Cytotoxic T cells increase leukemia stem cell numbers in a murine model of chronic myeloid leukemia.
Abstract
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasia arising from the oncogenic break point cluster region/Abelson murine leukemia viral oncogene homolog 1 translocation in hematopoietic stem cells (HSCs), resulting in a leukemia stem cell (LSC). Curing CML depends on the eradication of LSCs. Unfortunately, LSCs are resistant to current treatment strategies. The host’s immune system is thought to contribute to disease control, and several immunotherapy strategies are under investigation. However, the interaction of the immune system with LSCs is poorly defined. In the present study, we use a murine CML model to show that LSCs express major histocompatibility complex (MHC) and co-stimulatory molecules and are recognized and killed by leukemia-specific CD8+ effector CTLs in vitro. In contrast, therapeutic infusions of effector CTLs into CML mice in vivo failed to eradicate LSCs but, paradoxically, increased LSC numbers. LSC proliferation and differentiation was induced by CTL-secreted IFN-γ. Effector CTLs were only able to eliminate LSCs in a situation with minimal leukemia load where CTL-secreted IFN-γ levels were low. In addition, IFN-γ increased proliferation and colony formation of CD34+ stem/progenitor cells from CML patients in vitro. Our study reveals a novel mechanism by which the immune system contributes to leukemia progression and may be important to improve T cell–based immunotherapy against leukemia.
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that arises from break point cluster region/Abelson murine leukemia viral oncogene homolog 1 (BCR/ABL)–transformed hematopoietic stem (HSCs) or early progenitor cells known as leukemia stem cells (LSCs; Kavalerchik et al., 2008). LSCs have been first characterized as the tumor-initiating cells in acute myeloid leukemia (Lapidot et al., 1994) and have also been defined in other hematopoietic neoplasms since then (Cox et al., 2004; Matsui et al., 2004).
BCR/ABL-specific tyrosine kinase inhibitors (TKIs) such as Imatinib mesylate (Glivec) have revolutionized the therapy of CML (Druker et al., 2001a,b; Baccarani et al., 2006). Nevertheless, LSCs seem resistant to TKIs and traditional chemotherapy (Weiden et al., 1979; Deininger et al., 2000; Savona and Talpaz, 2008) and CML inevitably progresses to incurable acute leukemia (Faderl et al., 1999). Quiescent, self-renewing LSCs remain in the BM and are responsible for refractoriness and relapse of CML after treatment (Hughes et al., 2003). Therefore, novel cytotoxic agents that selectively target LSCs are under investigation (Jin et al., 2006; Guzman et al., 2007; Neviani et al., 2007; Ito et al., 2008; Bellodi et al., 2009; Majeti et al., 2009; Wang et al., 2010; Schürch et al., 2012).
Another promising approach in the treatment of CML is immunotherapy. In fact, currently, the only curative treatment for CML remains allogeneic stem cell transplantation (alloSCT). The graft-versus-leukemia effect of alloSCT is most likely executed by donor CD8+ effector CTLs specific for minor histocompatibility antigens (Weiden et al., 1979; Kolb et al., 1990; Gale et al., 1994; Druker et al., 2002). Patients who receive T cell–depleted alloSCT grafts have a higher risk of disease relapse, and donor lymphocyte infusions are able to induce complete remission after relapse (Thomas et al., 1979; Horowitz et al., 1990; Kolb et al., 1995; Sehn et al., 1999). Furthermore, endogenous CTLs directed against leukemia antigens have been detected in the peripheral blood of chronic phase CML patients (Molldrem et al., 2000; Butt et al., 2005). Several proteins may potentially act as potent leukemia-specific antigens for T cells, including BCR/ABL, Wilms’ tumor 1 protein (WT1), and proteinase 3 (Pr3; Van Driessche et al., 2005). Peptides from the junctional region of BCR/ABL are not present in healthy individuals and therefore are leukemia-specific. Yotnda et al. (1998) identified a BCR/ABL junctional nonapeptide that binds to human leukocyte antigen (HLA)-A2.1 and elicits specific CTL responses in vitro and in vivo. Additional studies confirmed and extended the finding of immunogenic BCR/ABL junction peptides (Bocchia et al., 1996; Clark et al., 2001).
CTLs have been shown to kill CML target cells in vitro via Fas-receptor triggering (Selleri and Maciejewski, 2000). In a BCR/ABL-induced murine CML model, we have shown that CD8+ T cells crucially contribute to disease control in vivo. However, programmed death ligand 1 (PD-L1) expression by the malignant cells induced T cell dysfunction leading to disease progression (Mumprecht et al., 2009b).
Despite these advances in the understanding of the immunosurveillance of CML and the development of immunotherapy strategies, the interaction of effector CTLs with the disease-originating LSCs has not been analyzed so far. In the present study, we analyzed the immunogenicity of LSCs in vitro and in vivo using the glycoprotein of lymphocytic choriomeningitis virus (LCMV) as model leukemia antigen. LSCs expressed MHC and co-stimulatory molecules. LSCs isolated from CD8+ T cell–depleted CML mice were more immunogenic than LSCs from control CML mice, indicating that CD8+ T cells interact with LSCs in vivo and select for low immunogenic variants. To analyze whether LSCs can be recognized and lysed by activated leukemia-specific effector CTLs, we treated CML mice with large numbers of specific T cells. Although these effector CTLs efficiently lysed LSCs in vitro, adoptive immunotherapy in vivo failed to eradicate LSCs but paradoxically increased LSC numbers. LSC proliferation was induced by CTL-secreted IFN-γ. Specific effector CTLs secreted high amounts of IFN-γ when transferred to CML mice in advanced disease stage with high antigen load, but not after transfer to CML mice in early stage of disease. Importantly, our results were confirmed in a humanized CML model using HLA-A2.1 transgenic mice and a BCR/ABL b3a2 junctional peptide. In addition, IFN-γ induced the proliferation of primary CD34+ stem/progenitor cells from newly diagnosed CML patients.
RESULTS
Immunogenicity of LSCs in vitro
To establish a CML-like disease, BM cells were retrovirally transduced with BCR/ABL-GFP, followed by transfer to sublethally irradiated (6.5 Gy) C57BL/6 (BL/6) recipient mice (Mumprecht et al., 2009a). As shown before (Mumprecht et al., 2009b), at this reduced irradiation dose the adaptive immune system, including CD8+ T cells, CD4+ T cells, and B cells, originates from the recipient mouse (Mumprecht et al., 2009b), allowing us to generate CML in a BL/6 host with a largely intact immune system. To analyze the interaction of CTLs with defined antigen specificity with LSCs, we used donor BM of H8 transgenic mice (Ehl et al., 1998) that ubiquitously express the LCMV glycoprotein epitope gp33 under the control of an MHC class I promoter to generate a CML in BL/6 mice (H8 CML). In this setting, BCR/ABL-GFP–expressing cells coexpress the LCMV-gp33 antigen.
