Abstract
Hyponatremia is the most common electrolyte abnormality encountered in clinical practice, but its optimal management is still evolving. While guidelines for infusion rates of hypertonic saline (HS) have been introduced, there is a risk of underestimating the response in serum sodium concentration after therapy. Guidelines also have evaluated the use of vasopressin receptor antagonists as alternatives or supplements to standard therapies. This single-center retrospective study from The Methodist Hospital (TMH) compared the effect of HS and conivaptan intervention in the management of 49 patients with hyponatremia from January 2009 through November 2010. Demographics, volume status, medical history, medication data, and serum sodium concentration correction over 48 hours were analyzed. No significant difference was noted with regard to age, ethnicity, gender, volume status, use of medications known to cause hyponatremia, or comorbidities. Baseline serum sodium concentration was not significantly different between HS (120.5 ± 3.8 mEq/L) and conivaptan (118.3 ± 6.7 mEq/L) groups. Regardless of whether the patient was euvolemic or hypervolemic, no significant difference was noted in serum sodium concentration at 4, 12, 24, or 48 hours after initiation of treatment or in frequency of over-correction between groups. This study compares the effect of HS to conivaptan intervention in the management of hyponatremia. No significant differences were identified in adherence to treatment guidelines. Further, based on this small retrospective study, neither agent poses a significant risk of over-correction at 4, 24, or 48 hours of therapy.
Keywords: conivaptan, hypertonic saline, hyponatremia, sodium correction

Introduction
Hyponatremia, defined as a serum sodium concentration ([Na+]) of ≤135 mEq/L (1 mEq/L=1 mmol/L), is the most common electrolyte abnormality encountered in clinical practice.1 Hyponatremia can occur with any degree of volume depletion or excess, and its severity is measured not only by the absolute [Na+] but also by the slope and rapidity of the decrease. Although most cases are mild and relatively asymptomatic, severe hyponatremia can manifest as cerebral edema leading to coma, irreversible neurological damage, and even death.2 Hypertonic saline was first used to treat hyponatremia in 1938.3 Although still accepted as the treatment of choice for symptomatic hyponatremia, its use demands careful monitoring to avoid osmotic demyelination syndrome (ODS), a feared neurologic disorder that manifests as progressive and sometimes permanent neurologic deficits and is thought to occur when the brain’s ability to recapture lost organic osmolytes is outpaced by rapid correction of [Na+].4 Patients with acute hyponatremia, developing in the course of ≤12 hours, are more likely to develop symptoms including seizures and coma than those with chronic hyponatremia (≥3 days).5
Optimal management of hyponatremia is still evolving despite awareness of this electrolyte disturbance since the mid 1900s.4, 5 Most authorities recommend correction of [Na+] in severely hyponatremic and symptomatic patients by 2 to 4 mEq/L within 2 to 4 hours, <12 mEq/L in 24 hours, and to <18 mEq/L in 48 hours.4, 5 Guidelines for infusion rates of hypertonic saline and monitoring procedures have been introduced, most notably the Adrogué-Madias formula.6 Caution in the use of these formulae has been recommended,7 especially given that they were designed for use in static conditions. Retrospective studies have found a risk of physicians underestimating the increase in [Na+] after hypertonic saline therapy, particularly in the setting of extracellular volume depletion.8, 9 A common criticism of these formulae is that they fail to account for ongoing renal and extrarenal fluid and electrolyte losses. Whereas the formulae apply to static conditions, the dynamic nature of the patient’s hospital course, including intravenous drips and gastrointestinal losses, affects the accuracy of [Na+] replacement by standard calculated deficits. To this effect, more elaborate formulae have been developed7 that better allow the clinician to follow [Na+] levels at close time intervals to adjust medical management.
