IL-1R1 signaling drives T cell activation in the CNS via effects on DC activation.
Abstract
Infections of the central nervous system (CNS) with cytopathic viruses require efficient T cell responses to promote viral clearance, limit immunopathology, and enhance survival. We found that IL-1R1 is critical for effector T cell reactivation and limits inflammation within the CNS during murine West Nile virus (WNV) encephalitis. WNV-infected IL-1R1−/− mice display intact adaptive immunity in the periphery but succumb to WNV infection caused by loss of virologic control in the CNS with depressed local Th1 cytokine responses, despite parenchymal entry of virus-specific CD8+ T cells. Ex vivo analysis of CD4+ T cells from WNV-infected CNS of IL-1R1−/− mice revealed impaired effector responses, whereas CD8+ T cells revealed no cell intrinsic defects in response to WNV antigen. WNV-infected, IL-1R1−/− mice also exhibited decreased activation of CNS CD11c+CD11b−CD103+ and CD11c+CD11b−CD8α+Dec-205+ cells with reduced up-regulation of the co-stimulatory molecules CD80, CD86, and CD68. Adoptive transfer of wild-type CD11c-EYFP+ cells from WNV-infected CNS into WNV-infected IL-1R1−/− mice trafficked into the CNS restored T cell functions and improved survival from otherwise lethal infection. These data indicate that IL-1R1 signaling promotes virologic control during WNV infection specifically within the CNS via modulation of CD11c+ cell–mediated T cell reactivation at this site.
Viral infections of the central nervous system (CNS) impose a challenge for host defenses because of limited immune surveillance, lack of resident cell MHC molecule expression, and restricted lymphocyte entry (Carson et al., 2006). Molecular mechanisms involved in viral clearance, especially those that regulate the recruitment and activation of APCs and antiviral T cells, must efficiently induce viral clearance while also limiting immunopathologic damage (McGavern and Kang, 2011). IL-1, which exists as two proinflammatory cytokines, IL-1α and IL-1β, is highly expressed within the CNS during neuroinflammatory diseases including viral encephalitis (Basu et al., 2004; Kanneganti, 2010). IL-1α and IL-1β signal through the type I IL-1 receptor (IL-1R1), leading to transcription of multiple inflammation-associated genes, including cytokines, chemokines, and adhesion molecules (Sims and Smith, 2010). In murine models of respiratory viruses, such as influenza A and rhinovirus, IL-1–mediated effects on leukocytes are critical for virologic control and survival but also cause inflammatory injury (Schmitz et al., 2005; Stokes et al., 2011). Currently, there are no studies addressing the role of IL-1 in viral infections of the CNS, a site in which immunopathology is an established consequence of leukocyte entry, even for the purpose of viral clearance (Hausmann et al., 2001; Alsharifi et al., 2006).
IL-1 is a key contributor to CNS autoimmune diseases (Dinarello, 2009), including multiple sclerosis (MS) and neuromyelitis optica, which are characterized by excessive autoreactive leukocyte entry (Bhat and Steinman, 2009). Studies confirm that chronic IL-1 expression within the CNS results in leukocyte accumulation (Shaftel et al., 2007), that IL-1 is critical for CD4+ T cell activation and IL-17 expression, and that targeted deletion of IL-1 or IL-1R1 results in protection from experimental autoimmune encephalitis, an animal model of MS (Schiffenbauer et al., 2000; Nakae et al., 2001; Matsuki et al., 2006; Sutton et al., 2006; McCandless et al., 2009). Overall, these results suggest that IL-1 contributes to CD4+ T cell trafficking and effector responses during CNS autoimmunity and suggest it might contribute to severe disease during viral encephalitis.
West Nile virus (WNV), an emerging significant human pathogen which causes encephalitis and has spread rapidly with major economic and public health consequences over the last decade worldwide (Petersen and Hayes, 2008; Kilpatrick, 2011), is an enveloped, single-stranded positive sense RNA virus member of the Flaviviridae family. Recently there has been a dramatic increase in the number of WNV disease outbreaks within the US (http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount12_detailed.htm), emphasizing the urgent necessity to understand the basic mechanisms of viral clearance within the CNS. After peripheral infection, WNV replicates within lymphoid tissues before entering the CNS, where it targets neurons within the cerebellum, brain stem, and cerebral cortex (Guarner et al., 2004; Kleinschmidt-DeMasters et al., 2004; Zhang et al., 2008). Studies in mice indicate that WNV clearance within the CNS compartment requires antiviral, effector T cell entry (Shrestha and Diamond, 2004; Sitati and Diamond, 2006; McCandless et al., 2008), whose presence may also contribute to immunopathology (Wang et al., 2003; King et al., 2007). Because intact adaptive cellular immune responses are integral for WNV clearance from the CNS, we sought to determine whether IL-1 contributes to neuroprotection versus immunopathology during WNV encephalitis.
Here we demonstrate a novel role for IL-1R1 in the CNS activation of a subpopulation of CD11c+ cells that are essential for T cell–mediated clearance of WNV in infected neurons. Thus, although WNV-infected IL-1R1−/− mice exhibit no defects in adaptive immune responses and viral clearance in the periphery, secondary effector T cell responses in the CNS were deficient with reduced CNS levels of Th1 cytokines, loss of virologic control, and survival compared with similarly infected WT animals. T cell–mediated viral clearance in IL-1R1–deficient mice was impaired despite their entry within the CNS parenchyma. Ex vivo analysis of CNS virus–specific CD8+ T cells revealed no cell-intrinsic defects associated with loss of IL-1R1 activity, whereas similarly evaluated CNS-derived CD4+ T cells exhibited diminished antiviral responses. Of interest, recruited CD11c+ cells exhibited dramatic reduction in expression of CD80, CD86, and CD68. These infiltrating CD11c+ cells were identified as CD11b−CD8α−CD103+ and CD11b−CD8α+DEC-205+ cells. The adoptive transfer of CD11c+ cells derived from CD11c–enhanced YFP (EYFP) transgenic WT mice demonstrated CNS infiltration of these particular CD11c+ subsets and led to increased survival of WNV-infected, IL-1R1–deficient mice as the result of a restoration in CD4+ T cell antiviral immune responses. Our results delineate an IL-1R1 signaling–dependent pathway for T cell reactivation within the CNS that is critical for virologic control in this organ.
RESULTS
IL-1R1 signaling promotes WNV clearance and protects against virus-associated mortality
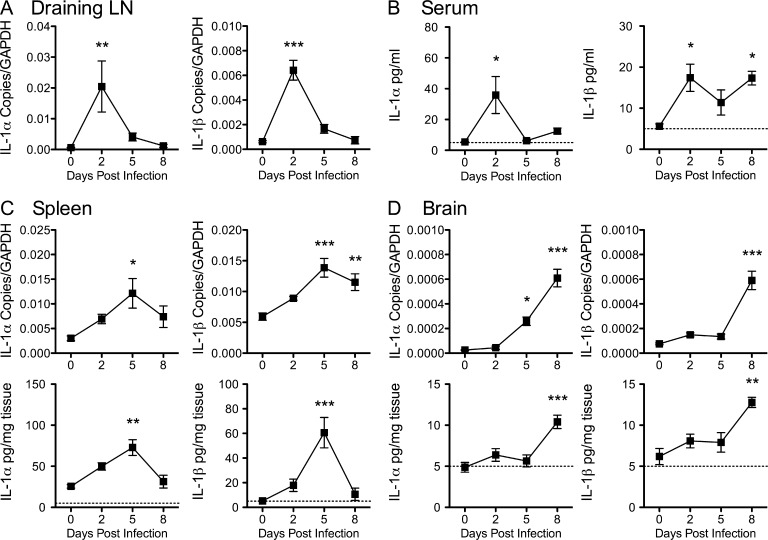
IL-1 promotes antiviral immunity outside the CNS in conjunction with immunopathologic injury (Schmitz et al., 2005; Stokes et al., 2011) and is associated with CNS injury during autoimmune diseases (Stanley et al., 1994; Mogi et al., 1996; McGuinness et al., 1997; Zhao et al., 2001). However, the precise role of IL-1 in antiviral immunity during viral encephalitis remains uncertain. To address this, we first examined IL-1 expression in peripheral and CNS tissues after footpad inoculation of 102 PFU of a virulent strain of WNV-NY (Ebel et al., 2001) in 8-wk-old C57BL/6 WT mice. In the draining LNs, IL-1α and IL-1β transcript levels peaked by day 2 after infection and then steadily returned to baseline levels (Fig. 1 A). In serum, protein levels of IL-1α and IL-1β also peaked at day 2 after infection, began to decline around day 5, and then reversed course to rise again at day 8 after infection (Fig. 1 B). In splenic tissues, however, IL-1α and IL-1β expression peaked at day 5 after infection and then precipitously returned to background levels by day 8 (Fig. 1 C). In the CNS, transcript levels of IL-1α were detected by day 5 after infection, whereas levels of IL-1β were not evident until day 8 after infection, at which time protein levels of both IL-1α and IL-1β began to increase (Fig. 1 D). Overall, IL-1 appears to be produced in response to WNV infection both in the periphery and in the CNS.
Figure 1.
