpoint of freedom from recurrent incessant VTs at the 6-month follow-up was achieved in 53% of the patients. The median of VT episodes was reduced from 11.5 to 0 at the 6-month follow-up compared to the 6-month period preceding RFCA. While 20% of patients had an increase in their VT episodes, 67% had a ≥75% reduction of VT episodes. The procedure mortality rate was 3% and the 1-year rate was 18%.
RFCA of VTs is effective in patients with HF and ICD both prophylactically and after multiple shocks. RFCA also is indicated acutely in patients with an electrical storm that is not responsive to intravenous AADs. Finally, RFCA is indicated in patients with incessant VTs that are slow and not detected by ICD. It is important to recognize that AADs are often continued in patients after RFCA. SMASH-VT is the only trial in which AAD therapy was stopped in patients following ablation. A direct comparison of optimal AAD therapy versus RFCA is being evaluated in the VT Ablation Versus Enhanced Drug Therapy (VANISH) trial that is currently in the enrollment phase.
Coronary Alcohol Ablation
Although RFCA with or without AAD therapy has proven effective for suppression of recurrent VTs, some patients have recurrent episodes and require repeat ablations.50 Some patients, however, remain refractory to multiple RFCAs and AAD therapy. These patients tend to have VTs with deep intramural circuits that an ablation catheter cannot access. In such cases, coronary ethanol ablation has shown great promise. In this method, ethanol is injected into a coronary artery branch proximal to the VT circuit. Since ethanol can penetrate into the myocardial tissue, it can ablate deeper circuits that are inaccessible to RFCA.
In a recent study, 27 consecutive patients with recurrent VTs and failed RFCA were considered for coronary artery alcohol ablation. Of these, 22 patients had a VT circuit that was mapped proximal to a coronary artery branch.51 Alcohol ablation successfully terminated the targeted VT in 82% of the patients. Recurrence of VT was observed in 14 (64%) of patients within a median of 16 days. However, 8 of these 14 patients had a VT storm or incessant VTs, and 6 of them remained free of these conditions. Therefore, coronary artery ethanol ablation successfully terminated or improved VTs in 63.6% of the patients. A complete heart block occurred in 5 patients (22.3%), and 3 patients (13.6%) with advanced HF died within 30 days of the operation.
We recently presented two cases in which a retrograde transcoronary venous approach was attempted for delivery of alcohol to the myocardium. Both patients had incessant VTs and had failed multiple RFCA attempts and AADs. Successful termination of all inducible VTs was achieved in both patients without any periprocedural complications.14
Alcohol ablation is a reasonable “last resort” for patients with incessant VTs or electrical storms who remain refractory to AAD and RFCA therapies. Major limitations of this procedure are unpredictability of alcohol delivery and risk of vascular and tissue damage in unwarranted regions.
Conclusion
In the past few decades, antiarrhythmic drug therapy has been widely used for suppression of ventricular arrhythmias in patients with heart failure. These drugs, however, are very poorly tolerated by patients due to their toxic side effects. Radiofrequency catheter ablation is a better option for prevention and suppression of ventricular arrhythmias in HF patients, and it has a very high safety margin. Coronary artery or venous ablations are reasonable in patients with refractory VTs who have failed previous RFCA attempts. However, they carry higher complication rates and are limited to a few large academic centers with very experienced clinicians.
Funding Statement
Funding/Support: The authors have no funding disclosures.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and the following was reported: Dr Valderrabano is a consultant to St Jude Medical, Hansen Medical, and Biosense-Webster.