Treatment guidelines published in 2007 evaluated the situations in which vasopressin receptor antagonists should be considered as alternatives or supplements to standard therapies.4 Conivaptan is one of these alternative therapies.10, 11 Conivaptan is a nonselective V1AR/V2R vasopressin receptor antagonist available in IV form and approved by the FDA to treat euvolemic hyponatremia in 2005 and hypervolemic hyponatremia in 2007. There is a caveat to the use of conivaptan in hypervolemia: although vasopressin receptor antagonism could have potentially beneficial effects in congestive heart failure by decreasing afterload, attenuating coronary vasoconstriction, and potentially diminishing cardiac remodeling, current data do not support its use in this condition.11 On the contrary, the potential exists for increasing portal pressure, resulting in bleeding in the hyponatremia of cirrhosis related to increased splanchnic blood flow. Conivaptan leads to an increase in [Na+] by blocking V2 receptors, thus promoting water excretion while sparing electrolyte excretion.2 Although rapid correction of [Na+] with use of conivaptan has been documented,12 its use is still thought to be a more effective method to treat hyponatremia by virtue of its unique ability to increase solute-free water excretion by the kidneys.4 There is a paucity of published retrospective and prospective studies analyzing the effectiveness of conivaptan in treating hyponatremia. To our knowledge, this is the first study to compare the effect of 3% saline (HS) and conivaptan intervention for the management of hyponatremia.
Subjects and Methods
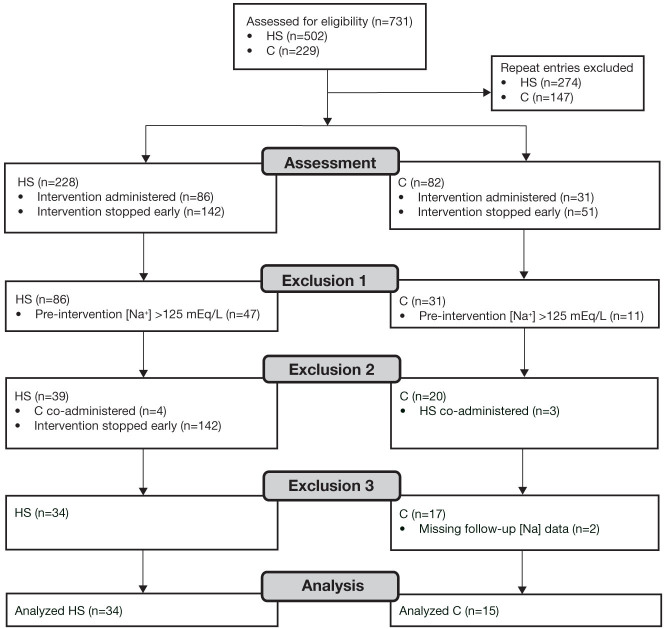
In this single-center retrospective study, we compared the efficacy of HS and conivaptan in achieving hyponatremia treatment goals according to expert guidelines.4 Inclusion criteria consisted of patients hospitalized at TMH in Houston, Texas, with computerized provider order entries (CPOE) for intravenous HS or conivaptan. Upon approval by TMH Institutional Review Board, the research team retrieved CPOE for HS and conivaptan from January 2009 through November 2010 (Figure 1). Of the total 731 CPOE identified, 310 were unique to single patients during a single hospitalization; of these, 117 were followed by administration of HS or conivaptan. Review of records from the excluded 193 patients revealed that administration of either HS or conivaptan was held after a pretreatment measurement of [Na+] showed an increase toward normal value (Figure 1).
Figure 1.
Flow diagram of patient identification, exclusion, and analysis.
C: conivaptan; DDAVP: 1-deamino-8-D-arginine vasopressin; HS: 3% saline.
Data including demographics, clinical presentation (e.g., postoperative state), clinically estimated volume status, medications known to cause hyponatremia, comorbid conditions, and suspected presence of the syndrome of inappropriate antidiuretic hormone secretion (SIADH) on clinical grounds as a cause of the patient’s hyponatremia were collected by chart review. Also retrieved were [Na+] at baseline and, when available, within 4, 12, 24, and 48 hours (± 1 hour) after the initiation of HS or conivaptan. All 49 patients analyzed had follow-up measurements of [Na+] within the expected 4, 12, 24, and 48 hour time frames. Over-correction was based on hyponatremia treatment guidelines4 and defined as exceeding the change in [Na+] of 4 mEq/L at 4 hours, 12 mEq/L at 24 hours, or 18 mEq/L at 48 hours after initiation of therapy.
Data analysis was performed using Intercooled Stata version 9.2 (Stata Corporation, College Station, Texas). Statistical significance was defined as P <0.05. Categorical data, summarized as percentages, were compared with the chi-square test. For quantitative data, 2-tailed Student’s t-test was performed. In cases of non-normally distributed data, Wilcoxon rank-sum analysis was performed. Results are presented as mean ± standard deviation.