IL-1 levels are increased in peripheral and CNS tissues during WNV encephalitis. 8-wk-old WT mice were inoculated with 102 PFU of WNV by footpad injection. (A–D) Draining LNs (A), sera (B), spleen (C), and brain (D) tissues were collected at the indicated time points. mRNA levels of IL-1α and IL-1β were analyzed via qRT-PCR, and protein levels of IL-1α and IL-1β were measured by ELISA. Data reflect the means of sera and tissue harvested from six to eight mice per time point from at least three independent experiments. Dotted lines represent assay sensitivity limit. Error bars are SEM. One-way ANOVA with Tukey-Kramer posttest was used to determine the statistical significance with respect to day 0 after infection. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
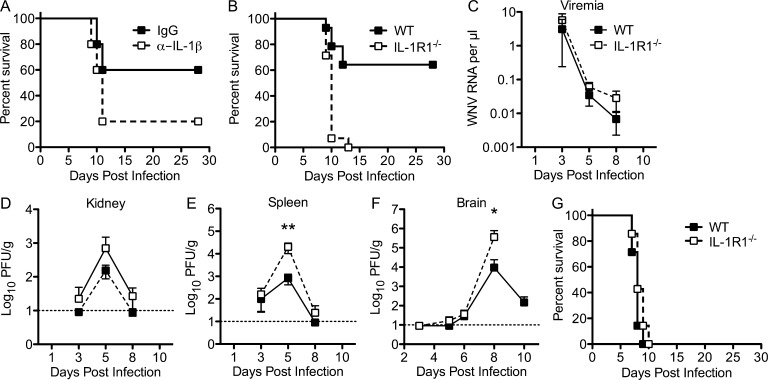
To assess the role of IL-1 in WNV infection and neuroinvasive disease, we compared survival rates in mice that had either an acquired (via anti–IL-1β mAb depletion) or a genetic (C57BL/6 IL-1R1−/− mice) deficiency in IL-1 signaling with untreated WT mice. 8-wk-old C57BL/6 WT mice were inoculated via footpad with 102 PFU of WNV that had been intravenously administered an IL-1β–neutralizing antibody at days 3 and 4 after infection, which resulted in a 30% increase in mortality compared with control antibody (P = 0.073; Fig. 2 A). Dramatically, 8-wk-old genetically IL-1R1–deficient mice succumbed universally (0% survival) to footpad inoculation with 102 PFU of WNV, whereas 65% of WT animals survived infection (P = 0.0001; Fig. 2 B). Thus, an absence of IL-1R1 signaling caused a more severe WNV infection, resulting in increased mortality after peripheral infection. To understand how an absence of IL-1 signaling increased the susceptibility of mice to WNV infection, the levels of virus in peripheral and CNS tissues were analyzed. Differences in viremia were not observed between IL-1R1−/− and WT mice at days 3, 5, and 8 after WNV infection (Fig. 2 C). Viral titers were higher in the kidneys and spleens of IL-1R1−/− mice by day 5 after infection compared with WT mice; however, these levels reached statistical significance only in the spleen (P < 0.01; Fig. 2, D and E). Splenic viral loads were cleared in both genotypes by day 8 after infection, indicating that IL-1R1 signaling is not required for virologic control in peripheral tissues. In CNS tissues, infectious virus was detected at similar levels on day 6 after infection in both genotypes; however, by day 8 after infection, viral titers were significantly higher (100-fold) in IL-1R1−/− mice (P = 0.0389), and by day 10 after infection, viral titers had dropped ∼2-log–fold in the WT mice (Fig. 2 F). There were no differences in the time points at which WNV was detected in the brain between the genotypes, indicating that IL-1R1 signaling does not impact neuroinvasion yet likely does impact viral clearance. Intracranial inoculation of 101 PFU of WNV into IL-1R1−/− and WT mice did not lead to differences in lethality (P = 0.203; Fig. 2 G), suggesting IL-1 does not directly impact viral replication within the CNS.
Figure 2.
WNV-infected IL-1R1−/− mice exhibit increased mortality and impaired CNS virologic control compared with WT mice. (A) WT mice were administered 0.2 mg anti–IL-1β (open squares) or an isotype control (closed squares) antibody via intravenous injection on days 3 and 4 after infection with 102 PFU of WNV via footpad and then monitored for mortality. Survival differences between isotype (n = 6)- and IL-1β antibody–treated (n = 6) mice were not statistically significant (P = 0.073). (B) WT and IL-1R1−/− mice were inoculated with 102 PFU of WNV by footpad injection and followed for mortality. Survival differences were statistically significant (14 mice per genotype; P < 0.0001). (C–F) Viral burden in serum (C), kidney (D), spleen (E), and brain (F) after WNV infection of WT and IL-1R1−/− mice at the indicated time points was determined using either qRT-PCR or standard viral plaque assay from at least two independent experiments. Dotted lines represent sensitivity limit. Error bars are SEM. Two-way ANOVA with Bonferroni posttest was used to determine the statistical significance. *, P < 0.05; **, P < 0.01 compared with WT. (G) WT and IL-1R1−/− mice were infected intracranially with 10 PFU of virulent WNV-NY and monitored for 28 d. The survival curves were constructed with data from two independent experiments (five mice per group). The survival differences between WT and IL-1R1−/− mice were not statistically significant.
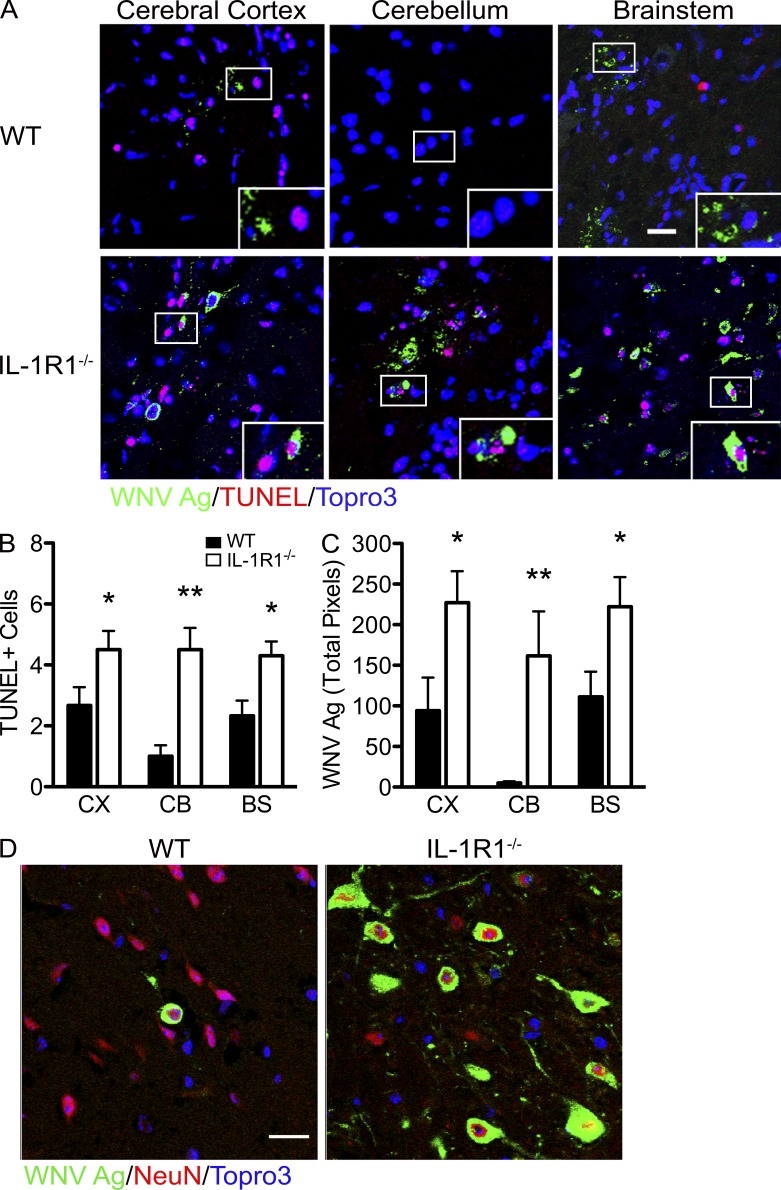
High viral loads in the CNS have previously been associated with extensive neuronal apoptosis (Samuel et al., 2007). In the absence of IL-1R1 signaling, TUNEL+ and WNV-infected (WNV-Ag+) cells were in abundance at day 8 after infection, detected across every region of the brain, and found in proximity to one another (Fig. 3 A, insets), whereas in WT animals, TUNEL+ cells were restricted to the cerebral cortex and brainstem (Fig. 3 A). Quantitative analysis of TUNEL+ and WNV-Ag+ cells revealed significant increases in IL-1R1–deficient mice compared with WT controls (P = 0.0031; Fig. 3, B and C), consistent with plaque assay data (Fig. 2 F). In addition, all WNV antigen positivity was detected within neurons in both the IL-1R1−/− and WT mice, indicating no apparent expanded tropism within the CNS in the setting of IL-1R1 deficiency (Fig. 3 D). These data suggest that IL-1R1 signaling is critical for controlling viral replication and concomitant virus-induced apoptotic cell death within neurons and that no additional CNS-resident cells become permissive for WNV infection in the absence of IL-1R1 signaling.
Figure 3.
IL-1R1 signaling protects against neuronal death during WNV infection. (A) Representative confocal microscopic images of WNV antigen, TUNEL+, and nuclei (blue) of different brain regions in WNV-infected (102 PFU) WT and IL-1R1−/− mice on day 8 after infection. Insets are high-power images of the boxed regions. The data are representative of results from four independent mice. (B) Quantification of confocal microscopic images for TUNEL+ cells in the cerebral cortex (CX), cerebellum (CB), and brainstem (BS) of WT and IL-1R1−/− mice. The number of TUNEL+ cells was quantified from 10 high-power fields per brain region per mouse for four independent mice. (C) Quantitation of WNV antigen in both IL-1R1−/− and WT mice in separate regions of the brain. Student’s t test was used to determine statistical significance. *, P < 0.05; **, P < 0.01 compared with WT. (D) Confocal microscopic images of NeuN+, WNV antigen, and nuclei (blue) from brainstem regions in IL-1R1−/− and WT WNV-infected mice on day 8 after infection. Images are representative of results from five independent mice. Data are from at least two experiments in which 10 images were analyzed in each of the mice and presented as mean ± SEM. Bars, 25 µm.
