Abstract
Ventricular assist devices are commonly utilized in the treatment of end-stage heart failure. Advances in continuous flow technology have improved efficiency, size, implantability, extended support, and overall patient outcomes. This has led to an expanded role of left ventricular assist device (LVAD) clinical use and applications. This review describes the advances and current state of LVAD devices and provides a future outlook for this technology.
Keywords: mechanical assist device, ventricular assist device, INTERMACS, bridge to transplant

Introduction
Cardiac transplantation remains the gold-standard treatment for end-stage heart failure refractory to medical therapy. However, its greatest limitation has been the number of donor hearts available. Coupled with ever-growing patient waiting lists and stringent eligibility criteria, transitory therapeutic measures (whether as a bridge or long term) to promote survival in end-stage heart failure have become a highly investigated field of medicine. As a consequence, implantable mechanical circulatory devices (MCDs) have emerged as a relevant option for improving survival in these patients. In general, patients receiving LVAD implantation are classified based on the expected outcome and device strategy. The most common strategies include bridge to transplant (BTT), bridge to candidacy (BTC), destination therapy (DT), and bridge to recovery (BTR) (Table 1). Established in June 2006, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) has been acquiring data on the vast majority of patients with implanted MCDs. To date, more than 6,000 patients have been entered into the database, with an annual growth of approximately 1,500 implants per year.1 Most importantly, INTERMACS has provided a tremendous amount of data on the current state of primary device placement and outcomes. In this review, we describe the types of implantable pumps, most recent outcomes, and future outlook.
Table 1.
Device strategy and total implants by year. Data derived from fourth and fifth INTERMACS report.1 MCS: mechanical circulatory support.
| Device Strategy | Definition | 2008 (%) | 2010 (%) | 2012* (%) |
| Bridge to Transplant (BTT) | Patient actively listed for a transplant. Would not survive without MCS. | 49.6 | 29.2 | 21.0 |
| Bridge to Candidacy (BTC) | Patient not actively listed for transplantation. Does not have absolute contraindication to transplant. Potential for recovery is unclear. | 41.2 | 38.3 | 33.1 |
| Destination Therapy (DT) | Patient is ineligible for transplant, but would not survive without MCS. Has absolute contraindication to transplant. | 5.4 | 30.6 | 44.0 |
| Bridge to Recovery (BTR) | Patient needs temporary support. Expected recovery. No transplant needed. | 2.1 | 0.8 | 1.0 |
| Total Number of Implants | 726 | 729 | 896 | |
*2012 (Jan-June, 6 months)
First-Generation LVADs
Also known as volume displacement devices, the first-generation of implantable devices pumped blood flow via a pulse generator, hence the term “pulsatile pump.” The systems that highlight this class are the HeartMate I® (Thoratec Corp., Pleasanton, CA), Thoratec PVAD™ (Thoratec Corp., Pleasanton, CA), and Novacor N100 (World Heart, Inc., Oakland, CA). Inherently, first-generation devices had larger tissue and blood contacting surfaces as well as multiple moving parts (i.e., pusher plates, pneumatic/electrical sacs, prosthetic valves).2 The HeartMate I (Figure 1) introduced a textured blood contacting surface, which is still being used in newer-generation devices to reduce thrombotic complications.3
Figure 1.
HeartMate I (left) and HeartMate II (right).
Reprinted with the permission of Thoratec Corporation.
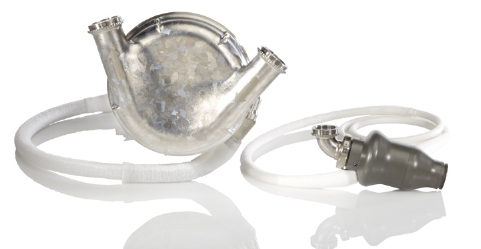
Implantation of these first-generation devices requires a median sternotomy, with inflow and outflow cannulation insertions made at the left ventricular apex and ascending aorta, respectively. Due to its size, the pumping chamber is located within the abdomen or preperitoneal space, and a single transcutaneous drive line exits the abdominal wall. As with all pulsatile systems, a compliance chamber is necessary to compensate for air displacement.4 Cardiac output that meets physiologic demand is standard, and they can be employed on a fixed or automatic setting. These devices are battery powered, providing 3 to 5 hours of charge.5 The native heart can usually provide systemic support in cases of device malfunction.