Results
Patient demographics are summarized in Table 1. The mean age for the HS group was 69.3 years and 77.7 years for the conivaptan group. Caucasians comprised the majority of the study’s population in both groups. In the HS group, 76% of patients were euvolemic, as were 66% in the conivaptan group. The remaining patients in each group were hypervolemic; no hypovolemic patients were identified. No significant difference was noted between groups with regard to age, ethnicity, gender, volume status, or use of medications known to cause hyponatremia.
Table 1.
Patient characteristics.
| Parameter | Hypertonic Saline (n=34) | Conivaptan (n=15) | P value |
| Age (y) | 69.3 ± 15.5 | 77.7 ± 10.7 | P = 0.06 |
| Ethnicity (%) | 27 (79.4%) Caucasian 7 (20.6%) Other |
12 (80%) Caucasian 3 (20%) Other |
P = 0.96 |
| Male (%) | 18 (52.9%) | 4 (26.7%) | P = 0.09 |
| CHF (%) | 5 (14.7%) | 3 (20%) | P = 0.64 |
| HTN (%) | 21 (61.8%) | 11 (73.3%) | P = 0.43 |
| SIADH (%) | 9 (26.5%) | 7 (46.7%) | P = 0.17 |
| Other comorbidities (%) | 19 (55.9%) | 5 (33.3%) | P = 0.15 |
| On medications causing hyponatremia (%) | 13 (38.2%) | 10 (66.7%) | P = 0.07 |
| Dextrose water (%) | 3 (8.8%) | 4 (26.7%) | P = 0.10 |
| *Euvolemic (%) | 26 (76.5%) | 10 (66.7%) | P = 0.47 |
CHF: congestive heart failure; HTN: hypertension; SIADH: syndrome of inappropriate antidiuretic hormone. *All remaining patients were hypervolemic.
In the HS group, three patients were taking hydrochlorothiazide (HCTZ), two were taking bumetanide, two were taking furosemide, one was taking torsemide, one was taking metolazone, one was taking spironolactone, and three were taking selective serotonin reuptake inhibitors (SSRI; one escitalopram, one citalopram, one fluoxetine). In the conivaptan group, five patients were taking furosemide, four were taking HCTZ, two were taking spironolactone, two were taking torsemide, one was taking bumetanide, and one was taking escitalopram. No significant difference between groups was noted when taking into account medical history such as congestive heart failure (CHF), hypertension (HTN), or the clinician’s estimation of SIADH as the cause of the patient’s hyponatremia. Similarly, no significant difference was found between the groups with regard to other comorbidities. In the HS group, at the time of initiating treatment, six patients were recovering from surgery, eight patients had been admitted for management of a central nervous system (CNS) lesion (one for normal pressure hydrocephalus, one for intracranial bleed, one for a pituitary tumor, one for medulloblastoma, one for leptomeningitis, one for skull fracture, one for subdural hematoma, and one for an unidentified CNS lesion), one patient had a pleural effusion, one had small cell lung carcinoma, one had scoliosis, one had undergone heart transplant, and one suffered from liver cirrhosis. In the conivaptan group, at the time of initiating treatment, two patients were recovering from surgery, one suffered from pulmonary arterial hypertension (PAH), one had unidentified lung carcinoma, and one had been admitted for acute respiratory distress syndrome (ARDS). A small percentage of patients received dextrose water shortly after the administration of either HS or conivaptan, but the difference in numbers between these two groups did not reach statistical significance.
The baseline [Na+] was not significantly different between the HS (120.5 ± 3.8 mEq/L) and conivaptan (118.3 ± 6.7 mEq/L) groups (Table 2). No significant difference was noted in [Na+] at 4, 12, 24, or 48 hours after initiation of treatment. Figure 2 displays the change in [Na+] at serial time points after initiation of therapy. There was no significant difference between HS and conivaptan groups at 4, 12, 24, or 48 hours after initiation of treatment. When stratified by volume status, the absence of significant change in [Na+] between the HS and conivaptan groups persisted. Figure 3 displays the percent of over-correctors based on expert guidelines, stratified by volume status. There was no significant difference between HS and conivaptan groups in [Na+] over-correction at 4, 24, or 48 hours after initiation of therapy.
Table 2.