Peripheral B and T cell responses are intact in the absence of IL-1R1 signaling
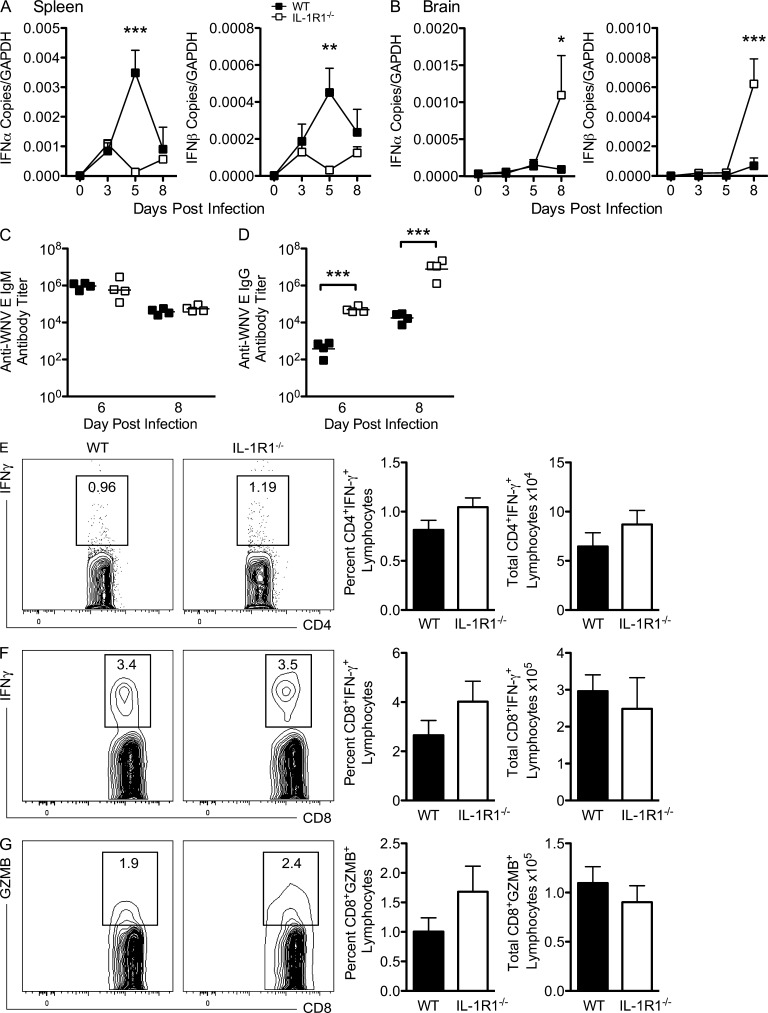
To understand the delay in viral clearance within the spleen in the IL-1R1−/− mice compared with similarly infected WT mice (Fig. 2 E), we measured transcript levels of type I IFNs because IFN-α/β has an established role in restricting viral replication in peripheral tissues during WNV infection (Samuel and Diamond, 2005). Peripheral levels of type I IFNs were significantly lower within IL-1R1–deficient mice compared with WT on day 5 after infection, whereas they were restored to WT levels by day 8 (P < 0.05; Fig. 4 A). Conversely, by day 8 after infection, IFN-α/β levels within the CNS were increased in the IL-1R1–deficient mice (P = 0.0001; Fig. 4 B), suggesting a disparate role for IL-1 in orchestrating immune-mediated viral clearance within the CNS compared with the periphery.
Figure 4.
Peripheral adaptive immune responses in WNV-infected IL-1R1−/− mice are intact. WT and IL-1R1−/− mice were inoculated with 102 PFU of WNV by footpad injection. (A and B) Spleen (A) and brain (B) tissues were evaluated for expression of type 1 IFN (IFN-α and IFN-β) mRNA via qRT-PCR at the indicated time points. All data are presented as the mean ± SEM for n = 6 mice/group. Asterisks indicate statistically significant values compared with WT controls. Two-way ANOVA with Bonferroni posttest was used to determine the statistical significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C and D) Serum samples were collected on days 6 and 8 after infection and assayed by ELISA for WNV E–specific IgM (C) and IgG (D) antibody. The data are means of five mice per group. (E–G) Spleens were harvested from WT and IL-1R1−/− mice inoculated with 102 PFU of WNV by footpad injection on day 8 after infection. Isolated leukocytes were stimulated ex vivo with I-Ab–restricted NS32066 and NS31616 or Db-restricted NS4B peptide, stained for CD4 or CD8 and intracellular IFN-γ and Granzyme B (GZMB), and then analyzed by flow cytometry. The data are shown as contour plots of gated CD4+ or CD8+ cells, and a summary is shown of the percentages and total numbers of CD4+ T cells positive for intracellular IFN-γ (E) or CD8+ T cells positive for intracellular IFN-γ (F) or Granzyme B (G). Statistically significant differences in IL-1R1−/− mice were measured by comparing with WT. Data in all panels represent the means of at least three independent experiments each with four to six mice.
Intact adaptive B and T cell immune responses are required to limit WNV replication in peripheral tissues (Samuel and Diamond, 2006). To determine whether IL-1R1 deficiency impacts antiviral antibody responses, we isolated sera from mice on days 6 and 8 after infection. For WNV-specific IgM, no appreciable differences in levels were observed between IL-1R1−/− and WT mice (Fig. 4 C). Higher levels of WNV-specific IgG were detected in IL-1R1−/− mice after infection at both days 6 and 8 after infection (P < 0.0001; Fig. 4 D). Thus, IL-1 appears to have a limited role for IgM and IgG antibody responses during WNV infection, and increased susceptibility of IL-1R1−/− mice was not caused by a B cell function defect.
Previous studies have shown that CD4+ and CD8+ T cells independently clear WNV from infected neurons in the CNS (Wang et al., 2003; Shrestha and Diamond, 2004; Shrestha et al., 2006; Sitati and Diamond, 2006; Brien et al., 2007, 2008). To determine whether a deficiency in IL-1R1 signaling altered WNV-specific CD4+ or CD8+ T cell responses, cells were harvested from the spleens of WNV-infected IL-1R1−/− and WT mice at days 6 and 8 after infection and restimulated ex vivo with I-Ab–restricted immunodominant NS32066 and NS31616 peptides for CD4+ T cells or a Db-restricted immunodominant NS4B peptide for CD8+ T cells (Brien et al., 2007, 2008; Purtha et al., 2007). Notably, by day 8 after infection, no significant differences were observed in the number or percentages of CD4+IFN-γ+ or CD8+IFN-γ+ and CD8+Granzyme B+ T cells after stimulation with NS32066 and NS31616 peptides or NS4B peptides, respectively (Fig. 4, E–G). Both CD4+ and CD8+ T cells showed little ex vivo restimulation with immunodominant peptides on day 6 after infection (not depicted). Additionally, there was no significant change in the levels of Th1 (IFN-γ, TNF, IL-1β), Th2 (IL-4 and IL-5), IL-10, or IL-17 inflammatory cytokines measured in the spleen on days 3, 5, and 8 after infection between WNV-infected IL-1R1−/− and WT mice (not depicted), confirming intact adaptive cellular immunity within the periphery. Thus, a deficiency in IL-1R1 signaling did not impair priming of WNV-specific CD4+ or CD8+ T cells in the spleen, nor was the higher viral burden in the brains of IL-1R1−/− mice a consequence of inadequate priming of adaptive immune responses in the periphery.
CD4 T cell effector responses fail to be reactivated in the IL-1R1−/− WNV-infected CNS
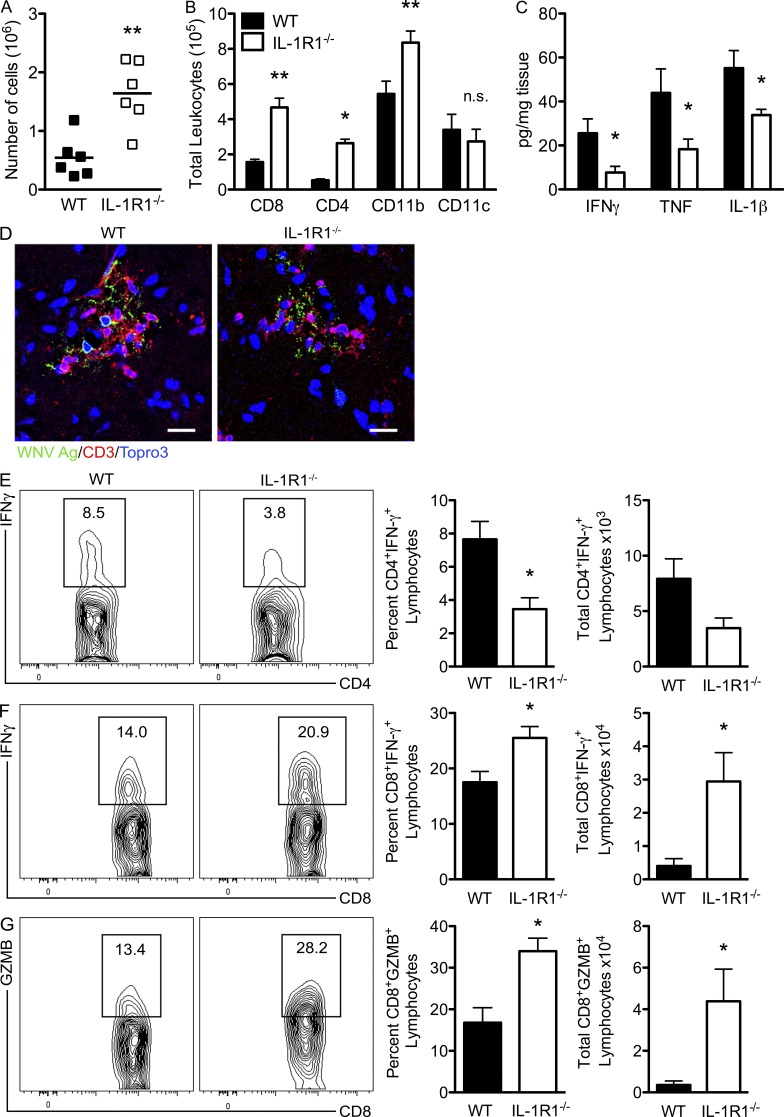
We and others have shown that clearance of WNV within the CNS compartment requires the presence of effector CD8+ T cells (Shrestha et al., 2006; McCandless et al., 2008). Therefore, we evaluated leukocyte infiltration and whether IL-1R1 signaling impacts T cell effector functions at this site. Flow cytometric analysis of leukocytes isolated from the brains of IL-1R1−/− and WT mice at day 8 after infection revealed a threefold increase in total numbers of leukocytes in the CNS tissues of IL-1R1−/− versus WT mice (P = 0.0022; Fig. 5 A), with significant increases in the total numbers of CD8+ and CD4+ T cells as well as CD11b+ leukocytes detected in the IL-1R1−/− brain compared with the WT (Fig. 5 B). There were no significant differences between the percentages of these cells within the IL-1R1−/− and WT brain. Although total numbers of CD11c+ leukocytes derived from WNV-infected WT and IL-1R1−/− brains did not differ significantly, the percentages of CD11c+ leukocytes were significantly lower (23.5% compared with 48.3%; P = 0.0005) in IL-1R1–deficient mice. Evaluation of CD3+ T cells within the CNS parenchyma revealed their juxtaposition with WNV-infected neurons in both IL-1R1–deficient and WT animals (Fig. 5 D). Despite this, protein levels of Th1 inflammatory cytokines, IFN-γ, TNF, and IL-1β, were significantly reduced by day 8 after infection within the brains of WNV-infected IL-1R1−/− mice compared with those from WT animals (P = 0.0459, 0.0453, and 0.0441, respectively; Fig. 5 C). These results suggest that IL-1R1–deficient mice do not efficiently reactivate local antigen-specific responses to WNV within the CNS. Ex vivo restimulation of CD4+ T cells isolated from the brains of WNV-infected IL-1R1−/− mice with immunodominant I-Ab–restricted NS32066 and NS31616 peptides revealed decreased percentages as well as total numbers of CD4+ T cells that express IFN-γ compared with similarly evaluated CD4+ T cells derived from WNV-infected WT animals (P = 0.0164 and 0.0569, respectively; Fig. 5 E). In contrast, ex vivo restimulation of CD8+ T cells with an immunodominant Db-restricted NS4B peptide revealed no cell-intrinsic defect in expression of IFN-γ or Granzyme B, with significant increases in the percentages and numbers of cells expressing these proteins compared with similarly evaluated WT CD8+ T cells (P = 0.046 and 0.011, respectively; Fig. 5, F and G). IL-1R1 expression was not detected by flow cytometry on either the CD4+ or CD8+ T cells infiltrating the WNV-infected brain (not depicted), suggesting that IL-1 does not directly impact T lymphocyte activation. These results suggest that the effector responses of CNS-infiltrating, antiviral CD4+, but not CD8+, T cells are diminished in WNV-infected IL-1R1–deficient mice.