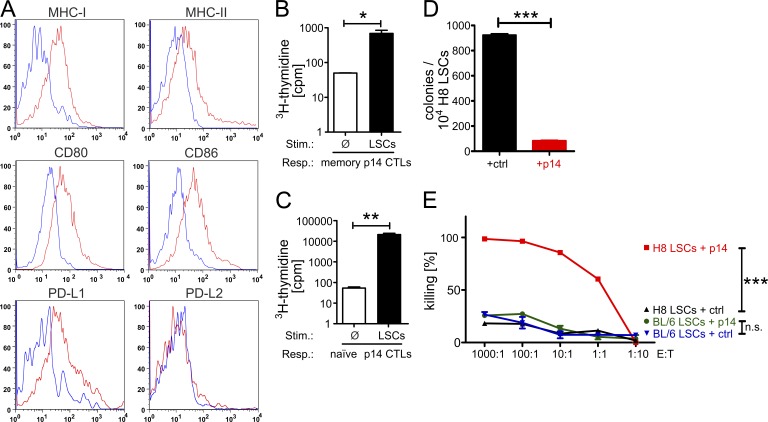
20 d after transplantation, H8 CML mice were sacrificed and BM was isolated. H8 LSCs were defined as BCR/ABL-GFP+, lineage negative (lin−), Sca-1+, c-kithi (Neering et al., 2007) and analyzed for expression of MHC class I and II and co-stimulatory molecules by FACS (Fig. 1 A). MHC-I, MHC-II, CD80, and CD86 were all strongly expressed on H8 LSCs. In addition, LSCs expressed PD-L1 but not PD-L2. We next sought to determine the capacity of H8 LSCs to activate naive or reactivate memory LCMV-gp33–specific, TCR transgenic CD8+ T cells (p14 CTLs; Pircher et al., 1989) in a standard 3H-thymidine incorporation assay. In the absence of additional cytokines, H8 LSCs induced proliferation not only in memory, but also in naive p14 CTLs (Fig. 1 B and C). These data indicate that LSCs possess the molecular repertoire to interact with T cells and are able to induce proliferation of naive and memory CTLs.
Figure 1.
Immunogenicity of CML LSCs in vitro. (A) Expression of MHC class I, MHC class II, CD80, CD86, PD-L1, and PD-L2 on H8 CML LSCs. 1 representative plot of 3–10 is shown. (B and C) 3H-thymidine assays. (B) 3 × 103 FACS-purified, irradiated (10 Gy) LSC stimulators were co-incubated with 1.5 × 104 MACS-purified CD8+ T cell responders from BL/6 mice infected with LCMV 6 wk earlier. One representative experiment out of two is shown. (C) 3 × 103 FACS-purified, irradiated (10 Gy) LSC stimulators were co-incubated with 1.5 × 104 MACS-purified CD8+ T cell responders from naive p14 TCR transgenic animals. One representative experiment out of five is shown. (D–E) In vitro killing assay. (D) 104 H8 LSCs FACS-purified from H8 CML mice at day 18 after transplantation were co-incubated with 2 × 104 naive BL/6 splenocytes (ctrl) or p14 effector splenocytes (p14) overnight, followed by transfer into methylcellulose. Colonies were enumerated 7 d later. One representative experiment out of two is shown. (E) 104 FACS-purified H8 or BL/6 LSCs from H8 or BL/6 CML mice (20 d after CML induction) were co-incubated with 0, 103, 104, 105, 106, or 107 MACS-purified naive CD8+ T cells (ctrl) or p14 effector CD8+ T cells (p14) overnight, followed by transfer into methylcellulose in duplicates. Colonies were enumerated 7 d later. Percent killing was calculated in relation to culture condition without CD8+ T cells (0). Data are displayed as mean ± SEM. Statistics: Student’s t test (B–D), two-way ANOVA (E). *, P < 0.05; **, P < 0.005; ***, P < 0.0001. Isotype controls, blue lines; stainings, red lines. E:T, effector/target ratio; LSCs, leukemia stem cells; Resp., responders; Stim., stimulators.
To test whether effector CTLs are able to kill LSCs in vitro, we isolated LSCs from H8 CML and BL/6 CML mice by FACS. LSCs were co-incubated with either naive cells (ctrl) or p14 effector cells (p14) overnight, followed by colony-forming assays. Co-incubation of H8 LSCs with p14 effector splenocytes resulted in significantly fewer colonies as compared with control naive splenocytes (Fig. 1 D). A titration of the effector/target ratio revealed an efficient lysis of H8 LSCs by MACS-purified p14 effector CD8+ CTLs in vitro. In contrast, p14 effector CD8+ CTLs did not lyse BL/6 LSCs (Fig. 1 E). These experiments revealed that LSCs are recognized and killed by activated CTLs in vitro and that this killing is antigen specific.
Immunoediting of LSCs by endogenous CD8+ T cells in vivo
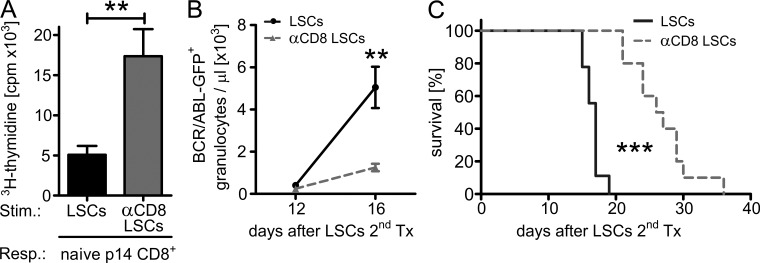
We next wanted to address the question of whether specific CTLs actually interact with LSCs in vivo. To this end, as an indirect parameter, we analyzed the immunogenicity of LSCs in the presence or absence of CD8+ T cells. We generated H8 CML mice and treated them i.p. with depleting anti-CD8 monoclonal antibody (mAb), starting 12 d after transplantation. This treatment depletes CD8+ T cells from peripheral blood to below detection limits of FACS (not depicted) and leads to rapid CML progression (Mumprecht et al., 2010). Control H8 CML mice were left untreated. 8 d later, we isolated LSCs from both groups of mice and performed a 3H-thymidine assay using naive p14 CD8+ T cells as responders (Fig. 2 A). Interestingly, LSCs isolated from CD8-depleted H8 CML mice (αCD8 LSCs) induced a more efficient expansion of naive p14 responder CD8+ T cells than LSCs from control H8 CML mice (LSCs).
Figure 2.
Immunoediting of LSCs by endogenous CD8+ T cells in vivo. (A) 3H-thymidine assay. 3 × 103 FACS-purified, irradiated (10 Gy) LSC stimulators from H8 CML mice (LSCs) or from CD8-depleted H8 CML mice (αCD8 LSCs) were co-incubated with 1.5 × 104 MACS-purified CD8+ T cell responders from naive p14 TCR transgenic animals. Pooled data from two independent experiments are shown. (B and C) 2 × 104 FACS-purified LSCs (solid line, n = 9) or αCD8 LSCs (dotted line, n = 10) were secondarily transplanted into irradiated (6.5 Gy) BL/6 recipient mice. (B) Numbers of BCR/ABL-GFP+ granulocytes/µl blood and (C) Kaplan-Meier-survival curves resulting from secondary transplantations. One representative out of three independent experiments is shown. Data are displayed as mean ± SEM. Statistics: Student’s t test (A), two-way ANOVA (B), and log-rank test (C). **, P < 0.005; ***, P < 0.0001. Resp., responders; Stim., stimulators.
To determine the immunogenicity of LSCs from CD8-depleted hosts in vivo, we secondarily transplanted 2 × 104 LSCs or αCD8 LSCs to irradiated (6.5 Gy) BL/6 recipients. 12 d after transplantation, secondary recipients had similar numbers of BCR/ABL-GFP+ granulocytes in peripheral blood, indicating similar engraftment of LSCs from both groups (Fig. 2 B). However, secondary CML originating from αCD8 LSCs progressed much slower than secondary CML originating from control LSCs. This was reflected in a slower increase of BCR/ABL-GFP+ granulocytes in blood (Fig. 2 B) and a significantly prolonged survival of secondary recipients (Fig. 2 C). These experiments indicate that CD8+ T cells interact with LSCs in vivo. This interaction leads to a selective pressure to develop poorly immunogenic LSCs, a process called immunoediting (Schreiber et al., 2011).