The major disadvantages that led to continued efforts to improve implantable LVADs included comfort/ease of use for patients and long-term mechanical durability of the pump. Furthermore, high risk of infection, thrombus formation, and blood trauma were significant complications that needed to be addressed if long-term support was to be achieved with LVAD therapy.2-6 In general, use of these devices requires long-term oral anticoagulation therapy. Despite these limitations, first-generation LVADs set the stage for implantable mechanical support. The landmark first-generation LVAD study, REMATCH, demonstrated a significant reduction in death and increased survival from any cause as compared to medical therapy alone. More specifically, endpoints of the study showed a 46% decrease in death from any cause and a 1-year survival of 52% with LVAD versus 25% with medical therapy.7
Second-Generation LVADS
To improve upon the limitations of their predecessors, the second-generation LVADs required a decrease in size and fewer complications while improving efficiency and durability. To accomplish these goals, researchers focused on the development and principles of continuous axial flow pumps. Systems that highlight the second-generation LVAD class include the HeartMate II® (Thoratec Corp., Pleasanton, CA) (Figure 1), Jarvik 2000 (Jarvik Heart, Inc., New York, NY), and Micromed DeBakey® (MicroMed Cardiovascular, Inc., Houston, TX).
A central skepticism of second-generation devices was whether “non-pulsatile/physiological” continuous blood flow could support long-term end-organ function. However, it has been well demonstrated that pulsatile blood flow primarily occurs at large/high-pressure arteries, as compared to capillary flow, where pulsatile flow is markedly reduced. In fact, the average velocity of blood flow in capillaries is about one-thousandth of that found in the aorta.8 This understanding, coupled with the need for LVAD improvement, fueled research in animal studies and ensuing clinical projects regarding continuous flow. The resulting studies were able to demonstrate successful long-term end-organ perfusion.9, 10
The key mechanical element was the implementation of a valveless axial pump with a rotary motor as the only moving part in the system. More specifically, the design introduced an internal rotor in the axial path of flow that was suspended via blood-immersed bearings (i.e., the rotor is in direct contact).11 The theoretical benefit of this design was further reduction of prothrombotic sites and minimization of wear and tear associated with multiple moving parts. Efficiency was further enhanced with elimination of the reservoir chamber and inflow/outflow valves. The blood contacting surfaces were specially designed with textured titanium lining as an additional antithrombotic measure.12
Surgical implantation is similar to the first-generation model, with inflow/outflow cannulation tracts practically unaltered (Figure 2).13 The inlet cannula is placed in the left ventricle, and the outflow cannula is anastamosed to the aorta via a Dacron graft. A single driveline exits the abdomen. The impeller blade is powered by an electromagnetic motor, which is driven by an external battery source similar to first-generation devices. The second-generation LVAD pump is designed to provide high-level cardiac output, with rotary speeds of 8,000 to a maximum of 15,000 rpm reported.14
Figure 2.
Schematic drawing of implanted HeartMate II LVAD.
Reprinted with the permission of Thoratec Corporation.
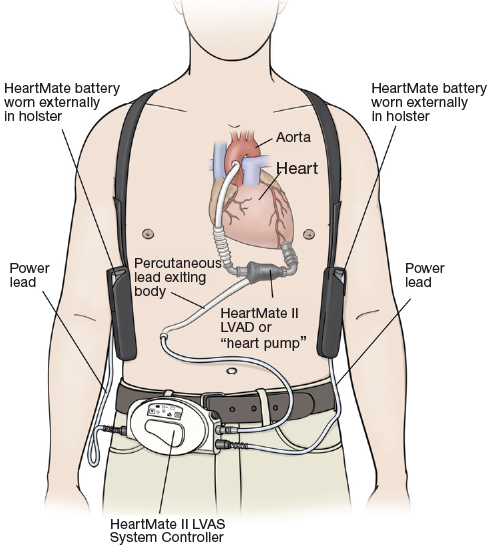
The benchmark predicted mechanical life of second-generation LVADs is 5 years, but longer support has been well documented.15 Long-term anticoagulation is still required, with emphasis on individual patient needs.16, 17 In 2007, the HeartMate II BTT trial became another landmark study for implantable LVADs, as it demonstrated survival rates of 75% at 6 months and 68% at 1 year, with significantly improved quality of life and functional capacity.18 In 2009, this was followed by the HeartMate II DT trial, which showed a significant increase in survival 2 years post-implantation when compared to HeartMate I.19 Despite the significant improvements in design, the second-generation device still left many theoretical concerns. Of importance were further enhancements of efficiency, durability, and patient outcomes. This may be a direct consequence of the increasing trend of LVAD use as destination/long-term therapy.