Figure 5.
T cell effector responses are impaired in the WNV-infected, IL-1R1−/− CNS. WT and IL-1R1−/− mice underwent footpad infection with 102 PFU of WNV, brains were harvested on day 8, and leukocytes were isolated after Percoll gradient centrifugation. (A) Total numbers of leukocytes isolated per brain from WT and IL-1R1−/− mice on day 8 after infection. (B) Cells derived from CNS tissues of WNV-infected WT and IL-1R1−/− mice at day 8 after infection were stained with mAbs against CD8, CD4, CD11b, and CD11c and analyzed after gating on leukocyte population. Data are presented as mean total numbers of leukocyte subsets ± SEM for n = 4–6 mice/group. The data for A and B are means of at least three independent experiments. (C) Brain tissue was also evaluated for protein levels of Th1 cytokine (IFN-γ, TNF, and IL-1β) at day 8 after infection in the brain. Data are presented as the mean pg/mg ± SEM for n = 6 WT and n = 6 IL-1R1−/− mice from at least two independent experiments. Student’s t test was used to determine statistical significance for A–C. *, P < 0.05; **, P < 0.01 compared with WT. (D) Representative confocal microscopic images from brain sections (brainstem region) from WT and IL-1R1−/− WNV-infected mice stained for WNV antigen, CD3, and nuclei (blue). Images are representative of results from five independent mice. Bars, 50 µm. (E–G) Isolated leukocytes from harvested brain tissue from WT and IL-1R1−/− mice inoculated with 102 PFU of WNV by footpad injection on day 8 after infection were stimulated ex vivo with I-Ab–restricted NS32066 and NS31616 peptides or Db-restricted NS4B peptide and then stained for CD4 and intracellular IFN-γ or CD8 and intracellular IFN-γ and Granzyme B (GZMB), respectively. The data were obtained via flow cytometry analysis and are shown as contour plots of gated CD4+ or CD8+ cells, and a summary is shown of the percentage and total number of CD4+ T cells positive for intracellular IFN-γ (E) and CD8+ T cells positive for intracellular IFN-γ (F) or Granzyme B (G). Statistically significant differences in IL-1R1−/− mice were determined by Student’s t test by comparing with WT. Data for E–G represent the means ± SEM of at least three independent experiments each with four to six mice/genotype. *, P < 0.05.
IL-1R1 signaling is required for CD11c+ cell activation within the CNS during WNV infection
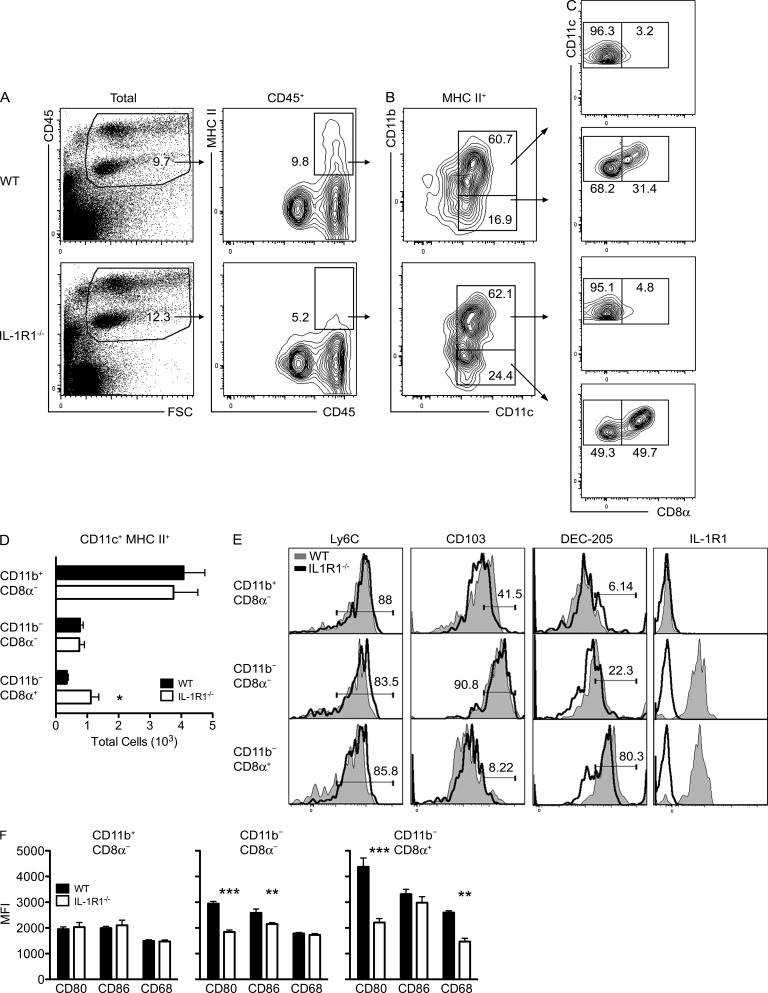
Our data identifying loss of secondary effector T cell function in the absence of IL-1R1 signaling indicate this receptor may be required for their proper reactivation locally by APCs within the virally infected CNS (Trifilo and Lane, 2004; Greter et al., 2005; Steel et al., 2009; Clarkson et al., 2012). To identify candidate APCs within the WNV-infected brain, we phenotyped CD45+ leukocytes in cell suspensions, comparing IL-1R1–deficient mice with WT on day 8 after infection with 102 PFU of WNV via footpad inoculation. In uninfected mice brains, there were minimal numbers of immune cells present in the brain, with CD45int cells (microglia) the dominant immune population (not depicted). Flow cytometry analysis for MHC class II (MHC II) and CD45 expression showed MHC II+ cells predominately in the CD45hi populations with a significant decrease in the percentage of these cells within the IL-1R1–deficient mice (Fig. 6 A). Analysis of CD11b and CD11c expression within this CD45hi population revealed that the largest subset of the CD11c+ fraction (∼60%) was CD11b+ (Fig. 6 B). The CD11b− fraction was increased (∼25% compared with 17%) within IL-1R1−/− mice. Two separate subsets within the CD11b− fraction were delineated by CD8α expression with an increase in the percentage of the CD8α+ subset (∼50% compared with 30%) and a decrease in the CD8α− subset (∼50% compared with 70%) within the IL-1R1−/− mice compared with WT (Fig. 6 C). Comparison of the total CD11c+MHC II+ cell subset numbers revealed no differences between the two genotypes except for an increase in the CD11b−CD8α+ subset compared with WT mice (P = 0.035; Fig. 6 D).
Figure 6.
Infiltrating CD11c+ APCs fail to be fully activated in the IL-1R1−/− WNV-infected CNS. (A–C) Flow cytometric plots of MHC II expression on CD45+ cells (A), CD11b/c expression on MHC II+ cells (B), and CD8α expression on CD11c+ cells (C) in brain leukocytes derived from WNV-infected WT versus IL-1R1−/− at day 8 after infection. Numbers indicate percentages of gated cells. Data are representative of at least three independent experiments. (D) Quantification of CNS-derived CD11b+ versus CD11b− CD11c+MHC II+ cells in WNV-infected IL-1R1−/− and WT mice at day 8 after infection. Data are presented as total cells ± SEM from two independent experiments with n = 5 mice/group. *, P < 0.05. (E) CNS cells were gated on CD11c+MHC II+ cells and subsetted based on expression of CD11b and CD8α. Histograms show expression levels of Ly-6C, CD103, Dec-205, and IL-1R1 from WNV-infected WT animals compared with similarly infected IL-1R1−/− animals within the indicated populations. Percentage of gated cells is indicated. (F) Levels of expression of CD80, CD86, and CD68 within each individual CD11c+MHC II+ population of isolated CNS tissues of WNV-infected IL-1R1−/− and WT at day 8 after infection. Data are presented as mean fluorescence intensities (MFI) ± SEM for five mice/group. Data represent means from two independent experiments. **, P < 0.01; ***, P < 0.001.