CTL killing of LSCs in vivo is dependent on antigen/leukemia load
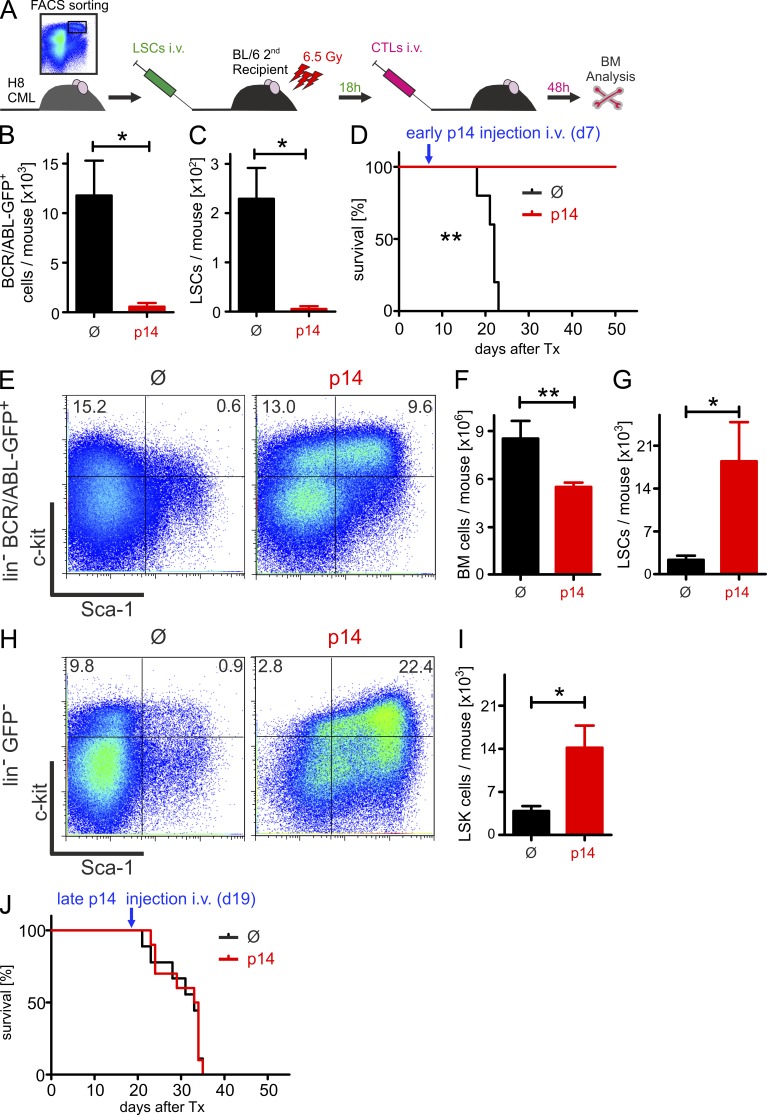
Our experiments thus far suggest that specific effector CTLs should be very efficient in the elimination of LSCs in vivo and therefore can be developed as a therapeutic strategy. To test the role of specific effector CTLs in a therapeutic setting with minimal leukemia load, we injected FACS-purified H8 LSCs into irradiated (6.5 Gy) secondary BL/6 recipient mice and adoptively transferred p14 effector CTLs 18 h later (Fig. 3 A). 2 d later, the numbers of BCR/ABL-GFP+ cells and LSCs in the BM were analyzed. In concordance with our in vitro experiments, the adoptive transfer of leukemia-specific CTLs drastically reduced the number of BCR/ABL-GFP+ cells and LSCs in the BM of p14 effector CTL-treated mice (Fig. 3, B and C). In line with these findings, primary H8 CML mice treated 7 d after CML induction survived, whereas untreated controls died after ∼3 wk (Fig. 3 D). These experiments indicate that LSCs can be recognized and killed by adoptively transferred specific CTLs in vivo.
Figure 3.
CTL-killing of LSCs in vivo depends on leukemia load. (A) Experimental design. 2 × 104 H8 LSCs from H8 CML mice were FACS-purified 20 d after transplantation and were transferred i.v. into irradiated (6.5 Gy) secondary BL/6 recipients. 18 h later, mice were either left untreated (Ø, n = 4) or treated with 3 × 106 FACS-purified p14 CTLs i.v. (n = 4). 48 h later, BM was harvested and analyzed for BCR/ABL-GFP+ cells (B) and LSCs (C) by FACS. Pooled data from two independent experiments are shown. (D) Kaplan-Meier survival curves resulting from H8 CML mice either left untreated (Ø, n = 5) or treated with 5 × 106 p14 effector CTLs (p14, n = 5) 7 d after transplantation. (E–I) H8 CML mice 18 d after transplantation were either left untreated (Ø, n = 4) or treated with 3 × 106 FACS-purified p14 effector CTLs (p14, n = 5). 2 d later, (E) the frequencies of LSCs in lin−BCR/ABL-GFP+ cells, the numbers of total BM cells (F), the numbers of LSCs in the BM (G), the frequencies of LSK cells in lin−GFP− cells (H), and the numbers of LSK cells in the BM (I) were analyzed. One representative plot of 4–5 is shown for each group. (J) Kaplan-Meier survival curves resulting from H8 CML mice either left untreated (Ø, n = 9) or treated with 5 × 106 p14 effector CTLs (p14, n = 10) 19 d after transplantation. Data are displayed as mean ± SEM. Statistics: Student’s t test (B, C, F, G, and I), log-rank test (D and J). *, P < 0.05; **, P < 0.005. LSCs, leukemia stem cells; LSK, lin−Sca-1+c-kithi.
To more closely model the clinical situation of leukemia, we treated H8 CML mice with p14 effector CTLs 20 d after primary leukemia transplantation. At this time point, the frequency of Gr-1+ BCR/ABL-GFP+ granulocytes in the peripheral blood was 43 ± 9% (not depicted), indicating a high leukemia load. Injections of p14 effector CTLs significantly reduced total BM cells compared with untreated mice (Fig. 3 F). Surprisingly, when analyzing the lineage-negative fraction and LSCs in these mice, we found a drastic increase in total LSC numbers (Figs. 3 E, 3G) in p14 effector CTL-treated H8 CML mice. This increase in LSCs was accompanied by a similar increase in host BCR/ABL-GFP−, lin−, Sca-1+, c-kithi (LSK) cells (Fig. 3, H and I) suggesting that a soluble factor secreted by effector CTLs, rather than a direct cell–cell interaction, is responsible for the observed effect. However, treatment with p14 T cells at this late stage of disease did not improve survival (Fig. 3 J).