References
- 1.Francis GS. Development of arrhythmias in the patient with congestive heart failure: pathophysiology prevalence and prognosis. Am J Cardiol. 1986 Jan 31;57(3):3B–7B. doi: 10.1016/0002-9149(86)90991-4. [DOI] [PubMed] [Google Scholar]
- 2.Holmes J, Kubo SH, Cody RJ, Kligfield P. Arrhythmias in ischemic and nonischemic dilated cardiomyopathy: prediction of mortality by ambulatory electrocardiography. Am J Cardiol. 1985 Jan 1;55(1):146–51. doi: 10.1016/0002-9149(85)90317-0. [DOI] [PubMed] [Google Scholar]
- 3.Hynes BJ, Luck JC, Wolbrette DL, Boehmer J, Naccarelli GV. Arrhythmias in patients with heart failure. Curr Treat Options Cardiovasc Med. 2002 Dec;4(6):467–85. doi: 10.1007/s11936-002-0041-1. [DOI] [PubMed] [Google Scholar]
- 4.The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997 Nov 27;337(22):1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. New Engl J Med. 2002 Mar 21;346(12):877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005 Jan 20;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Kamphuis HC, de Leeuw JR, Derksen R, Hauer RN, Winnubst JA. Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace. 2003 Oct;5(4):381–9. doi: 10.1016/s1099-5129(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 8.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008 Sep 4;359(10):1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KP. Sudden cardiac death unresponsive to implantable defibrillator therapy: an urgent target for clinicians industry and government. J Interv Card Electrophysiol. 2005 Nov;14(2):71–8. doi: 10.1007/s10840-005-4547-9. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007 Dec 27;357(26):2657–65. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz E, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010 Jan 2;375(9708):31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 12.Gepstein L, Hayam G, Ben-Haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation. 1997 Mar 18;95(6):1611–22. doi: 10.1161/01.cir.95.6.1611. [DOI] [PubMed] [Google Scholar]
- 13.Brugada P, de Swart H, Smeets JL, Wellens HJ. Transcoronary chemical ablation of ventricular tachycardia. Circulation. 1989 Mar;79(3):475–82. doi: 10.1161/01.cir.79.3.475. [DOI] [PubMed] [Google Scholar]
- 14.Baher A, Shah DJ, Valderrabano M. Coronary venous ethanol infusion for the treatment of refractory ventricular tachycardia. Heart Rhythm. 2012 Oct;9(10):1637–9. doi: 10.1016/j.hrthm.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000 Aug 15;102(7):748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 16.Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. The Cardiac Arrhythmia Suppression Trial II Investigators. N Engl J Med. 1992 Jul;327(4):227–33. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- 17.Echt DS, Leibson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991 Mar 21;324(12):781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 18.International Mexiletine and Placebo Antiarrhythmic Coronary Trial (IMPACT): II. Results from 24-hour electrocardiograms. IMPACT Research Group. Eur Heart J. 1986 Sep;7(9):749–59. [PubMed] [Google Scholar]
- 19.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982 Mar 26;247(12):1707–14. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 20.McMurray J, Kober L, Robertson M, Dargie H, Colucci W, Lopez-Sendon J, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol. 2005 Feb 15;45(4):525–30. doi: 10.1016/j.jacc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 21.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999 Jan 2;353(9146):9–13. [PubMed] [Google Scholar]
- 22.Tepper D. Frontiers in congestive heart failure: Effect of Metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Congest Heart Fail. 1999 Jul-Aug;5(4):184–5. [PubMed] [Google Scholar]
- 23.Køber L, Torp-Pederson C, McMurray JJ, Gøtzche O, Levy S, Crijns H, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008 Jun 19;358(25):2678–87. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 24.Burkart F, Pfisterer M, Kiowski W, Follath F, Burckhardt D. Effect of antiarrhythmic therapy on mortality in survivors of myocardial infarction with asymptomatic complex ventricular arrhythmias: Basel Antiarrhythmic Study of Infarct Survival (BASIS). J Am Coll Cardiol. 1990 Dec;16(7):1711–8. doi: 10.1016/0735-1097(90)90324-i. [DOI] [PubMed] [Google Scholar]
- 25.Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet. 1997 Mar 8;349(9053):675–82. doi: 10.1016/s0140-6736(96)08171-8. [DOI] [PubMed] [Google Scholar]
- 26.Singh SN, Carson PE, Fisher SG. Nonsustained ventricular tachycardia in severe heart failure. Circulation. 1997 Nov 18;96(10):3794–5. [PubMed] [Google Scholar]
- 27.Kober L, Bloch Thomsen PE, Moller M, Torp-Pederson C, Carlsen J, Sandoe E, et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000 Dec 16;356(9247):2052–8. doi: 10.1016/s0140-6736(00)03402-4. [DOI] [PubMed] [Google Scholar]
- 28.Julian DG, Camm AJ, Frangin G, Janse ML, Munoz A, Schwartz PJ, et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997 Mar 8;349(9053):667–74. doi: 10.1016/s0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- 29.Doval HC, Nul DR, Grancelli HO, Varini SD, Soufer S, Corrado G, et al. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA-GEMA Investigators. Circulation. 1996 Dec 15;94(12):3198–203. doi: 10.1161/01.cir.94.12.3198. [DOI] [PubMed] [Google Scholar]