Further phenotypic analysis of the CD11c+ subsets revealed they were all Ly6c+. Increased CD103 expression was observed on CD11c+CD11b−CD8α− cells, and increased DEC-205 expression was detected on CD11c+CD11b−CD8α+ cells (Fig. 6 E). IL-1R1 expression was detected by flow cytometry only within the CD11b− fractions of the CD11c+ subsets (i.e., CD11b−CD8α− and CD11b−CD8α+; Fig. 6 E), suggesting that these two populations are the main targets of IL-1. The evaluation of the expression levels of the co-stimulatory molecules CD80 (B-7.1), CD86 (B-7.2), and CD68 within the CD11b−CD8α− and CD11b−CD8α+ subsets revealed depressed levels of CD80 and CD86 and CD80 and CD68, respectively, in the absence of IL-1R1 signaling compared with WT mice (Fig. 6 F). Levels of expression of these molecules in the CD11b+CD8α− did not differ between the two genotypes. To determine whether these APCs function principally by locally reactivating T cells or by capturing antigen and transporting them to draining LNs for priming, we also assessed percentages of CD11c+ cells within the cervical draining LNs. We were unable to detect any significant difference in the percentages of CD11c+ cells, including CD11c+CD11b+ or CD11c+CD8α+ cells, within the cervical LNs by day 8 after infection within IL-1R1−/− and WT mice, suggesting that IL-1R1 signaling had little effect on trafficking of APCs to secondary LN organs (not depicted). Overall, these data suggest that IL-1R1 deficiency impacts the expression of co-stimulatory molecules on CD11c+ cells, leading to direct and indirect impairment of CD4+ and CD8+ T cell reactivation, respectively, and antiviral function within the CNS.
Adoptively transferred CD11c+ cells infiltrate the WNV-infected CNS
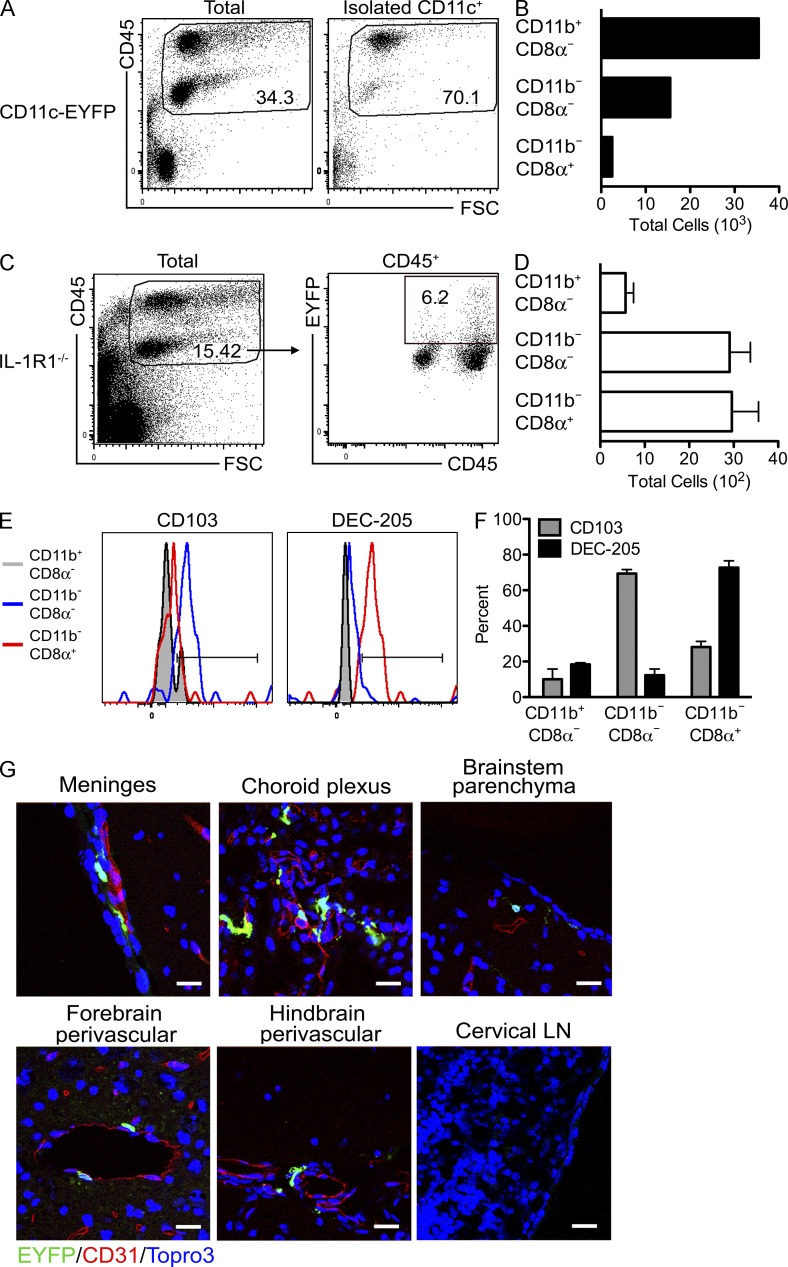
To determine whether the presence and the activation state of infiltrating CD11c+ APCs within the CNS is key to an optimal reactivation of infiltrating WNV-specific T lymphocytes, we used CD11c-EYFP reporter mice (Lindquist et al., 2004; Choi et al., 2009) to phenotype and detect EYFP cells in adoptive transfer experiments. Brains were collected from 8-wk-old CD11c-EYFP donor mice infected with 102 WNV via footpad injection on day 9 after infection. After preparation of single cell suspensions from WNV-infected CD11c-EYFP donor mice brains, CD11c+ cells were isolated by positive selection (MACS) and yielded ∼70% CD45+ leukocytes (Fig. 7 A). The phenotypic characterization of these cells revealed the same three CD11c+ subsets previously identified, listed in decreasing total numbers: CD11c+CD11b+CD8α−, CD11c+CD11b−CD8α−, and CD11c+CD11b−CD8α+ (Fig. 7 B). Approximately 5 × 104 of the isolated donor CD11c+ cells were adoptively transferred into congenic IL-1R1−/− mice 4 d after WNV infection, when virus is being cleared in the periphery but replicating in the CNS (Fig. 2; Klein and Diamond, 2008). The percentage of EYFP+ cells isolated on day 8 after infection from WNV-infected recipient IL-1R1−/− mice, whose gating was based on data obtained from nontransferred animals, was ∼6% (Fig. 7 C), suggesting few cells trafficked into the CNS. However, the phenotypic analysis showed an enrichment of CD11c+CD11b−CD8α− and CD11c+CD11b−CD8α+ subsets (Fig. 7 D). Flow analysis also confirmed that the CD11c+CD11b−CD8α− expressed CD103 and that the CD11c+CD11b−CD8α+ expressed DEC-205 (Fig. 7, E and F). To determine the localization of the cells, we examined the brains of the IL-1R1−/− recipient mice on day 8 after infection for EYFP expression. We detected EYFP+ cells predominately along the meninges and in the choroid plexus with few cells in the perivascular locations or parenchyma of the brain (Fig. 7 G). We were unable to detect EYFP+ cells within the cervical LN of the IL-1R1−/− mice (Fig. 7 G). Therefore, adoptively transferred IL-1R1+/+ CD11c+ cells, specifically the CD103+ and CD8α+DEC-205+ subsets, are found within the WNV-infected CNS of IL-1R1−/− mice.
Figure 7.
Adoptively transferred CD11c+ cells infiltrate the WNV-infected CNS. CD11c+ cells were obtained via positive selection (MACS) from brain leukocytes isolated from the CNS of WNV-infected CD11c-EYFP transgenic mice at day 9 after infection. (A) Flow cytometric analysis of CD45 expression in brain leukocytes before (total) and after CD11c+ cell isolation. Numbers indicate percentage of gated cells. (B) Bar graph shows total numbers of the indicated subsets among CD11c+ cells. (C–F) Isolated CD11c+EYFP+ cells were transferred intravenously into WNV-infected IL-1R1−/− mice on day 4 after infection. (C) Flow cytometric analysis of EYFP expression of brain leukocytes obtained from IL-1R1−/− mice on day 8 after infection. Percentage of gated cells is shown. (D) Absolute numbers of the indicated subsets among EYFP+ cells. (E) CD103 and DEC-205 expression for CD11b+CD8α−, CD11b−CD8α−, and CD11b−CD8α+ populations. (F) Percentages of cells expressing CD103 or DEC-205 within the indicated populations. (D and F) Error bars are SEM. (G) Representative confocal images of sagittal brain sections from various regions and the cervical draining LN at day 8 after infection from four independent IL-1R1−/− mice that received EYFP+ cells on day 4 after infection. EYFP+ cell locations were evaluated after amplification with anti-GFP antibodies and in relation with the microvasculature with detection of CD31. All nuclei were counterstained with ToPro3. Bars, 25 µm.
Adoptively transferred CD11c+ cells restore T cell reactivation within the WNV-infected CNS
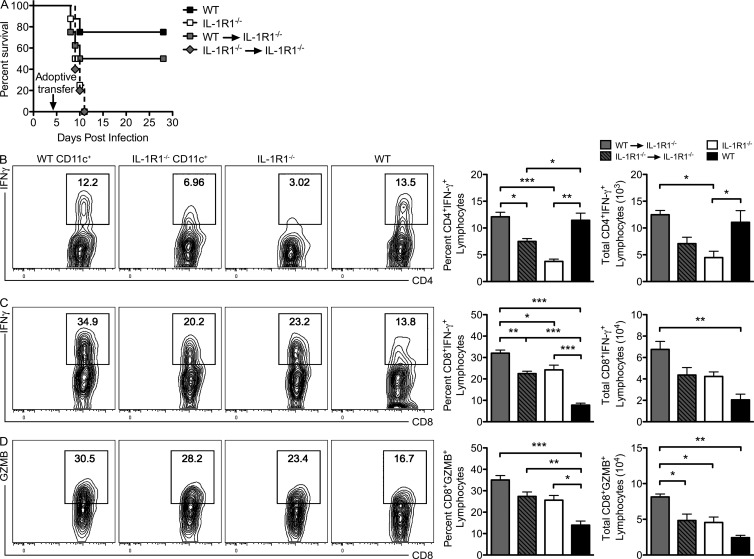
To evaluate whether CD11c+ cells contributed to protection against WNV infection, additional adoptively transferred mice were followed for survival. Transfer of CNS-derived, WT CD11c+ cells rescued IL-1R1−/− mice, resulting in a 50% survival rate compared with the 0% survival observed when IL-1R1−/− CD11c+ or no CD11c+ cells were transferred (Fig. 8 A). These data suggest that CNS-infiltrating CD11c+ cells are critical for survival from lethal WNV encephalitis.