IFN-γ enhances proliferation of LSCs and leukemia progenitor cells in vivo
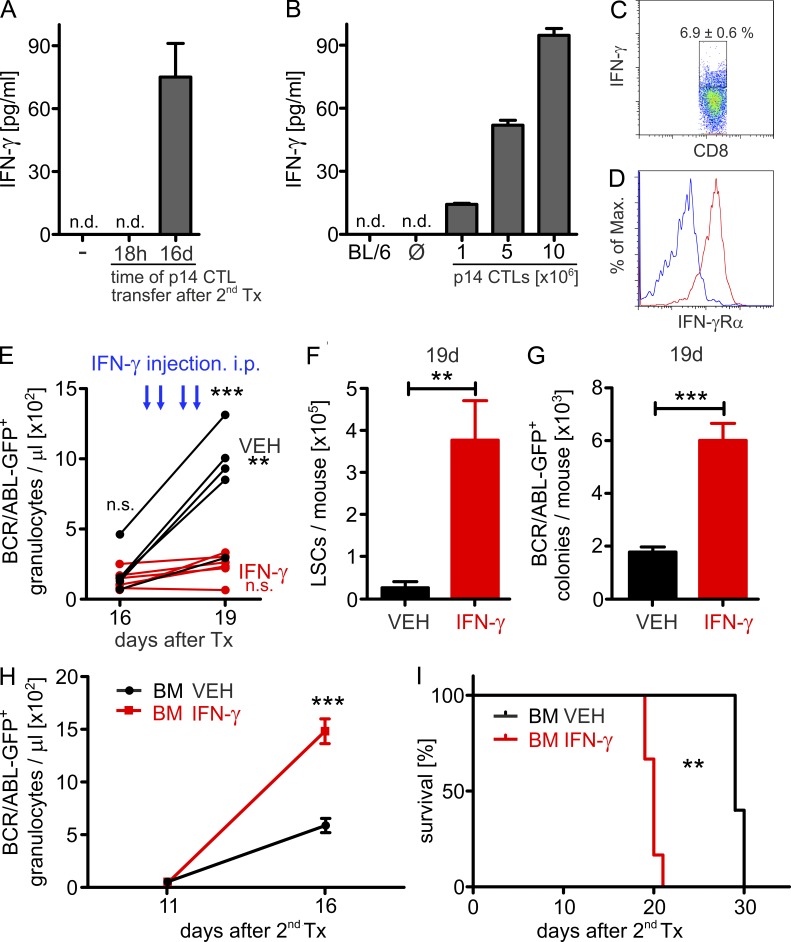
IFN-γ is a major effector cytokine secreted by effector CTLs (Farrar and Schreiber, 1993). In addition, recent studies have shown that interferons have the capacity to activate HSCs (Essers et al., 2009; Baldridge et al., 2010). Therefore, we analyzed the concentration of IFN-γ in the sera of p14 effector CTL-treated CML mice. H8 LSCs were transplanted into secondary BL/6 recipient mice and p14 effector CTLs were adoptively transferred either 18 h or 16 d after LSC transplantation. 2 d after adoptive transfer of p14 effector CTLs, serum IFN-γ levels were analyzed. IFN-γ concentrations were below the detection limit in sera of untreated CML mice and of CML mice that were treated with p14 effector CTLs 18 h after H8 LSC transplantation (Fig. 4 A). In contrast, in CML animals with an established leukemia 16 d after secondary transplantation, adoptively transferred p14 effector CTLs produced IFN-γ, resulting in detectable serum concentrations of IFN-γ (Fig. 4 A). The level of IFN-γ increased in dependence of the number of transferred p14 effector CTLs (Fig. 4 B). Intracellular stainings revealed that BM-infiltrating CTLs produced IFN-γ in CML mice (Fig. 4 C). Moreover, the IFN-γ receptor was expressed on H8 LSCs, suggesting that LSCs are able to respond to IFN-γ (Fig. 4 D).
Figure 4.
IFN-γ increases LSC numbers in CML. (A and B) IFN-γ serum levels of CML mice 48 h after p14 CTL treatment. (A) 2 × 104 H8 LSCs were FACS-purified 20 d after primary transplantation and were transferred i.v. into irradiated (6.5 Gy) secondary BL/6 hosts. 18 h or 16 d later, mice were either left untreated (Ø, n = 10, 18h) or treated with 3 × 106 FACS-purified p14 CTLs i.v. (18h: n = 10; 16d: n = 5). (B) H8 CML mice were treated with titrated numbers of MACS-purified p14 CTLs (106, 5 × 106 or 10 × 106 cells) 16 d after primary transplantation or were left untreated (n = 6–7 mice per group). (C) IFN-γ production of BM-infiltrating CTLs in BL/6 hosts that were treated 16 d after secondary LSC transplantation (n = 5). (D) Expression of IFN-γ receptor α chain (IFN-γRα) on H8 CML LSCs. One representative plot of 5 is shown. (E-I) BL/6 CML mice were treated with either vehicle (VEH, n = 5) or 2.5 × 105 U of rm-IFN-γ (n = 6) i.p. twice daily on two consecutive days (days 17 and 18 after primary transplantation). (E) Numbers of BCR/ABL-GFP+ granulocytes/µl blood and (F) LSC numbers were determined on day 19 after transplantation. (G) Equal numbers of lin− cells were plated in methylcellulose and BCR/ABL-GFP+ colonies were enumerated 7 d later by fluorescence microscopy. (H-I) 3 × 106 BM cells from vehicle-treated (black line, n = 5) or IFN-γ–treated (red line, n = 6) BL/6 CML mice were secondarily transplanted into irradiated (4.5 Gy) BL/6 mice. (H) Numbers of BCR/ABL+ granulocytes/µl blood and (I) Kaplan-Meier survival curves resulting from secondary transplantations. Data are displayed as mean ± SEM. Statistics: Student’s t test (F and G), two-way ANOVA (E and H), log-rank test (I). *, P < 0.05; **, P < 0.01; ***, P < 0.0005.
To investigate the effect of IFN-γ on leukemia development and the behavior of LSCs, we injected recombinant murine (rm)-IFN-γ or vehicle i.p. into BL/6 CML mice on 2 consecutive days, starting 17 d after CML induction. IFN-γ treatment stabilized the number of Gr-1+ BCR/ABL-GFP+ granulocytes in the peripheral blood (before IFN-γ, 164 ± 28/µl; after IFN-γ, 264 ± 38/µl; ns), whereas in vehicle-treated mice, Gr-1+ BCR/ABL-GFP+ granulocyte numbers significantly increased during this time period (before vehicle: 194 ± 69/µl; after vehicle: 879 ± 166/µl; P = 0.005, Fig. 4 E). In contrast, FACS analysis of BM after IFN-γ treatment (day 19) revealed a fivefold increase in LSC numbers compared with vehicle-treated mice (Fig. 4 F), a finding that was confirmed by colony forming assays of lin− cells (Fig. 4 G). Similar results with IFN-γ treatment were obtained in H8 CML mice expressing the LCMV-gp33 as a model tumor antigen (not depicted).
To verify that the increase in LSCs detected in FACS analysis and in methylcellulose cultures in vitro actually represents an increase in cells that can induce leukemia in vivo, we secondarily transplanted 3 × 106 BM cells from vehicle- and IFN-γ–treated CML mice (day 19) into sublethally irradiated (4.5 Gy) BL/6 mice. Mice that received BM from IFN-γ–treated leukemia mice developed a more severe course of the disease and died significantly faster than control mice (Fig. 4, H and I).
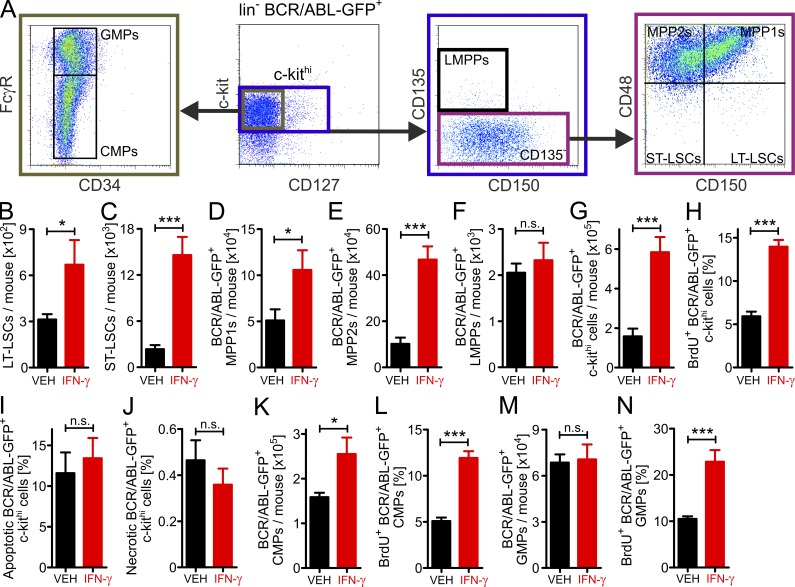
LSCs are a heterogeneous population consisting of long-term and short-term LSCs and leukemia multipotent progenitor cells (MPPs; Li et al., 2012). We therefore determined the LSC subpopulation preferentially responding to rm-IFN-γ (Fig. 5 A). IFN-γ increased numbers of primitive long-term and short-term LSCs, as well as early myeloid leukemia MPPs (LMPPs) in BM (Fig. 5, B–E). In contrast, there was no difference in lymphoid-primed LMPPs (Fig. 5 F). IFN-γ increased leukemia c-kithi cells by enhancing their proliferation (Fig. 5, G and H); cell survival was not affected (Fig. 5, I–J). In addition, we analyzed more differentiated leukemia common myeloid progenitors (CMPs) and leukemia granulocyte-macrophage progenitors (GMPs). Upon IFN-γ treatment, leukemia CMP numbers increased as a result of proliferation (Fig. 5, K and L). Interestingly, although IFN-γ increased proliferation of leukemia GMPs, their absolute numbers in BM were not increased (Fig. 5, M and N). This suggests that leukemia GMPs rapidly differentiate into more mature myeloid cells.