Serial serum sodium concentrations.
| Parameter |
Hypertonic Saline (n=34 unless otherwise specified) |
Conivaptan (n=15 unless otherwise specified) |
P value |
| [Na+] 0 h (mEq/L) | 120.5 ± 3.8 | 118.3 ± 6.7 | P = 0.16 |
| [Na+] 4 h (mEq/L) | (n=27) 122.9 ± 4.5 | 121.2 ± 7.7 | P = 0.37 |
| [Na+] 12 h (mEq/L) | (n=32) 125.8 ± 3.9 | (n=14) 123.6 ± 7.7 | P = 0.21 |
| [Na+] 24 h (mEq/L) | (n=31) 128.5 ± 4.4 | 126.1 ± 7.2 | P = 0.17 |
| [Na+] 48 h (mEq/L) | (n=21) 130.7 ± 6.2 | 129.1 ± 6.3 | P = 0.44 |
Figure 2.
Change in [Na+] from baseline. Error bars represent standard error of the mean. Numbers inside bars indicate number of patients. Dotted lines represent targets based on expert guidelines. No significant differences at any time point. C: conivaptan; HS: 3% saline.
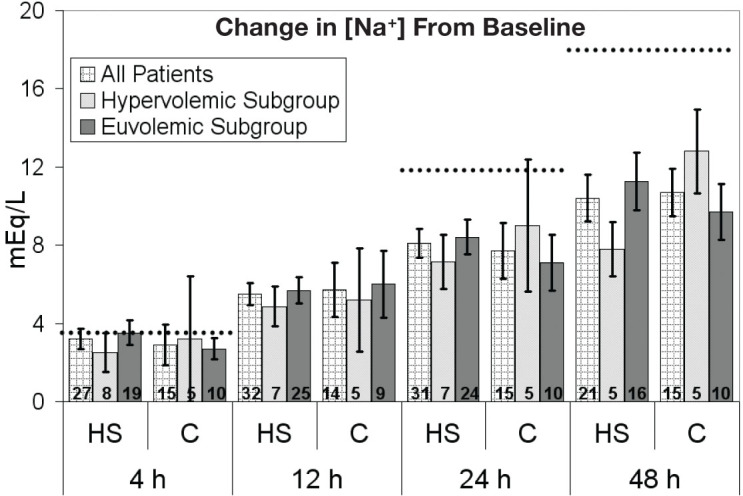
Figure 3.
Incidence of [Na+] over-correction based on expert guidelines. No significant differences at any time point. C: conivaptan; HS: 3% saline.
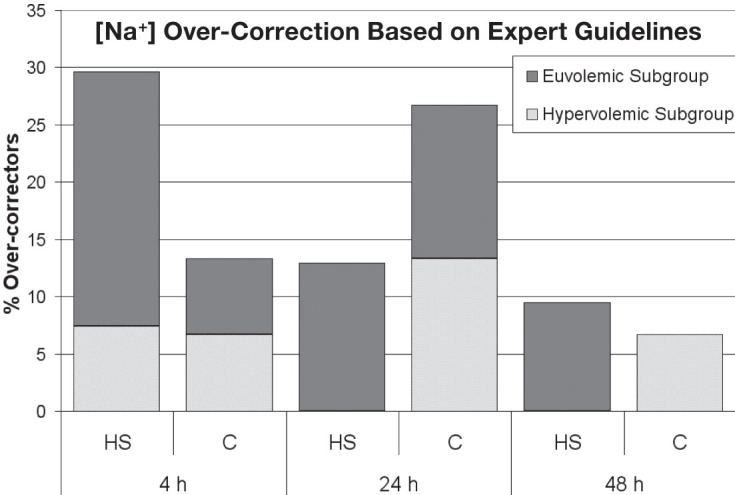
A small percentage of patients received 5% dextrose in water shortly after the administration of either HS (n=3, 8.8%) or conivaptan (n=4, 26.7%), and there was no difference in the incidence of [Na+] over-correction between HS and conivaptan groups related to administration of dextrose water.
Discussion
With an estimated incidence of 1% and prevalence of 2.5%, hyponatremia is the most common electrolyte abnormality in clinical practice and, as such, is often encountered by primary care and subspecialty physicians, e.g., nephrologists, geriatricians, endocrinologists, etc.13 Optimal management of hyponatremia is evolving. In the setting of symptomatic hyponatremia, treatment options include HS and conivaptan. A known benefit of HS is that it is less expensive.14 However, it carries the risk of volume overload in oliguric or anuric patients, and guidelines for rates of infusion have been criticized for posing a risk of underestimating an increase in [Na+], particularly in the setting of extracellular volume depletion where normal saline is the crystalloid of choice. Conversely, conivaptan treatment involves a significantly lower volume of medication but carries a higher cost.14 These are factors that must be considered when deciding which agent to use in treating euvolemic or hypervolemic hyponatremia.