Figure 8.
CD11c+ cell adoptive transfer rescues T cell responses in the brains of WNV-infected mice. (A) WT and IL-1R1−/− mice or IL-1R1−/− recipients adoptively transferred with WT or IL-1R1−/− CD11c+ cells (5 × 104) isolated from the CNS of WNV-infected mice at day 9 after infection were infected with 100 PFU of WNV, and survival was monitored. (B–D) Leukocytes were isolated from harvested brain tissue from the indicated mice, stimulated ex vivo with CD4 or CD8 WNV-specific peptides, stained for intracellular IFN-γ and CD4 and CD8 or Granzyme B (GZMB) and CD8, and analyzed via flow cytometry. Contour plots of gated CD4+ or CD8+ cells are shown, and bar graphs show percentages and total numbers ± SEM of CD4+ T cells positive for intracellular IFN-γ (B) and CD8+ T cells positive for intracellular IFN-γ (C) or Granzyme B (D). Data represent the means of two independent experiments each with four to six mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether the transfer of CD11c+ cells impacted the antigen-specific T cell response to WNV within the CNS, ex vivo restimulation of CD4+ and CD8+ T cells isolated from the brains of WNV-infected IL-1R1−/− mice was performed as previously detailed (Figs. 4 and 5) after CD11c+ cell adoptive transfers. The percentages and total cell numbers of CD4+ T cells that express IFN-γ were restored in IL-1R1−/− mice that received WT CD11c+ cells, whereas IL-1R1−/− CD11c+ or no CD11c+ failed to fully activate antigen-specific CD4+ T cell responses (Fig. 8 B). Ex vivo restimulation of CD8+ T cells isolated from WT CD11c+ cell recipient IL-1R1−/− mice revealed significant increases in the percentages and numbers of cells expressing IFN-γ or Granzyme B compared with similarly evaluated IL-1R1−/− CD11c+ or no CD11c+ recipient IL-1R1−/− mice (Fig. 8, C and D). These results continue to highlight that there is no cell-intrinsic defect in CD8+ T cells within the WNV-infected CNS and that the antiviral CD4+ T cell responses may aid in the proper reactivation of CD8+ T cell responses.
DISCUSSION
The integrity of adaptive cellular immune responses is essential for WNV clearance, especially within the CNS where efficient T cell–mediated immunity has been shown to both clear virus and dampen immunopathology (McCandless et al., 2008). However, the cellular and molecular mechanisms directing T cell reactivation in the CNS during viral infections are incompletely understood. In this study, we show that IL-1R1 signaling is critical for the full activation of infiltrating CD11c+ APCs specifically within the CNS. WNV-infected, IL-1R1–deficient mice exhibit fulminant and fatal encephalitis, characterized by loss of virologic control caused by lack of effective CD4+ T cell effector responses. Thus, although IL-1R1 is dispensable for normal peripheral adaptive immune responses during WNV infection, it is essential for proper reactivation of WNV-specific effector T cells within the CNS.
The identity of APCs within the CNS in the context of neuroinflammatory diseases has been controversial. Although earlier studies focused on the role of resident microglia and recruited macrophages (Olson and Miller, 2004; Rock et al., 2004; Murray and Wynn, 2011), more recent analyses indicate that DCs are more likely to provide the signals required for T cell reactivation and retention in the CNS, especially in the context of viral infections. DCs have been shown to accumulate in the CNS in various mouse models of viral encephalitis (Trifilo and Lane, 2004; McMahon et al., 2006; Steel et al., 2009; D’Agostino et al., 2012); however, their antiviral function within the CNS was not defined. Our study is the first to clearly indicate that IL-1R1 signaling dictates the activation of CD11c+ APCs within the CNS, which locally optimizes the effector function of antigen-specific T cells. DCs have been shown to be permissive to WNV infection (Suthar et al., 2010), and a previous study has detected plasmacytoid DCs in the CNS during WNV infection as early as day 4 after infection (Bréhin et al., 2008), suggesting a potential antigen-presenting role for these cells within the CNS. As all infiltrating leukocytes enter the CNS via leptomeningeal vessels (Bartholomäus et al., 2009), DCs and WNV-primed T cells may encounter each other before entering the CNS parenchyma proper. Our study indicates that IL-1R1 signaling is a critical part of these encounters through promoting recruitment and activation of at least two populations of DC-like APCs: the CD11c+CD11b−CD8α+Dec-205+ cells, similar phenotypically to lymphoid-derived DCs, and the CD11c+CD11b−CD103+, similar to migratory DCs (Liu and Nussenzweig, 2010; Belz and Nutt, 2012). Our study is in agreement with those using intranasally introduced vesicular stomatitis virus, which similarly demonstrated that CD103+ DCs play a functional a role in T cell proliferation and cytokine production (D’Agostino et al., 2012). In addition, we observed the CD11c+ cells predominately within the meninges and choroid plexus. The existence of CD11c+MHC II+ DC-like cells within these same areas of the steady-state mouse brain has recently been demonstrated (Anandasabapathy et al., 2011). In addition, studies using the MS model experimental autoimmune encephalitis have demonstrated that these areas act as avenues of effector T cell entry (Kivisäkk et al., 2003; Ransohoff et al., 2003). Therefore, in our model, adoptively transferred DC-like APCs may be positioned as sentinels to meet the infiltrating effector T cells. Interestingly, one of these populations contains a subset of CD8α+DEC-205+ cells that may be analogous to conventional DC subsets associated with cross-presentation and in priming antiviral CD8+ T cells (den Haan et al., 2000; Belz and Nutt, 2012).
Our results illustrate that severe WNV infection and the lack of CNS viral clearance in the absence of IL-1R1 signaling is not related to peripheral adaptive immune responses or neuroinvasiveness. After peripheral inoculation, WNV initially replicates in LNs and then disseminates via hematogenous spread into peripheral organs such as the spleen and kidney, where it is largely cleared via humoral and T cell immune responses (Klein and Diamond, 2008). Indeed, in IL-1R1–deficient mice, increased peripheral viral loads are met with an elevated total IgG response. In response to viral infection, IL-1 is produced both within the peripheral organs as well as in the CNS, suggesting fundamental contributions during antiviral immune responses. During WNV infection, type I IFNs and γδ T cells have previously been reported to impact peripheral DC activation, which impacts the subsequent peripheral adaptive immune response in the periphery (Asselin-Paturel et al., 2005; Wang et al., 2006). Similarly, our results indicate that by day 5 after infection, type I IFN levels are diminished in the IL-1R1−/− animal compared with WT, suggesting that DC activation may be impacted. However, by day 8 after infection, peripheral CTL and antibody responses were unaffected, resulting in effective WNV clearance from the sera and peripheral organs of both the 8-wk-old IL-1R1−/− and WT mice, suggesting that IL-1R1 signaling is ultimately dispensable for peripheral viral control. Although diminished within the periphery, type I IFN responses increased within the CNS by day 8 after infection in the absence of IL-1R1 signaling. This disparity may be in response to increased viral titer within the IL-1R1–deficient CNS (Daffis et al., 2009). In addition, recent work suggests that type I IFN signaling shapes the maturation of effector T cells, and therefore, it may have a synergistic role with IL-1 in the CNS (Pinto et al., 2011). Our results suggest that IL-1R1 signaling impacted two specific CD11c+ APCs within the CNS. Potentially, IL-1R1 signaling may not have an impact on specific DC subsets, which are necessary for an effective activation of an adaptive immune response within the periphery. A study with an influenza virus model also showed that IL-1 has little role in peripheral CD8+ T cell responses (Schmitz et al., 2005). Our results also demonstrate that WNV was no more neuroinvasive in the absence of IL-1R1 signaling than with WT. In rodent models, infectious WNV can be recovered from CNS tissues ∼4–5 d after infection (Diamond et al., 2003; Shrestha and Diamond, 2004). Although viral titer peaked within the peripheral tissues by day 5 after infection, we saw no statistical difference in viral titer within the CNS between IL-1R1−/− and WT mice at this time point, suggesting that IL-1 does not have a role in limiting WNV neuroinvasion. However, because IL-1R1 signaling in astrocytes may impact restoration of blood–brain barrier (BBB) permeability after injury, the observed increase in leukocyte trafficking in WNV-infected, IL-1R1–deficient mice might also be caused by persistent changes at the BBB (Argaw et al., 2006). Further studies are needed to evaluate the role of IL-1R1 at the BBB in CNS viral infections.
Although we cannot rule out that IL-1 may instigate some pathology, our results confirm its salient role in viral clearance and survival. After adoptive transfer, neuronal injury in the brain of IL-1R1−/− mice appears to be caused by increased viral titer. Previous studies report that WNV replication directly induces neuronal injury, followed by apoptosis of neurons (Samuel et al., 2007; Kobayashi et al., 2012). The proximity of WNV-Ag+ and TUNEL+ cells suggest that viral spread and cell death are a contiguous process. Another potential path to neuronal injury is the increase in CD8+ T cell activity within the CNS after adoptive transfer. However, our findings clearly support previous results showing that increased CD8+ T cell presence improves survival and reduces immunopathology during WNV encephalitis (McCandless et al., 2008). Similarly, a previous study demonstrates that IL-1β–deficient mice were more susceptible to HSV1-mediated encephalitis caused by increased viral load (Sergerie et al., 2007). These results also suggest that IL-1–targeted therapeutics, such as in rheumatoid arthritis (Lane and Lachmann, 2011), may increase the susceptibility to and morbidity from viral encephalitis.