Figure 5.
IFN-γ enhances the proliferation of early LSCs and leukemia progenitor cells in vivo. (A) Gating strategy to identify early LSCs and leukemia progenitor cells in the BM of CML mice. (B–N) BL/6 CML mice were treated with either vehicle (VEH, n = 7) or 2.5 × 105 U of rm-IFN-γ (n = 5) i.p. twice daily on two consecutive days (days 17 and 18 after primary transplantation). On the same days, mice also received BrdU (0.8 mg/ml in drinking water and 1 mg i.p./day). 1 d later, lin−BCR/ABL-GFP+ BM cells were analyzed for LSCs and leukemia progenitor cells. (B) Long-term LSCs (c-kithiCD135−CD48−CD150+). (C) Short-term LSCs (c-kithiCD135−CD48-CD150−). (D) Leukemia MPP1s (c-kithiCD135−CD48+CD150+). (E) Leukemia MPP2s (c-kithiCD135−CD48+CD150−). (F) Leukemia LMPPs (c-kithiCD135+CD150−). (G) Numbers, (H) proliferation, and (I-J) viability of c-kithi leukemia progenitor cells. Numbers (K) and proliferation (L) of leukemia CMPs (c-kithiCD127−CD34+FcγR−). Numbers (M) and proliferation (N) of leukemia GMPs (c-kithiCD127−CD34+FcγR+). Data are displayed as mean ± SEM. Statistics: (B–N) Student’s t test. *, P < 0.05; ***, P < 0.0005. Apoptotic cells, Annexin V+ cells; necrotic cells, AnnexinV+7-AAD+ cells. CMPs, common myeloid progenitors; GMPs, granulocyte-macrophage progenitors; LMPPs, lymphoid-primed multipotent progenitors; LT, long-term; MPPs, multipotent progenitors; ST, short-term.
In summary, these experiments indicate that IFN-γ increases the numbers of LSCs and leukemia progenitor cells by enhancing their proliferation.
Adoptively transferred CTLs increase the proliferation of leukemia progenitor cells via IFN-γ
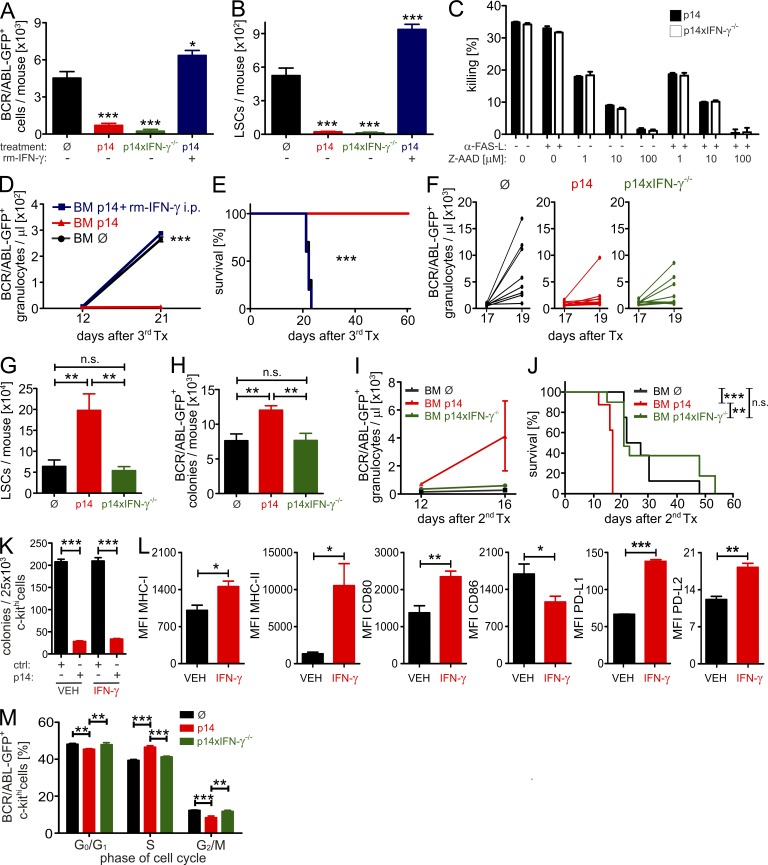
To analyze if the observed increase of LSCs in CML mice after p14 effector CTL-treatment is indeed caused by IFN-γ secretion from CTLs, treatment protocols early and late after LSC transfer were repeated using IFN-γ–deficient p14 effector CTLs. First, secondary recipients were treated 18 h after LSC transplantation, either with IFN-γ–competent (p14) or –deficient (p14xIFN-γ−/−) p14 effector CTLs. 2 d later, numbers of BCR/ABL-GFP+ cells and LSCs were analyzed in the BM. P14 and p14xIFN-γ−/− effector CTLs were equally able to eradicate BCR/ABL-GFP+ cells and LSCs at this early time point after LSC transfer (Fig. 6, A and B). In vitro cytotoxicity assays revealed that target cell lysis by p14 and p14xIFN-γ−/− CTLs could be inhibited by addition of the granzyme B inhibitor Z-aminoactinomycin D (AAD)-CMK, but not by blocking the FAS–FASL interaction using mAb (Fig. 6 C). Importantly, additional injection of rm-IFN-γ in the same experimental setting prevented the rejection of leukemia cells by p14 effector CTLs and increased LSC numbers. In addition, 3 × 106 BM cells from untreated, p14-treated, or p14+rm-IFN-γ–treated H8 CML mice were transplanted into sublethally (6.5 Gy) irradiated tertiary BL/6 recipient mice. Mice that received p14-treated BM did not develop leukemia, therefore they survived (Fig. 6, D and E). In contrast, mice receiving untreated BM or p14+rm-IFN-γ–treated BM developed leukemia and died from classical symptoms of leukemia (Fig. 6, D and E). These experiments indicate that IFN-γ increases LSC numbers and prevents the elimination of LSCs by CTLs. The increase of leukemia cells in the BM in response to IFN-γ is in contrast to the reduction of malignant granulocytes after IFN-γ treatment in the circulation (Fig. 4 E), indicating that the effect on mature granulocytes and on leukemia stem/progenitor cells is different.
Figure 6.