- 30.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999 Dec 16;341(25):1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 31.Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996 Jul 6;348(9019):7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg JS, Martins J, Sadanandan S, Goldner B, Menchavez E, Domanski M, et al. Antiarrhythmic drug use in the implantable defibrillator arm of the Antiarrhythmics Versus Implantable Defibrillators (AVID) Study. Am Heart J. 2001 Sep;142(3):520–9. doi: 10.1067/mhj.2001.117129. [DOI] [PubMed] [Google Scholar]
- 33.Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence management and prognostic implications. J Am Coll Cardiol. 1998 Dec;32(7):1909–15. doi: 10.1016/s0735-1097(98)00495-1. [DOI] [PubMed] [Google Scholar]
- 34.Izquierdo M, Ruiz-Granell R, Ferrero A, Martinez A, Sanchez-Gomez J, Bonanad C, et al. Ablation or conservative management of electrical storm due to monomorphic ventricular tachycardia: differences in outcome. Europace. 2012 Dec;14(12):1734–9. doi: 10.1093/europace/eus186. [DOI] [PubMed] [Google Scholar]
- 35.Silva RM, Mont L, Nava S, Rojel U, Matas M, Brugada J. Radiofrequency catheter ablation for arrhythmic storm in patients with an implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2004 Jul;27(7):971–5. doi: 10.1111/j.1540-8159.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 36.Pacifico A, Hohnloser SH, Williams JH, Tao B, Saksena S, Henry PD, et al. Prevention of implantable-defibrillator shocks by treatment with sotalol. d,l-Sotalol Implantable Cardioverter-Defibrillator Study Group. N Engl J Med. 1999 Jun 17;340(24):1855–62. doi: 10.1056/NEJM199906173402402. [DOI] [PubMed] [Google Scholar]
- 37.Kuhlkamp V, Mewis C, Mermi J, Bosch RF, Seipel L. Suppression of sustained ventricular tachyarrhythmias: a comparison of d,l-sotalol with no antiarrhythmic drug treatment. J Am Coll Cardiol. 1999 Jan;33(1):46–52. doi: 10.1016/s0735-1097(98)00521-x. [DOI] [PubMed] [Google Scholar]
- 38.Seidl K, Hauer B, Schwick NG, Zahn R, Senges J. Comparison of metoprolol and sotalol in preventing ventricular tachyarrhythmias after the implantation of a cardioverter/defibrillator. Am J Cardiol. 1998 Sep 15;82(6):744–8. doi: 10.1016/s0002-9149(98)00478-0. [DOI] [PubMed] [Google Scholar]
- 39.Kettering K, Mewis C, Dornberger V, Vonthein R, Bosch RF, Kuhlkamp V. Efficacy of metoprolol and sotalol in the prevention of recurrences of sustained ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2002 Nov;25(11):1571–6. doi: 10.1046/j.1460-9592.2002.01571.x. [DOI] [PubMed] [Google Scholar]
- 40.Singer I, Al-Khalidi H, Niazi I, Tchou P, Simmons T, Henthorn R, et al. Azimilide decreases recurrent ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators. J Am Coll Cardiol. 2004 Jan 7;43(1):39–43. doi: 10.1016/j.jacc.2003.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Dorian P, Borggrefe M, Al-Khalidi HR, Hohnloser SH, Brum JM, Tatla DS, et al. Placebo-controlled, randomized clinical trial of azimilide for prevention of ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillator. Circulation. 2004 Dec 14;110(24):3646–54. doi: 10.1161/01.CIR.0000149240.98971.A8. [DOI] [PubMed] [Google Scholar]
- 42.Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES, et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA. 2006 Jan 11;295(2):165–71. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 43.Piccini JP, Berger JS, O’Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J. 2009 May;30(10):1245–53. doi: 10.1093/eurheartj/ehp100. [DOI] [PubMed] [Google Scholar]
- 44.Hohnloser SH, Dorian P, Roberts R, Gent M, Israel CW, Fain E, et al. Effect of amiodarone and sotalol on ventricular defibrillation threshold: the optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation. 2006 Jul 11;114(2):104–9. doi: 10.1161/CIRCULATIONAHA.106.618421. [DOI] [PubMed] [Google Scholar]
- 45.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000 Mar 21;101(11):1288–96. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 46.Volkmer M, Ouyang F, Deger F, Ermst S, Goya M, Bansch D, et al. Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia: impact on outcome of catheter ablation. Europace. 2006 Nov;8(11):968–76. doi: 10.1093/europace/eul109. [DOI] [PubMed] [Google Scholar]
- 47.Soejima K, Delacretaz E, Suzuki M, Brunckhorst CB, Maisel WH, Friedman PL, et al. Saline-cooled versus standard radiofrequency catheter ablation for infarct-related ventricular tachycardias. Circulation. 2001 Apr 10;103(14):1858–62. doi: 10.1161/01.cir.103.14.1858. [DOI] [PubMed] [Google Scholar]
- 48.Valderrábano M, Dave AS, Baez-Escudero JL, Rami T. Robotic catheter ablation of left ventricular tachycardia: initial experience. Heart Rhythm. 2011 Dec;8(12):1837–46. doi: 10.1016/j.hrthm.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DM, et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol. 2000 Jun;35(7):1905–14. doi: 10.1016/s0735-1097(00)00615-x. [DOI] [PubMed] [Google Scholar]
- 50.Kosmidou I, Inada K, Seiler J, Koplan B, Stevenson WG, Tedrow UB. Role of repeat procedures for catheter ablation of postinfarction ventricular tachycardia. Heart Rhythm. 2011 Oct;8(10):1516–22. doi: 10.1016/j.hrthm.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Tokuda M, Sobieszcyzk P, Eisenhauer AC, Kojodjojo P, Inada K, Koplan BA, et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhythm Electrophysiol. 2011 Dec;4(6):889–96. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]