In summary, the results of our experiments suggest that IL-1 is critical in modulating the host immune response to WNV infection. IL-1 in the CNS appears to positively regulate CD11c+ APC activation, which in turn reactivates the effector function of infiltrating virus-specific T cells. These experiments illuminate the host complexity of IL-1R1 signaling and enhance our understanding of the balance between an effective immune response and immunopathology with injury to neurons.
MATERIALS AND METHODS
Ethics statement.
All experiments were performed in strict compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and according to the international Guiding Principles for Biomedical Research Involving Animals. The protocol was approved by the Washington University School of Medicine in St. Louis Animal Safety Committee (#20100278).
Viruses.
The WNV strain 3000.0259 was isolated in New York in 2000 (Ebel et al., 2001) and passaged once in C6/36 Aedes albopictus cells to generate an insect cell–derived stock. The stock titer was determined by using BHK21 cells for viral plaque assay as previously described (Diamond et al., 2003).
Mouse experiments.
C57BL/6 WT and IL-1R1−/− inbred mice were obtained commercially (The Jackson Laboratory). All mice were housed and bred in the pathogen-free animal facilities of the Washington University School of Medicine in St. Louis. Matched 8-wk-old mice were inoculated subcutaneously via footpad injection (50 µl) or intracranially (10 µl) with either 102 or 101 PFU of WNV, respectively, as previously described (Engle and Diamond, 2003).
Viral tissue burden and viremia quantification.
For in vivo virus replication experiments, mice were infected with WNV via footpad and euthanized at specific time points. Organs were harvested, weighed, and homogenized after complete cardiac perfusion with PBS. Virus was titrated by standard plaque assay as described previously (Diamond et al., 2003), and WNV infection levels in serum were measured by analyzing positive-strand viral RNA levels using quantitative RT-PCR (qRT-PCR) as described previously (Samuel et al., 2006).
WNV-specific antibody and T cell responses.
The levels of WNV-specific IgM and IgG were determined using an ELISA against purified WNV E protein (gift of M.S. Diamond, Washington University in St. Louis) as previously described (Mehlhop and Diamond, 2006). Intracellular IFN-γ and Granzyme B staining was performed on splenocytes as well as isolated leukocytes from brains of day 6 and 8 postinfection animals in an I-Ab–restricted NS32066 and NS31616 peptide and Db-restricted NS4B peptide (gift of M.S. Diamond) restimulation assay as previously described (Purtha et al., 2007; Brien et al., 2008). Samples were processed by multicolor flow cytometry on an LSR flow cytometer (BD) using FlowJo software (Tree Star).
CNS leukocyte isolation.
Cells were isolated from the CNS of WT and IL-1R1−/− mice at day 8 after infection and stained with fluorescently conjugated antibodies to CD4, CD8β, CD11b, CD11c, CD45, CD8α, CD68, CD86, CD80, CD103, DEC-205, Ly-6C, MHC I, and MHC II as previously described (McCandless et al., 2006). Data collection and analysis were performed with an LSR flow cytometer using FlowJo software.
Real-time qRT-PCR.
Total RNA was prepared from the spleens and brains of WNV-infected WT and IL-1R1−/− mice using the RNeasy kit (QIAGEN) according to the manufacturer’s instructions. qRT-PCR was performed as previously described (Klein et al., 2005). Calculated copies were normalized against copies of the housekeeping gene GAPDH. All oligonucleotide primers used have been previously reported (Klein et al., 2005).
Immunohistochemistry and confocal microscopy.
Mice were infected with 102 PFU of WNV and sacrificed at day 8 after infection. CNS tissues were then isolated, and frozen sections were permeabilized, blocked, and stained as previously described (McCandless et al., 2006). Immunohistochemistry detection of CD3, CD31, NeuN, and WNV with nuclear ToPro3 counterstaining was performed as previously described (McCandless et al., 2008). Sections were analyzed by using an LSM 510 laser-scanning confocal microscope and accompanying software (Carl Zeiss). Quantitative analyses of TUNEL staining were performed in a blinded fashion by counting the numbers of TUNEL+ cells per high-power field. ImageJ image analysis software (National Institutes of Health) was used to analyze total pixels per high-power field. Detection of EYFP was performed using a monoclonal rat anti–mouse GFP (Invitrogen) as previously described (Cruz-Orengo et al., 2011).
Statistical analysis.
Graphs were made and statistical analysis was performed via computerized software (Prism; GraphPad Software). Depending on the data, an unpaired, two-tailed Student’s t test, one-way ANOVA with Tukey-Kramer posttest, or two-way ANOVA with Bonferroni posttest was performed, with P < 0.05 considered to be significant. Kaplan-Meier survival curves were analyzed by the log-rank test.
Acknowledgments
We would like to thank Dr. Michael S. Diamond for gifts of reagents, Eric Ford for technical assistance, and Dr. Greg Wu for critical reading of the manuscript.
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS052632 and P01NS059560 (to R.S. Klein).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BBB
- blood–brain barrier
- CNS
- central nervous system
- EYFP
- enhanced YFP
- MS
- multiple sclerosis
- qRT-PCR
- quantitative RT-PCR
- WNV
- West Nile virus
References
- Alsharifi M., Lobigs M., Simon M.M., Kersten A., Müller K., Koskinen A., Lee E., Müllbacher A. 2006. NK cell-mediated immunopathology during an acute viral infection of the CNS. Eur. J. Immunol. 36:887–896 10.1002/eji.200535342 [DOI] [PubMed] [Google Scholar]
- Anandasabapathy N., Victora G.D., Meredith M., Feder R., Dong B., Kluger C., Yao K., Dustin M.L., Nussenzweig M.C., Steinman R.M., Liu K. 2011. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 208:1695–1705 10.1084/jem.20102657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw A.T., Zhang Y., Snyder B.J., Zhao M.L., Kopp N., Lee S.C., Raine C.S., Brosnan C.F., John G.R. 2006. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 177:5574–5584 [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C., Brizard G., Chemin K., Boonstra A., O’Garra A., Vicari A., Trinchieri G. 2005. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 201:1157–1167 10.1084/jem.20041930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomäus I., Kawakami N., Odoardi F., Schläger C., Miljkovic D., Ellwart J.W., Klinkert W.E., Flügel-Koch C., Issekutz T.B., Wekerle H., Flügel A. 2009. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 462:94–98 10.1038/nature08478 [DOI] [PubMed] [Google Scholar]
- Basu A., Krady J.K., Levison S.W. 2004. Interleukin-1: a master regulator of neuroinflammation. J. Neurosci. Res. 78:151–156 10.1002/jnr.20266 [DOI] [PubMed] [Google Scholar]
- Belz G.T., Nutt S.L. 2012. Transcriptional programming of the dendritic cell network. Nat. Rev. Immunol. 12:101–113 10.1038/nri3149 [DOI] [PubMed] [Google Scholar]
- Bhat R., Steinman L. 2009. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 64:123–132 10.1016/j.neuron.2009.09.015 [DOI] [PubMed] [Google Scholar]
- Bréhin A.C., Mouriès J., Frenkiel M.P., Dadaglio G., Desprès P., Lafon M., Couderc T. 2008. Dynamics of immune cell recruitment during West Nile encephalitis and identification of a new CD19+B220-BST-2+ leukocyte population. J. Immunol. 180:6760–6767 [DOI] [PubMed] [Google Scholar]
- Brien J.D., Uhrlaub J.L., Nikolich-Zugich J. 2007. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur. J. Immunol. 37:1855–1863 10.1002/eji.200737196 [DOI] [PubMed] [Google Scholar]
- Brien J.D., Uhrlaub J.L., Nikolich-Zugich J. 2008. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J. Immunol. 181:8568–8575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M.J., Doose J.M., Melchior B., Schmid C.D., Ploix C.C. 2006. CNS immune privilege: hiding in plain sight. Immunol. Rev. 213:48–65 10.1111/j.1600-065X.2006.00441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Do Y., Cheong C., Koh H., Boscardin S.B., Oh Y.S., Bozzacco L., Trumpfheller C., Park C.G., Steinman R.M. 2009. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J. Exp. Med. 206:497–505 10.1084/jem.20082129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson B.D., Héninger E., Harris M.G., Lee J., Sandor M., Fabry Z. 2012. Innate-adaptive crosstalk: how dendritic cells shape immune responses in the CNS. Adv. Exp. Med. Biol. 946:309–333 10.1007/978-1-4614-0106-3_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orengo L., Holman D.W., Dorsey D., Zhou L., Zhang P., Wright M., McCandless E.E., Patel J.R., Luker G.D., Littman D.R., et al. 2011. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J. Exp. Med. 208:327–339 10.1084/jem.20102010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino P.M., Kwak C., Vecchiarelli H.A., Toth J.G., Miller J.M., Masheeb Z., McEwen B.S., Bulloch K. 2012. Viral-induced encephalitis initiates distinct and functional CD103+ CD11b+ brain dendritic cell populations within the olfactory bulb. Proc. Natl. Acad. Sci. USA. 109:6175–6180 10.1073/pnas.1203941109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S., Suthar M.S., Szretter K.J., Gale M., Jr, Diamond M.S. 2009. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 5:e1000607 10.1371/journal.ppat.1000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan J.M., Lehar S.M., Bevan M.J. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1696 10.1084/jem.192.12.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M.S., Shrestha B., Marri A., Mahan D., Engle M. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578–2586 10.1128/JVI.77.4.2578-2586.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. 2009. Interleukin-1beta and the autoinflammatory diseases. N. Engl. J. Med. 360:2467–2470 10.1056/NEJMe0811014 [DOI] [PubMed] [Google Scholar]
- Ebel G.D., Dupuis A.P., II, Ngo K., Nicholas D., Kauffman E., Jones S.A., Young D., Maffei J., Shi P.Y., Bernard K., Kramer L.D. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle M.J., Diamond M.S. 2003. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J. Virol. 77:12941–12949 10.1128/JVI.77.24.12941-12949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M., Heppner F.L., Lemos M.P., Odermatt B.M., Goebels N., Laufer T., Noelle R.J., Becher B. 2005. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11:328–334 10.1038/nm1197 [DOI] [PubMed] [Google Scholar]