Third-Generation LVADS
The critical distinguishing factor between the second- and third-generation LVADs is the employment of contact versus noncontact bearings, respectively. The latter employs the technology known as magnetic levitation (MAGLEV), which allows for rotation without friction or wear.20 The goal of this design is to further minimize prothrombotic sites while enhancing efficiency and durability. The devices that highlight the latest generation include DuraHeart™ (Terumo Heart, Inc., Ann Arbor, MI), HeartWare HVAD® (HeartWare International, Inc., Framingham MA), Incor® (Berlin Heart, Inc., Berlin, Germany), Levacor® (World Heart Inc., Salt Lake City, UT), and HeartMate III (Thoratec Corp., Pleasanton, CA) (Figure 3).
Figure 3.
Third-generation LVADS. (A) HeartMate III. ©Thoratec Corp. Reprinted with permission. (B) HeartWare HVAD. ©Heartware International.
Reprinted with permission.

Third-generation LVADS remain classified as continuous-flow pumps but can be broadly differentiated as follows: 1) centrifugal versus axial flow pumps, and 2) magnetically levitated impeller +/- hydrodynamic support (Figure 4).21 Also known as radial flow pumps, centrifugal pumps afford certain advantages that include lower rotational speeds, higher efficiency, and further enhanced anatomic design. For example, the Levacor achieves physiologic cardiac output at a rotational speed of 2,000 rpm.22 This is a significant difference when compared to Micromed DeBakey, which requires almost quadruple the rate (9,500±600 rpm) to achieve similar outputs.23 The Incor is the only third-generation axial pump under active clinical investigation, with the remaining pumps listed as centrifugal. In terms of levitation, the DuraHeart employs a dual hydrodynamic and magnetic system for levitation compared to the Levacor, which is completely magnetically levitated. Unique to the HeartWare (FDA approval November 2012) is that implantation is completely intrapericardial (Figure 5). This is attributed to the smaller pump size (Figure 3) and is significant because the need for an abdominal pocket is eliminated.24
Figure 4.
HeartWare HVAD design and flow patterns. (A) HVAD assembly: inflow cannula within front housing, magnetic center-post, and rotating impeller. (B) Primary and secondary flow paths. The primary flow path enters via the inflow cannula and into four impeller flow channels that point flow centrifugally (black arrows). Blood collects in the housing assembly and exits via the outflow graft. The secondary flow path starts under the impeller at the center post, which creates an axial flow (black arrows) and re-enters the primary flow path.
Image reproduced with permission from Larose JA, Tamez D, Ashenuga M, Reyes C. Design concepts and principle of operation of the HeartWare ventricular assist system. ASAIO J. 2010 Jul-Aug;56(4):285-9.38
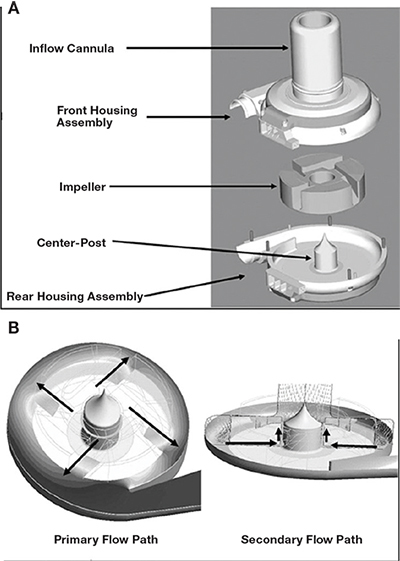
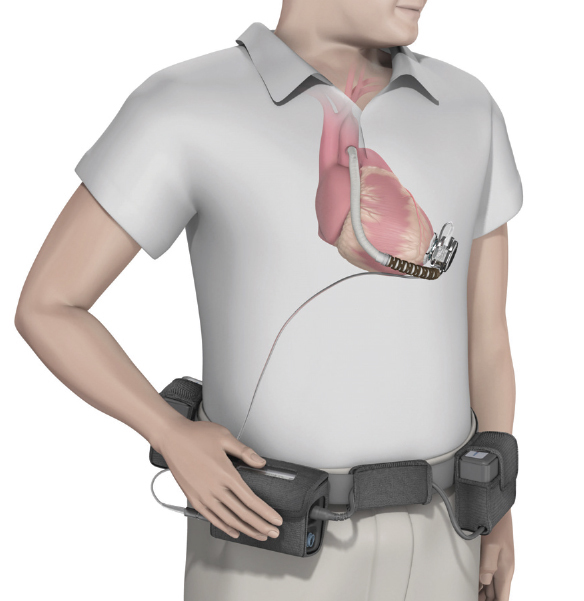
Figure 5.