CTL-secreted IFN-γ increases LSC numbers. (A) 2 × 104 H8 LSCs were FACS-purified 20 d after transplantation and were transferred i.v. into irradiated (6.5 Gy) secondary BL/6 recipients. 18 h later, mice were either left untreated (Ø, n = 11) or treated with 3 × 106 FACS-purified p14 (n = 12) or p14xIFNγ−/− (n = 3) CTLs i.v. One group of mice received p14 CTLs and was treated with 2.5 × 105 U of rm-IFN-γ twice daily for two consecutive days (n = 5). After 48 h, BM was harvested and analyzed for BCR/ABL-GFP+ (A) cells and LSCs (B) by FACS. Pooled data from two independent experiments are shown. (C) Duplicates of bulk BM cells from H8 CML mice were either incubated alone or were co-cultured with CTLs at a ratio of 5:1 with or without 10 µg/ml anti–FAS-ligand, in the presence or absence of the indicated concentrations of the granzyme B inhibitor Z-AAD-CMK. After 4 h, the killing of BCR/ABL-GFP+ cells was determined. (D and E) 3 × 106 BM cells from p14-treated (red line, n = 5), p14+rm-IFN-γ–treated (blue line, n = 5), or untreated (black line, n = 10) H8 CML mice were tertiarily transplanted into irradiated (6.5 Gy) BL/6 mice. Numbers of BCR/ABL-GFP+ granulocytes/µl blood (D) and Kaplan-Meier survival curves (E) resulting from tertiary transplantations are shown. (F) BCR/ABL-GFP+ granulocytes in blood of primary H8 CML mice either left untreated (Ø) or treated with 3 × 106 FACS-purified p14 CTLs or p14xIFN-γ−/− CTLs (n = 8 each). LSC numbers (G) and BCR/ABL-GFP+ colonies per mouse (H) 2 d after adoptive transfer. (I–J) 3 × 106 BM cells from primary H8 CML mice left untreated (black line, n = 8) or treated with p14 CTLs (red line, n = 8) or p14xIFN-γ−/− CTLs (green line, n = 11) were secondarily transplanted into irradiated (6.5 Gy) BL/6 mice. (I) Numbers of BCR/ABL+ granulocytes/µl blood and (J) Kaplan-Meier survival curves resulting from secondary transplantations. (K) H8 CML mice were treated with either vehicle (VEH, n = 5) or 2.5 × 105 U of rm-IFN-γ (n = 5) i.p. twice daily on two consecutive days (d17 and d18 after primary transplantation). 2.5 × 104 FACS-purified H8 BCR/ABL-GFP+ c-kithi cells from VEH- or IFN-γ–treated H8 CML mice were co-incubated with 2.5 × 104 naive BL/6 CD8+ T cells (ctrl) or p14 CTLs (p14) overnight, followed by transfer into methylcellulose in duplicates. Colonies were enumerated 7 d later. (L) Mean fluorescent intensity (MFI) of MHC class I, MHC class II, CD80, CD86, PD-L1 and PD-L2 on LSCs from VEH- or IFN-γ–treated H8 CML mice. (M) Cell cycle analysis by DAPI stainings of total c-kithi cells from primary H8 CML mice left untreated (Ø, n = 6) or treated with p14 (n = 5) or p14xIFN-γ−/− (n = 5) CTLs. Data are displayed as mean ± SEM. Statistics: one-way ANOVA (A, B, G–H, and M), two-way ANOVA (D and I), Student’s t test (K and L), and log-rank test (E and J). *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Next, we adoptively transferred p14 and p14xIFN-γ−/− effector CTLs to primary H8 CML mice in an advanced stage of the disease 17 d after transplantation. Control H8 CML mice were left untreated. Before adoptive transfer, numbers of Gr-1+ BCR/ABL-GFP+ granulocytes in the peripheral blood were comparable (untreated, 728 ± 59/µl; p14-treated, 719 ± 143/µl; p14x IFN-γ−/−–treated, 977 ± 139/µl; ns). Blood and BM were analyzed 2 d after adoptive transfer. In untreated mice, peripheral blood BCR/ABL-GFP+ granulocyte numbers rapidly increased (Fig. 6 F). In contrast, p14 effector CTLs and, to a lesser extent, p14xIFN-γ−/− effector CTLs reduced the increase of BCR/ABL-GFP+ granulocytes after these 2 d (Fig. 6 F). Strikingly, we found a marked increase in total LSC numbers in p14-treated, but not p14xIFN-γ−/−–treated CML mice (Fig. 6 G). This finding was confirmed by performing colony forming assays from lin− BM and assessing the numbers of BCR/ABL-GFP+ colonies by fluorescence microscopy (Fig. 6 H). CML mice treated at this late stage of disease died rapidly a few days after treatment (Fig. 3 J), thus the effect on CML disease cannot be analyzed directly. To further confirm the increase in malignant progenitors in the BM of p14-treated mice, we secondarily transplanted 3 × 106 BM cells from untreated, p14-treated, or p14xIFN-γ−/−–treated H8 CML mice into sublethally irradiated (6.5 Gy) BL/6 mice. Monitoring secondary CML by repetitive bleeding revealed that mice receiving p14-treated BM developed CML and succumbed to lethal disease by day 18 after transplantation (Fig. 6, I and J). In contrast, mice receiving untreated BM or p14xIFN-γ−/−–treated BM developed CML substantially slower and survived significantly longer (Fig. 6, I and J).
We next sought to analyze how IFN-γ protects LSCs from CTL-mediated lysis, or even increases their numbers. Therefore, we analyzed the lysis of H8 LSCs from vehicle- or IFN-γ–treated H8 CML mice in vitro. We isolated BCR/ABL-GFP+ c-kithi cells by FACS and co-incubated them directly ex vivo either with naive BL/6 or p14 effector CD8+ T cells overnight, followed by colony-forming assays (Fig. 6 K). These experiments revealed that BCR/ABL-GFP+ c-kithi cells from vehicle- and IFN-γ–treated CML mice were similarly killed by effector CTLs. In addition, the mean fluorescence intensities of MHC-I, MHC-II, CD80, and PD-L1 and PD-L2 were significantly up-regulated on BCR/ABL-GFP+ c-kithi cells from IFN-γ–treated CML mice (Fig. 6 L).
A similar lysis of IFN-γ–treated and control LSCs excludes the possibility that IFN-γ prevents CTL-mediated lysis of LSCs. Therefore, the observed IFN-γ–dependent increase in LSC numbers might be caused by an increase in LSC proliferation. To investigate whether adoptively transferred p14 effector CTLs induce proliferation of myeloid leukemia progenitor cells, we purified lin− BCR/ABL-GFP+ c-kithi cells by FACS and performed an analysis of the cell cycle using DAPI staining. BCR/ABL-GFP+ c-kithi cells from p14-treated H8 CML mice were highly significantly more in S-phase than BCR/ABL-GFP+ c-kithi cells from untreated or p14xIFN-γ−/−–treated mice (Fig. 6 M), indicating that CTL-secreted IFN-γ increases the proliferation of leukemia progenitor cells. A similar increase of cells in S-phase was observed for nonmalignant c-kithi in these mice (not depicted).
The experiments so far suggested that the increased IFN-γ production after CTL transfer to a CML mouse with high leukemia load but not with low leukemia load is caused by differences in antigen load. To confirm this experimentally, we transferred H8 LSCs into sublethally irradiated (6.5 Gy) H8 recipient mice and analyzed the effects of specific CTL therapy 18 h after adoptive transfer (Fig. 7 A).
Figure 7.