In a retrospective analysis of patients treated with HS or conivaptan for hyponatremia, no significant differences were identified in adherence to treatment guidelines established in 2007 by expert panel recommendations.4 Although drawn from a small sample size originating from a single center, to our knowledge this study is the first to compare the effect of HS and conivaptan intervention for the management of hyponatremia in a sample of population otherwise similar in all parameters evaluated. Findings of the present study suggest that neither agent poses a significant risk of over-correction at 4, 24, or 48 hours regardless of whether the patient is euvolemic or hypervolemic. This must be tempered by the fact that in this retrospective study, it was found that 73.3% of the patients receiving conivaptan received it as a continuous infusion with the recommended loading dose whereas the remainder of the patients did not. This may be due to the fact that the prescribing conivaptan was available to any attending-level physician at our institution, regardless of department. The observed rate of mean [Na+] correction with conivaptan at 4 (2.9 mEq/L) and 24 (7.7 mEq/L) hours in this study is consistent with previously published findings of 2–3.5 mgEq/L and 6–8 mEq/L, respectively. However, the rate of correction at 12 (5.7 mEq/L) and 48 (10.7 mEq/L) hours exceeds that of previous findings of 3–5 mEq/L and 3.5–8 mEq/L, respectively.2, 15-17 This may be explained by the small sample size of our study.
This study has a number of limitations, including its small sample size, retrospective design, ethnic homogeneity, absence of post-treatment follow-up to monitor potential complications (e.g., ODS), and loss of data at later time points after initiation of treatment. In addition, although both treatments were shown to be comparable regarding the rate of sodium correction, they were comparably slow. A further limitation of the study is that the majority of patients had surgical, neurological, or neurosurgical conditions, constituting a complex patient population. It is possible that the results from these patients may differ from those of a different inpatient population. A meta-analysis of randomized controlled trials comparing vasopressin receptor antagonist use to placebo or no treatment in the setting of hyponatremia showed that, within 3–7 days of initiating therapy, the [Na+] correction of vasopressin antagonists was significantly increased (5.27 mEq/L) compared to the control, and the relative risk of rapid [Na+] overcorrection (2.52) was significant with vasopressin antagonists without significantly increasing the rate of hypernatremia.18
Since the most serious criticism of early formulae suggesting hyponatremia correction rates is that they fail to account for ongoing fluid and electrolyte losses, and therefore underestimate actual increases in [Na+], additional retrospective or prospective analyses using newer formulae are warranted. Future studies focusing on the adherence of HS and conivaptan to expert guidelines in the treatment of hyponatremia should include a larger sample size; they also should analyze the extent to which prescribed rates of HS IV administration are derived from accurate calculations of patients’ ideal body weight or the extent to which conivaptan infusion rates adhere to manufacturer-established prescription rates.4 Although the focus of this study was not to assess the accuracy of commonly used formulas to predict a rise in [Na+] or to assess the accuracy of established prescription dosages for conivaptan, prescribed rates for administering HS were not uncommonly lower than those calculated by the Adrogué-Madias formula. This may partly explain the paucity of over-correction among patients treated with HS. The rationale of the prescribing physicians’ orders for a lower rate of HS than that suggested by the Adrogué-Madias formula is unclear — assuming goal [Na+] at interval points of 2 to 4 mEq/L within 2 to 4 hours, <12 mEq/L in 24 hours, and to <18 mEq/L in 48 hours; however, caution is advised in using these formulae in clinical care, as there is no consensus on a universally adopted standard of care but only guidelines to achieve normonatremia. Perhaps the observed lower infusion rate of HS was prescribed in response to recommendations made in previous studies.9 Although the protocol advises the use of a 20-mg loading dose of conivaptan followed by an infusion of 20 mg in 24 hours, some of the treating physicians elected to use the infusion alone in 23% of the cases; this was based on previous experience whereby the loading dose resulted in massive diuresis requiring intravenous dextrose in water. As such, the slow infusion provides a safer control of the diuretic response since the magnitude of the diuresis varies by patient. It is hoped that the approval of tolvaptan in May 2009 will ease the treatment of euvolemic and hypervolemic hyponatremia since tolvaptan can be administered orally, eliminating the possibility of infusion site reactions that come with the current method of central line administration.19
Acknowledgments
We are indebted to Wadi Suki, M.D., for his editorial assistance, and Michael Sirimaturos, Pharm.D., for his guidance in gathering data.