Schematic drawing of implanted HeartWare HVAD.
©Heartware International. Reprinted with permission.
In terms of survival, reports demonstrate expected success with third-generation devices. For example, one European single-center study reported very promising long-term outcomes in 68 patients implanted with the DuraHeart. Overall survival at 3, 6, 12, and 24 months was 87%, 81%, 77%, and 61%, respectively.25 Another more recent study of 34 patients implanted with the HeartWare LVAD demonstrated an overall mortality rate of 24% and overall cumulative survival of 56% at 2 years.26 Table 2 illustrates the currently active second- and third-generation devices.
Table 2.
Second- and Third-Generation LVADs.
| Device | Specs (grams, flow, placement, levitation) | Unique Features |
| Thoratec HeartMate II | 375, AX, IA | most widely studied > 200 publications |
| Jarvik 2000 | 85, AX, IP | documented survival 7.5 years |
| Micromed DeBakey 5 | 93, AX, IP | direct flow measurement |
| Terumo Dura-Heart | 540, RA, IA, ML | hydrodynamic backup system |
| HeartWare HVAD | 145, RA, IP, ML+ | uni/biventricular capabilities smallest design |
| Berlin Heart Incor | 200, AX, IP, ML | only 3rd generation AX >500 implants |
| Levacor VAD | 440, RA, IA, ML | complete magnetic suspension |
| Thoratec HeartMate III | 500, RA, IA, ML | artificial pulse generator, sensorless flow estimator |
AX: axial flow; BTT: bridge to transplant; C: clinical; CE: European approved; DT: destination therapy; FDA: Food and Drug Administration; HDE: humanitarian device exemption; HF: heart failure; IA: intra-abdominal; IDE: investigational device exemption; IP: intrapericardial; ML: magnetically levitated; ML+: mixed ML with hydrodynamics; RA: radial flow.
Current State
As mentioned, the INTERMACS registry provides a wealth of information on the current state of LVADs actively implanted throughout the world. Since commencing in June 2006, more than 6,000 patients have been entered, and 145 institutions are actively participating in the registry. The most recent publication, the Fifth INTERMACS Annual Report, demonstrated notable changes in the culture of LVAD employment.1 There was a significant increase in LVAD placement after the HeartMate II BTT trial, which has resulted in a virtual replacement of pulsatile pumps with continuous pumps (Figure 6). This cultural change was reflected in 2010 when nearly 99% of LVADs placed were continuous.
Figure 6.
Distribution of LVADs placed from 2006–2011. Data derived from 4th INTERMACS report.1
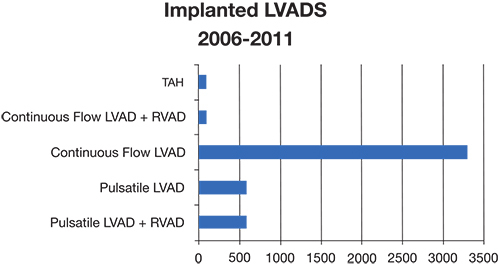
There also has been a noted change in risk factor analysis and decision regarding when to place an LVAD. To understand this notion, one must understand the INTERMACS risk levels, which range from 1–7. In general, levels 1–5 fall under New York Heart Association (NYHA) functional class IV, with level 1 classifying the worst state, cardiogenic shock. Levels 6–7 are patients in the advanced NYHA class III subset and can be viewed as relatively lower risk. The latest trends demonstrate a significant shift towards LVAD use as DT and in patients in higher (less compromised) INTERMACS brackets. From most to least common, the device strategies that are currently being employed are DT (44%), BTC (30%), BTT (21%), and BTR (1%).