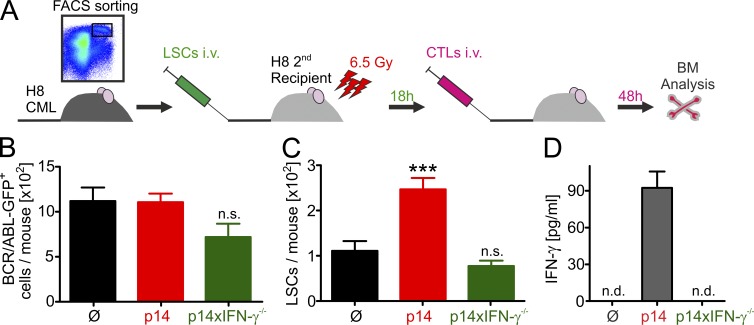
CML treatment with CTLs specific for a ubiquitously expressed antigen increases LSC numbers. (A) Experimental design. 2 × 104 H8 LSCs were FACS-purified 20 d after transplantation and were transferred i.v. into irradiated (6.5 Gy) secondary H8 hosts. 18 h later, mice were either left untreated (Ø, n = 15) or treated with 3 × 106 FACS-purified p14 (n = 16) or p14xIFN-γ−/− (n = 11) CTLs i.v. After 48 h, BM was harvested and analyzed for BCR/ABL-GFP+ cells (B) and LSCs (C) by FACS. (D) In one experiment, serum IFN-y levels were determined 48 h after CTL transfer (n = 4–7 mice per group). (B and C) Pooled data from four independent experiments are shown. Data are displayed as mean ± SEM. Statistics: one-way ANOVA (B and C). ***, P < 0.0001.
In this experimental setup, the leukemia load is low, but all host cells express gp33 on MHC-I. Transfer of p14 and p14xIFNγ−/− effector CTLs had no or only a marginal effect on the numbers of BCR/ABL-GFP+ cells in the BM (Fig. 7 B). In contrast, LSC numbers increased in p14-treated mice (Fig. 7 C). This increase in LSC numbers is attributed to CTL-secreted IFN-γ as indicated by elevated IFN-γ serum levels (Fig. 7 D). Treatment with p14xIFN-γ−/− effector CTLs neither increased LSC numbers nor IFN-γ serum levels (Fig. 7 C, D).
In summary, our experiments indicate that CTLs are able to eradicate LSCs in vitro and in vivo in a setting with minimal leukemia load. In contrast, LSC numbers increase because of CTL-secreted IFN-γ if T cell therapy is applied in a situation with high leukemia load or in a situation where the targeted antigen is not leukemia-specific but widely expressed in other tissues.
Adoptive immunotherapy using BCR/ABL junctional peptide-specific CTLs increases LSC numbers in a humanized CML model
The use of the LCMV-gp33 as a model tumor antigen has the advantage that TCR transgenic mice are available and that the expression of the antigen can be clearly controlled using transgenic animals. However, results may differ if a relevant leukemia antigen is targeted. Therefore, we made use of β2-microglobulin (β2m)/HLA-A2.1 monochain transgenic, H2-Db/β2m double knock-out mice (HLA-A2tg mice; Pascolo et al., 1997). HLA-A2tg mice were repeatedly immunized with autologous DCs pulsed with a HLA-A2.1–binding junctional fusion oncoprotein (SSKALQRPV, b3a2) derived from BCR/ABL (Yotnda et al., 1998) to induce b3a2-specific CTLs. Spleens were harvested after 14 d, and CD8+ T cells were purified, cultured in vitro, and were stimulated (weekly) with autologous, b3a2 peptide-pulsed DCs to select for b3a2-specific T cell lines. After 3 wk of restimulation, MACS-purified b3a2-specific CTLs were injected into HLA-A2tg CML mice, and control mice were left untreated. Again, we observed a block in the increase of BCR/ABL-GFP+ granulocytes in the peripheral blood after adoptive T cell therapy (Fig. 8 A). FACS analysis of BM revealed that LSCs increased in frequency (Fig. 8 B) and in absolute numbers (Fig. 8 C) after b3a2-specific CTL treatment, which was confirmed by colony-forming assays of lin− cells (Fig. 8 D). Most importantly, secondary transplantations of 3 × 106 BM cells from CTL-treated HLA-A2tg CML mice into sublethally irradiated (6.5 Gy) HLA-A2tg mice led to rapid death of secondary recipients, whereas secondary recipients receiving BM from untreated HLA-A2tg CML mice survived substantially longer (Fig. 7 E).
Figure 8.
CTL treatment paradoxically increases LSCs in a humanized CML model. HLA-A2tg CML mice 19 d after transplantation were either left untreated (Ø, n = 5) or treated with BCR/ABL b3a2 junction peptide-specific HLA-A2tg CD8+ T cells (+b3a2-CTLs, n = 4). In a similar, independent experiment, mice were left untreated (Ø, n = 8) or treated with HIV SL9 peptide-specific HLA-A2tg CD8+ T cells (+SL9-CTLs, n = 6). 1 d later, mice were bled and sacrificed for analysis. (A) Numbers of BCR/ABL-GFP+ granulocytes/µl blood before and 1 d after adoptive transfer of b3a2-CTLs. (B) BM was lineage depleted and analyzed by FACS for the expression of c-kit and Sca-1 after adoptive transfer of b3a2-CTLs. One representative plot of four untreated and five b3a2-CTL–treated HLA-A2tg CML mice is shown. (C and F) LSC numbers per mouse 1 d after adoptive transfer. (D and G) Equal numbers of lin− cells were plated in methylcellulose and BCR/ABL-GFP+ colonies were enumerated 7 d later by fluorescence microscopy. (E and H) 3 × 106 BM cells from HLA-A2tg CML mice left untreated (n = 4–8), treated with BCR/ABL b3a2-junction peptide-specific HLA-A2tg CTLs (n = 4), or treated with HIV SL9 peptide-specific HLA-A2tg CD8+ T cells (+SL9-CTLs, n = 6) were secondarily transplanted into irradiated (6.5 Gy) HLA-A2tg mice. Kaplan-Meier survival curves resulting from secondary transplantations are shown. Data are displayed as mean ± SEM. Statistics: Student’s t test (C, D, F, and G) and log-rank test (E and H). *, P < 0.05; **, P < 0.01.
To analyze if the effects on LSCs after CTL treatment require antigen specificity for the leukemia antigen, we performed a control experiment using CTLs specific for the HLA-A2.1–binding HIV peptide (SLYNTVATL, SL9; Brander et al., 1998). The number of LSCs in BM (Fig. 8 F) and BCR/ABL-GFP+ colony-forming capacity of lin− cells (Fig. 8 G) was not affected by SL9-specific CTL treatment. In addition, we observed similar survival in mice secondarily transplanted with BM from untreated or SL9 CTL-treated primary HLA-A2tg CML (Fig. 8 H).
Therefore, BCR/ABL fusion protein–specific effector CTLs specifically increase the numbers of LSCs and confirm the results obtained with LCMV-gp33 as a model leukemia antigen.