- Guarner J., Shieh W.J., Hunter S., Paddock C.D., Morken T., Campbell G.L., Marfin A.A., Zaki S.R. 2004. Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum. Pathol. 35:983–990 10.1016/j.humpath.2004.04.008 [DOI] [PubMed] [Google Scholar]
- Hausmann J., Schamel K., Staeheli P. 2001. CD8(+) T lymphocytes mediate Borna disease virus-induced immunopathology independently of perforin. J. Virol. 75:10460–10466 10.1128/JVI.75.21.10460-10466.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T.D. 2010. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10:688–698 10.1038/nri2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A.M. 2011. Globalization, land use, and the invasion of West Nile virus. Science. 334:323–327 10.1126/science.1201010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N.J., Getts D.R., Getts M.T., Rana S., Shrestha B., Kesson A.M. 2007. Immunopathology of flavivirus infections. Immunol. Cell Biol. 85:33–42 10.1038/sj.icb.7100012 [DOI] [PubMed] [Google Scholar]
- Kivisäkk P., Mahad D.J., Callahan M.K., Trebst C., Tucky B., Wei T., Wu L., Baekkevold E.S., Lassmann H., Staugaitis S.M., et al. 2003. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. USA. 100:8389–8394 10.1073/pnas.1433000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.S., Diamond M.S. 2008. Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus. Trends Mol. Med. 14:286–294 10.1016/j.molmed.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Klein R.S., Lin E., Zhang B., Luster A.D., Tollett J., Samuel M.A., Engle M., Diamond M.S. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457–11466 10.1128/JVI.79.17.11457-11466.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters B.K., Marder B.A., Levi M.E., Laird S.P., McNutt J.T., Escott E.J., Everson G.T., Tyler K.L. 2004. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch. Neurol. 61:1210–1220 10.1001/archneur.61.8.1210 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Orba Y., Yamaguchi H., Kimura T., Sawa H. 2012. Accumulation of ubiquitinated proteins is related to West Nile virus-induced neuronal apoptosis. Neuropathology. 32:398–405 10.1111/j.1440-1789.2011.01275.x [DOI] [PubMed] [Google Scholar]
- Lane T., Lachmann H.J. 2011. The emerging role of interleukin-1β in autoinflammatory diseases. Curr. Allergy Asthma Rep. 11:361–368 10.1007/s11882-011-0207-6 [DOI] [PubMed] [Google Scholar]
- Lindquist R.L., Shakhar G., Dudziak D., Wardemann H., Eisenreich T., Dustin M.L., Nussenzweig M.C. 2004. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5:1243–1250 10.1038/ni1139 [DOI] [PubMed] [Google Scholar]
- Liu K., Nussenzweig M.C. 2010. Development and homeostasis of dendritic cells. Eur. J. Immunol. 40:2099–2102 10.1002/eji.201040501 [DOI] [PubMed] [Google Scholar]
- Matsuki T., Nakae S., Sudo K., Horai R., Iwakura Y. 2006. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int. Immunol. 18:399–407 10.1093/intimm/dxh379 [DOI] [PubMed] [Google Scholar]
- McCandless E.E., Wang Q., Woerner B.M., Harper J.M., Klein R.S. 2006. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J. Immunol. 177:8053–8064 [DOI] [PubMed] [Google Scholar]
- McCandless E.E., Zhang B., Diamond M.S., Klein R.S. 2008. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc. Natl. Acad. Sci. USA. 105:11270–11275 10.1073/pnas.0800898105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless E.E., Budde M., Lees J.R., Dorsey D., Lyng E., Klein R.S. 2009. IL-1R signaling within the central nervous system regulates CXCL12 expression at the blood-brain barrier and disease severity during experimental autoimmune encephalomyelitis. J. Immunol. 183:613–620 10.4049/jimmunol.0802258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern D.B., Kang S.S. 2011. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 11:318–329 10.1038/nri2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness M.C., Powers J.M., Bias W.B., Schmeckpeper B.J., Segal A.H., Gowda V.C., Wesselingh S.L., Berger J., Griffin D.E., Smith K.D. 1997. Human leukocyte antigens and cytokine expression in cerebral inflammatory demyelinative lesions of X-linked adrenoleukodystrophy and multiple sclerosis. J. Neuroimmunol. 75:174–182 10.1016/S0165-5728(97)00020-9 [DOI] [PubMed] [Google Scholar]
- McMahon E.J., Bailey S.L., Miller S.D. 2006. CNS dendritic cells: critical participants in CNS inflammation? Neurochem. Int. 49:195–203 10.1016/j.neuint.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Mehlhop E., Diamond M.S. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J. Exp. Med. 203:1371–1381 10.1084/jem.20052388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Harada M., Narabayashi H., Inagaki H., Minami M., Nagatsu T. 1996. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 211:13–16 10.1016/0304-3940(96)12706-3 [DOI] [PubMed] [Google Scholar]
- Murray P.J., Wynn T.A. 2011. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11:723–737 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S., Asano M., Horai R., Sakaguchi N., Iwakura Y. 2001. IL-1 enhances T cell-dependent antibody production through induction of CD40 ligand and OX40 on T cells. J. Immunol. 167:90–97 [DOI] [PubMed] [Google Scholar]
- Olson J.K., Miller S.D. 2004. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 173:3916–3924 [DOI] [PubMed] [Google Scholar]
- Petersen L.R., Hayes E.B. 2008. West Nile virus in the Americas. Med. Clin. North Am. 92:1307–1322: ix 10.1016/j.mcna.2008.07.004 [DOI] [PubMed] [Google Scholar]
- Pinto A.K., Daffis S., Brien J.D., Gainey M.D., Yokoyama W.M., Sheehan K.C.F., Murphy K.M., Schreiber R.D., Diamond M.S. 2011. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 7:e1002407 10.1371/journal.ppat.1002407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtha W.E., Myers N., Mitaksov V., Sitati E., Connolly J., Fremont D.H., Hansen T.H., Diamond M.S. 2007. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur. J. Immunol. 37:1845–1854 10.1002/eji.200737192 [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M., Kivisäkk P., Kidd G. 2003. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 3:569–581 10.1038/nri1130 [DOI] [PubMed] [Google Scholar]
- Rock R.B., Gekker G., Hu S., Sheng W.S., Cheeran M., Lokensgard J.R., Peterson P.K. 2004. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 17:942–964 10.1128/CMR.17.4.942-964.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Diamond M.S. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350–13361 10.1128/JVI.79.21.13350-13361.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Diamond M.S. 2006. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80:9349–9360 10.1128/JVI.01122-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Whitby K., Keller B.C., Marri A., Barchet W., Williams B.R., Silverman R.H., Gale M., Jr, Diamond M.S. 2006. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 80:7009–7019 10.1128/JVI.00489-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Morrey J.D., Diamond M.S. 2007. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 81:2614–2623 10.1128/JVI.02311-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffenbauer J., Streit W.J., Butfiloski E., LaBow M., Edwards C., III, Moldawer L.L. 2000. The induction of EAE is only partially dependent on TNF receptor signaling but requires the IL-1 type I receptor. Clin. Immunol. 95:117–123 10.1006/clim.2000.4851 [DOI] [PubMed] [Google Scholar]
- Schmitz N., Kurrer M., Bachmann M.F., Kopf M. 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79:6441–6448 10.1128/JVI.79.10.6441-6448.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie Y., Rivest S., Boivin G. 2007. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J. Infect. Dis. 196:853–860 10.1086/520094 [DOI] [PubMed] [Google Scholar]
- Shaftel S.S., Carlson T.J., Olschowka J.A., Kyrkanides S., Matousek S.B., O’Banion M.K. 2007. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 27:9301–9309 10.1523/JNEUROSCI.1418-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B., Diamond M.S. 2004. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 78:8312–8321 10.1128/JVI.78.15.8312-8321.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B., Samuel M.A., Diamond M.S. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J. Virol. 80:119–129 10.1128/JVI.80.1.119-129.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J.E., Smith D.E. 2010. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10:89–102 10.1038/nri2691 [DOI] [PubMed] [Google Scholar]
- Sitati E.M., Diamond M.S. 2006. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J. Virol. 80:12060–12069 10.1128/JVI.01650-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley L.C., Mrak R.E., Woody R.C., Perrot L.J., Zhang S., Marshak D.R., Nelson S.J., Griffin W.S. 1994. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 53:231–238 10.1097/00005072-199405000-00003 [DOI] [PubMed] [Google Scholar]
- Steel C.D., Hahto S.M., Ciavarra R.P. 2009. Peripheral dendritic cells are essential for both the innate and adaptive antiviral immune responses in the central nervous system. Virology. 387:117–126 10.1016/j.virol.2009.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C.A., Ismail S., Dick E.P., Bennett J.A., Johnston S.L., Edwards M.R., Sabroe I., Parker L.C. 2011. Role of interleukin-1 and MyD88-dependent signaling in rhinovirus infection. J. Virol. 85:7912–7921 10.1128/JVI.02649-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar M.S., Ma D.Y., Thomas S., Lund J.M., Zhang N., Daffis S., Rudensky A.Y., Bevan M.J., Clark E.A., Kaja M.K., et al. 2010. IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 6:e1000757 10.1371/journal.ppat.1000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilo M.J., Lane T.E. 2004. The CC chemokine ligand 3 regulates CD11c+CD11b+CD8alpha- dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 327:8–15 10.1016/j.virol.2004.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Gao Y., Scully E., Davis C.T., Anderson J.F., Welte T., Ledizet M., Koski R., Madri J.A., Barrett A., et al. 2006. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J. Immunol. 177:1825–1832 [DOI] [PubMed] [Google Scholar]
- Wang Y., Lobigs M., Lee E., Müllbacher A. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 77:13323–13334 10.1128/JVI.77.24.13323-13334.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Chan Y.K., Lu B., Diamond M.S., Klein R.S. 2008. CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J. Immunol. 180:2641–2649 [DOI] [PubMed] [Google Scholar]
- Zhao M.L., Kim M.O., Morgello S., Lee S.C. 2001. Expression of inducible nitric oxide synthase, interleukin-1 and caspase-1 in HIV-1 encephalitis. J. Neuroimmunol. 115:182–191 10.1016/S0165-5728(00)00463-X [DOI] [PubMed] [Google Scholar]