In terms of risk factors for death post-implantation, cardiogenic shock and development of right heart failure (RHF) requiring biventricular support were among the most significant noted. The latter factor was depicted as “a major challenge and thrust” for future investigations. In fact, multiple studies have documented this complication, with RHF onset occurring in 10–40% of patients implanted with an LVAD.27-30 Additionally, parameters such as age, blood urea nitrogen, pulmonary hypertension, and pulsatile LVAD were all significant risk factors for death (P <0.05). Despite these risk factors, the overall expected survival rate is currently 81% at 1 year and nearly 70% at 2 years.
Future Progress
As outcomes improve with advances in pump technology, minimizing long-term morbidities will likely become the focus and challenge of future LVAD design. Some experts propose that the optimal LVAD, especially for use in DT, is one that is durable, biocompatible, and totally implantable (i.e., no transcutaneous machinery).31 Perhaps the most attainable upgrade will be the elimination of the driveline as a measure to reduce infection.
Patented in 1994, the technology known as transcutaneous energy transfer (TET) allows energy to be noninvasively transmitted inside the body.32 The fundamental mechanism of energy formation is an induction-heating (IH) system, which creates an electromagnetic charge between two sets of coils located inside and outside the body.33 Certain low-energy requiring devices, such as pacemakers and defibrillators, have already incorporated this technology. However, reports of electromagnetic interference with household devices have been documented.34 This interference ultimately results in device dysfunction and is a factor that has limited its commercial use in high-risk devices such as the LVAD. Thus, further investigations are warranted to improve upon these limitations. Interestingly, there are two third-generation LVADs, AbioCor® (Abiomed, Inc., Danvers, MA) and LionHeart® (Arrow International Inc., Research Triangle Park, NC), under clinical investigation that employ TET technology and have demonstrated TET reliability at 1 and 2 years.31
As described, DT is on the rise as a device strategy and has the highest potential benefit in older patients with end-stage heart failure. Currently, the majority of these patients are medically managed. The prospective trials REVIVE-IT and ROADMAP are well underway and are designed to investigate the benefits of a more “aggressive” LVAD implant strategy in these patients compared to medical management. REVIVE-IT is a randomized controlled trial to evaluate DT employing the HeartWare HVAD versus optimal medical management (OMM) in NYHA IIIb/IV patients who have not needed ionotropic support.35 ROADMAP is a multicenter nonrandomized observational trial of outcomes with HeartMate II versus OMM as DT in patients with INTERMACS 4–7 profiles.36 The primary objective of these studies is to show that strategic placement of implantable mechanical support before onset of higher risk heart failure can improve survival and functional status in moderate to severe heart failure patients.
As the use of LVADs increases, physicians must be prepared to readily assess and manage complications associated with these devices. Of importance, thrombotic events and right heart failure are serious potential complications of LVAD, and task forces are developing guidelines for managing complications in the post-implantation period.37 Thus, we can expect more refined and timely interventions, both pharmaceutical and mechanical, in the years to come.
Conclusion
Implantable LVADs have revolutionized the treatment of late-stage heart failure. They are the result of more than 60 years of research and investigation. Prospective studies in the last 10 years have consistently demonstrated the significant improvement in clinical status that can be achieved with strategic employment of LVAD therapy. Moreover, the durability and efficiency of these devices has allowed DT to become a viable option for patients who are not candidates for transplantation. At the moment, continuous-flow LVAD therapy has become the standard when implantation is warranted. Its success has opened the door for multiple devices to become FDA approved and for exponential growth in total implantations per year. There is a large market for potential candidates with a focus on providing safe, effective, and long-term care to a high-risk population. Understanding the patient population from a logistical level and employing preimplantation measures will decrease complications and further improve efficiency, cost, reliability, and durability of the devices. In conclusion, innovations in implantable heart devices have allowed the technology to become relevant and clinically applicable, offering an effective long-term therapeutic option for the management of patients with late-stage cardiac disease.