Funding Statement
Funding/Support: The authors have no funding disclosures.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006 Jul;119(7 Suppl 1):S30–5. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized controlled study. Clin Endocrinol (Oxf). 2008 Jul;69(1):159–68. doi: 10.1111/j.1365-2265.2007.03149.x. [DOI] [PubMed] [Google Scholar]
- 3.Helwig FC, Schutz CB, Kuhn HP. Water intoxication: Moribund patient cured by administration of hypertonic salt solution. JAMA. 1938;110:644–645. [Google Scholar]
- 4.Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007 Nov;120(11 Suppl 1):S1–21. doi: 10.1016/j.amjmed.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009 May;29(3):282–99. doi: 10.1016/j.semnephrol.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000 May 25;342(21):1581–9. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 7.Barsoum NR, Levine BS. Current prescriptions for the correction of hyponatraemia and hypernatraemia: are they too simple? Nephrol Dial Transplant. 2002 Jul;17(7):1176–80. doi: 10.1093/ndt/17.7.1176. [DOI] [PubMed] [Google Scholar]
- 8.Liamis G, Kalogirou M, Saugos V, Elisaf M. Therapeutic approach in patients with dysnatraemias. Nephrol Dial Transplant. 2006 Jun;21(6):1564–9. doi: 10.1093/ndt/gfk090. [DOI] [PubMed] [Google Scholar]
- 9.Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol. 2007 Nov;2(6):1110–7. doi: 10.2215/CJN.00910207. [DOI] [PubMed] [Google Scholar]
- 10.Gross PA, Wagner A, Decaux G. Vaptans are not the mainstay of treatment in hyponatremia: perhaps not yet. Kidney Int. 2011 Sep;80(6):594–600. doi: 10.1038/ki.2011.78. [DOI] [PubMed] [Google Scholar]
- 11.Schrier RW, Bansal S. Diagnosis and management of hyponatremia in acute illness. Curr Opin Crit Care. 2008 Dec;14(6):627–34. doi: 10.1097/MCC.0b013e32830e45e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velez JC, Dopson SJ, Sanders DS, Delay TA, Arthur JM. Intravenous conivaptan for the treatment of hyponatraemia caused by the syndrome of inappropriate secretion of antidiuretic hormone in hospitalized patients: a single-centre experience. Nephrol Dial Transplant. 2010 May;25(5):1524–31. doi: 10.1093/ndt/gfp731. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RJ, Chung HM, Kluge R, Schrier RW. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med. 1985 Feb;102(2):164–8. doi: 10.7326/0003-4819-102-2-164. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson-Myrthil N. Novel agents for the treatment of hyponatremia: a review of conivaptan and tolvaptan. Cardiol Rev. 2010 Nov-Dec;18(6):313–21. doi: 10.1097/CRD.0b013e3181f5b3b7. [DOI] [PubMed] [Google Scholar]
- 15.Annane D, Decaux G, Smith N. Conivaptan Study Group. Efficacy and safety of oral conivaptan a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am J Med Sci. 2009 Jan;337(1):28–36. doi: 10.1097/MAJ.0b013e31817b8148. [DOI] [PubMed] [Google Scholar]
- 16.Koren MJ, Hamad A, Klasen S, Abeyratne A, McNutt BE, Kalra S. Efficacy and safety of 30-minute infusions of conivaptan in euvolemic and hypervolemic hyponatremia. Am J Health Syst Pharm. 2011 May 1;68(9):818–27. doi: 10.2146/ajhp100260. [DOI] [PubMed] [Google Scholar]
- 17.Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Conivaptan Study Group. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447–57. doi: 10.1159/000106456. [DOI] [PubMed] [Google Scholar]
- 18.Rozen-Zvi B, Yahav D, Gheorghiade M, Korzets A, Leibovici L, Gafter U. Vasopressin receptor antagonists for the treatment of hyponatremia: systematic review and meta-analysis. Am J Kidney Dis. 2010 Aug;56(2):325–37. doi: 10.1053/j.ajkd.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Wright WL, Asbury WH, Gilmore JL, Samuels OB. Conivaptan for hyponatremia in the neurocritical care unit. Neurocrit Care. 2009;11(1):6–13. doi: 10.1007/s12028-008-9152-1. [DOI] [PubMed] [Google Scholar]