Acknowledgments
The authors thank Amanda Hodgson, Ph.D., for critical reading of the manuscript. We would also like to thank The Methodist Hospital Research Institute and the Methodist Cardiovascular Surgery Associates for their support throughout the formation of this comprehensive review.
Funding Statement
Funding/Support: The authors have no funding disclosures.
Footnotes
Conflict of Interest Disclosure: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Contributor Information
Limael E. Rodriguez, Methodist DeBakey Heart & Vascular Center, The Methodist Hospital, Houston, Texas
Erik E. Suarez, Methodist DeBakey Heart & Vascular Center, The Methodist Hospital, Houston, Texas
Matthias Loebe, Methodist DeBakey Heart & Vascular Center, The Methodist Hospital, Houston, Texas
Brian A. Bruckner, Methodist DeBakey Heart & Vascular Center, The Methodist Hospital, Houston, Texas
References
- 1.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Fifth INTERMACS Annual Report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013 Jan;32(2):141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998 Nov 19;339(21):1522–33. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 3.Farrar DJ, Bourque K, Dague CP, Cotter CJ, Poirier VL. Design features developmental status, and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO J. 2007 May-Jun;53(3):310–5. doi: 10.1097/MAT.0b013e3180536694. [DOI] [PubMed] [Google Scholar]
- 4.El-Banayosy A, Arusoglu L, Kizner L, Tenderich G, Minami K, Inoue K, et al. Novacor left ventricular assist system versus Heartmate vented electric left ventricular assist system as a long-term mechanical circulatory support device in bridging patients: a prospective study. J Thorac Cardiovasc Surg. 2000 Mar;119(3):581–7. doi: 10.1016/s0022-5223(00)70140-1. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson C, Paz JC. Total artificial heart and physical therapy management. Cardiopulm Phys Ther J. 2010 Jun;21(2):13–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Frazier OH, Myers TJ, Radovancevi′c B. The HeartMate left ventricular assist system. Overview and 12-year experience. Tex Heart Inst J. 1998;25(4):265–71. [PMC free article] [PubMed] [Google Scholar]
- 7.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001 Nov 15;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Prothero J, Burton AC. The physics of blood flow in capillaries. I. The nature of the motion.”. Biophys J. 1961;1:565–79. doi: 10.1016/s0006-3495(61)86909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito S, Westaby S, Piggot D, Dudnikov S, Robson D, Catarino PA, et al. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg. 2002;74(4):1080–5. doi: 10.1016/s0003-4975(02)03846-8. [DOI] [PubMed] [Google Scholar]
- 10.Kamdar F, Boyle A, Liao K, Colvin-Adams M, Joyce L, John R. Effects of centrifugal axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009 Apr;28(4):352–9. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Burke DJ, Burke E, Parsaie F, Poirier V, Butler K, Thomas D, et al. The Heartmate II: design and development of a fully sealed axial flow left ventricular assist system. Artif Organs. 2001 May;25(5):380–5. doi: 10.1046/j.1525-1594.2001.06770.x. [DOI] [PubMed] [Google Scholar]
- 12.Frazier OH, Delgado RM, 3rd, Kar B, Patel V, Gregoric ID, Myers TJ. First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report. Tex Heart Inst J. 2004;31:157–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007 Aug 30;357(9):885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 15.Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg. 2010 Jul;25(4):490–4. doi: 10.1111/j.1540-8191.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009 Sep;28(9):881–7. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 17.John R, Kamdar F, Liao K, Colvin-Adams M, Miller L, Joyce L, et al. Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. J Thorac Cardiovasc Surg. 2008 Nov;136(5):1318–23. doi: 10.1016/j.jtcvs.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 18.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007 Aug 30;357(9):885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 19.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen DQ, Thourani VH. Third-generation continuous flow left ventricular assist devices. Innovations (Phila). 2010 Jul-Aug;5(4):250–8. doi: 10.1097/IMI.0b013e3181ee77a1. [DOI] [PubMed] [Google Scholar]
- 21.Hoshi H, Shinshi T, Takatani S. Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs. 2006 May;30(5):324–38. doi: 10.1111/j.1525-1594.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 22.Timms D. A review of clinical ventricular assist devices. Med Eng Phys. 2011 Nov;33(9):1041–7. doi: 10.1016/j.medengphy.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DJ. Worldwide experience with the MicroMed DeBakey Ventricular Assist Device as a bridge to transplantation. Circulation. 2003 Sep 9;108 Suppl 1:II272–7. doi: 10.1161/01.cir.0000087387.02218.7e. [DOI] [PubMed] [Google Scholar]
- 24.Garbade J, Bittner HB, Barten MJ, Mohr FW. Current trends in implantable left ventricular assist devices. Cardiol Res Pract. 2011;2011:290561. doi: 10.4061/2011/290561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morshuis M, El-Banayosy A, Arusoglu L, Koerfer R, Hetzer R, Wieselthaler G, et al. European Experience of DuraHeartTM Magnetically Levitated. Eur J Cardiothorac Surg. 2009 Jun;35(6):1020-7; Discussion 1027–8. doi: 10.1016/j.ejcts.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Popov AF, Hosseini MT, Zych B, Mohite P, Hards R, Krueger H, et al. Clinical experience with HeartWare left ventricular assist device in patients with end-stage heart failure. Ann Thorac Surg. 2012 Mar;93(3):810–5. doi: 10.1016/j.athoracsur.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 27.Kukucka M, Stepanenko A, Potapov E, Krabatsch T, Redlin M, Mladenow A, et al. Right-to-left ventricular end-diastolic diameter ratio and prediction of right ventricular failure with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2011 Jan;30(1):64–9. doi: 10.1016/j.healun.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick JR, 3rd, Frederick JR, Hiesinger W, Hsu VM, McCormick RC, Kozin ED, et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg. 2009 Apr;137(4):971–7. doi: 10.1016/j.jtcvs.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potapov EV, Stepanenko A, Dandel M, Kukucka M, Lehmkuhl HB, Weng Y, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant. 2008 Dec;27(12):1275–81. doi: 10.1016/j.healun.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Puwanant S, Hamilton KK, Klodell CT, Hill JA, Schofield RS, Cleeton TS, et al. Tricuspid annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2008 Oct;27(10):1102–7. doi: 10.1016/j.healun.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Slaughter MS, Myers TJ. Transcutaneous energy transmission for mechanical circulatory support systems: history current status, and future prospects. J Card Surg. 2010 Jul;25(4):484–9. doi: 10.1111/j.1540-8191.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller JA. inventor; The University of Ottawa, assignee. Transcutaneous Energy Transfer Device. United States patent US 5350413. 1994 Sept 27. [Google Scholar]
- 33.Yamamoto T, Koshiji K. Magnetic-field immunity examination and evaluation of transcutaneous energy-transmission system for a totally implantable artificial heart. Advances in Power Electronics. 2012: Article ID 421639 5 pages. [Google Scholar]
- 34.Nagatomo T, Abe H, Kohno R, Toyoshima T, Fujimoto H, Kondo S, et al. Electromagnetic interference with a bipolar pacemaker by an induction heating (IH) rice cooker. Int Heart J. 2009 Jan;50(1):133–7. doi: 10.1536/ihj.50.133. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin JT, Mann DL. NHLBI’s program for VAD therapy for moderately advanced heart failure: the REVIVE-IT pilot trial. J Card Fail. 2010 Nov;16(11):855–8. doi: 10.1016/j.cardfail.2010.06.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ClinicalTrials [Internet]. Bethesda MD: National Institutes of Health; c2012. Risk assessment and comparative effectiveness of left ventricular assist device (LVAD) and medical management (ROADMAP); 2012 Dec 10 [cited 2013 Jan 2]. Available from: http://clinicaltrials.gov/ct2/show/NCT01452802. [Google Scholar]
- 37.Wever-Pinzon O, Stehlik J, Kfoury AG, Terrovitis JV, Diakos NA, Charitos C, et al. Ventricular assist devices: pharmacological aspects of a mechanical therapy. Pharmacol Ther. 2012 May;134(2):189–99. doi: 10.1016/j.pharmthera.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larose JA, Tamez D, Ashenuga M, Reyes C. Design concepts and principle of operation of the HeartWare ventricular assist system. ASAIO J. 2010 Jul-Aug;56(4):285–9. doi: 10.1097/MAT.0b013e3181dfbab5. [DOI] [PubMed] [Google Scholar]