IFN-γ increases proliferation and colony formation of primary CD34+ stem/progenitor cells from CML patients
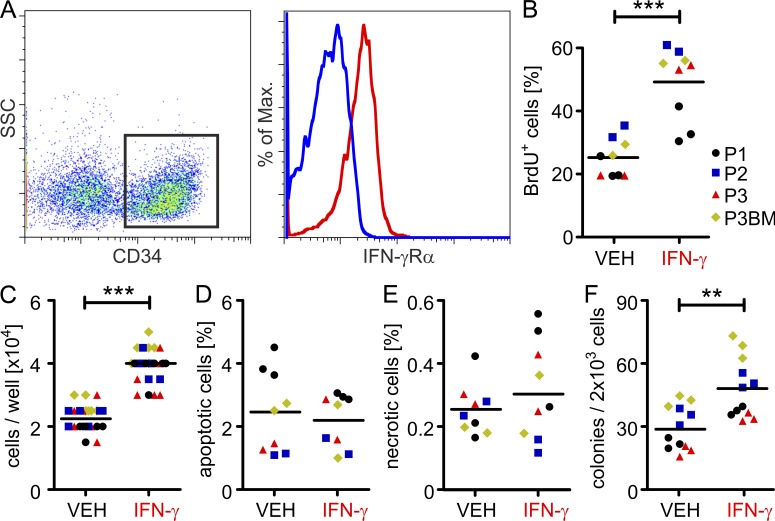
To extend our findings to primary human CML stem/progenitor cells, we stained the IFN-γ receptor on CD34+ cells from newly diagnosed CML patients (initial leukocyte count, 169.9 ± 93.2 × 109/liter). The IFN-γ receptor was expressed on CD34+ stem/progenitor cells in peripheral blood and BM, suggesting that CML stem cells are able to respond to IFN-γ (Fig. 9 A). Recombinant human (rh)-IFN-γ enhanced the proliferation of FACS-purified CD34+ stem/progenitor cells from CML patients in liquid cultures in vitro, resulting in increased BrdU incorporation and elevated cell numbers after 7 d of culture (Fig. 9, B and C). Cell death was not affected by rh-IFN-γ (Fig. 9, D and E). Furthermore, rh-IFN-γ increased the colony-forming capacity of CD34+ stem/progenitor cells from CML patients (Fig. 9 F).
Figure 9.
IFN-γ increases proliferation and colony formation of primary CD34+ stem/progenitor cells from CML patients. (A) Expression of IFN-γ receptor α chain (IFN-γRα) on CD34+ stem/progenitor cells from newly diagnosed CML patients. One representative plot of four is shown. (B–E) 104 FACS-sorted CD34+ cells from the peripheral blood or BM were cultured in the presence or absence of rh-IFN-γ for 7 d in sextuplicates. At day 7 of culture, BrdU incorporation (B), cell numbers (C), apoptosis (D), and necrosis (E) were assessed in duplicates or triplicates. (F) 2 × 103 FACS-sorted CD34+ cells were cultured in methylcellulose in triplicates and colony-forming capacity was determined after 14 d. Data are displayed as mean ± SEM. Statistics: Student’s t test. **, P < 0.005; ***, P < 0.0001. Apoptotic cells, Annexin V+ cells; necrotic cells, Annexin V+7-AAD+ cells. P, Patient; VEH, vehicle.
In summary, these data indicate that IFN-γ increases the numbers of primary CD34+ stem/progenitor cells from blood and BM from newly diagnosed CML patients by enhancing their proliferation.
DISCUSSION
The long-term success of a leukemia treatment depends on its effect on LSCs. Unfortunately, LSCs are selectively resistant against most current treatment strategies, including chemotherapy and TKIs (Weiden et al., 1979; Deininger et al., 2000; Savona and Talpaz, 2008). In contrast, the susceptibility of LSCs to immune therapies is less clear, and it is suspected that T cells and NK cells are able to eliminate residual tumor cells after cytoreduction in the BM (Kurz et al., 2010). However, although LSCs were identified more than a decade ago, the interaction of the adaptive immune system with LSCs is poorly defined. Therefore, we addressed the interaction of CTLs with LSCs in vitro and in vivo. Surprisingly, LSCs expressed high levels of MHC class I and II, as well as co-stimulatory molecules. Therefore, they acted very efficiently as antigen-presenting cells and were able to induce the proliferation of naive CTLs even in the absence of additional co-stimulatory molecules or cytokines. Similarly, effector CTLs efficiently eliminated LSCs in vitro. These characteristics suggest that a T cell–mediated therapy could efficiently target LSCs also in vivo. However, immunotherapy with identically activated CTLs did not eliminate, and in fact increased, the number of LSCs in vivo.
LSCs retain many characteristics of HSCs and reside in and probably depend on the HSC niche in the BM. For example, blocking CD44 suppresses both AML and CML progression and induces differentiation, probably by disrupting the interaction between LSCs and the BM niche (Jin et al., 2006; Krause et al., 2006). BM niche cells express membrane-bound and secreted factors that regulate HSC quiescence, differentiation, and migration (Kiel and Morrison, 2008; Wilson et al., 2007). Therefore, the microenvironment in the HSC niche might be protective for LSCs in vivo and prevent CTL-mediated lysis. Two experiments argue against this possibility. First, CTLs were able to eliminate LSCs 18 h after transfer in vivo, a time period that is sufficient for HSCs and LSCs to migrate to the niche (Colvin et al., 2007; Bhattacharya et al., 2009; Xie et al., 2009; Capron et al., 2010). Second, CD8+ T cell depletion in CML mice increased the immunogenicity of LSCs, as documented by a more efficient stimulation of naive T cells in vitro and a more efficient rejection in secondary transplantation experiments. This strongly suggests that LSCs in vivo are accessible to effector CTLs and that effector CTLs select for low immunogenic variants. For solid tumors, it has been demonstrated that in equilibrium, the immune system is able to control occult tumor cells over a prolonged period of time while selecting for lower immunogenic variants that ultimately escape immune surveillance and lead to tumor progression (Schreiber et al., 2011). Our data suggest that LSCs undergo a similar immunoediting process. In the experimental conditions chosen in our murine CML model, progression and death occur after 3–4 wk. In contrast, in human CML, the chronic phase lasts 3–5 yr before outgrowth of blasts that have acquired additional mutations (Sawyers, 1999).
Type I IFNs (IFN-α and -β) and type II IFN (IFN-γ) are important cytokines in the control of infections (Kurz et al., 2010). As the elimination of an infectious pathogen requires a concerted action of different elements of the immune system it is not surprising that IFNs have direct effects on hematopoiesis. IFN-α activates dormant HSCs to enter proliferation. In contrast, chronic activation of the IFN-α pathway in HSC impairs their function, probably as a result of exhaustion (Essers et al., 2009). Before the introduction of TKIs, IFN-α was used as standard therapy for CML (Sawyers, 1999). In addition, T cell–derived cytokines have been described to influence hematopoiesis (Metcalf, 2008). IFN-γ induces apoptosis of erythroid progenitors and BM cultures in vitro (Means, 1995; Wang et al., 1995) and may be a central cytokine in the induction of acquired aplasia (Young, 2006) and in anemia of chronic disease (Weiss and Goodnough, 2005). In contrast, in combination with SCF and IL-3, IFN-γ stimulates differentiation of the granulocyte-monocyte and erythropoietic lineages in vitro (Kurz et al., 2010). More recently, IFN-γ was recognized as important mediator of infection-induced myelopoiesis (MacNamara et al., 2011). In a murine model of malaria infection, IFN-γ induced a unique myelolymphoid progenitor population that gave rise to mostly myeloid cells (Belyaev et al., 2010). Other studies have shown that IFN-γ directly induces the expansion of lin− Sca-1+ c-kit+ cells (Zhao et al., 2010) and that IFN-γ–induced genes are critical for HSC survival and maintenance (Baldridge et al., 2010). We now documented a comparable effect of IFN-γ on LSCs. IFN-γ induced proliferation of leukemia progenitor cells in our CML model as well as in primary CD34+ CML stem/progenitor cells from newly diagnosed patients. In the murine model, an increase in disease-initiating LSCs was documented in a secondary transplantation experiment where CML progression was faster after transplantation of IFN-γ–treated LSCs. In addition, IFN-γ treatment up-regulated the inhibitory molecules PD-L1 and PD-L2 on LSCs, probably contributing to LSC immune escape in vivo (Zou and Chen, 2008). Thus, IFN-γ evolutionary evolved as an important regulator of infection- or stress-induced myelopoiesis. However, the same IFN-γ–mediated effects on LSCs induce their proliferation and disease progression.
Therefore, the adoptive transfer of specific CTLs affected leukemia development in different ways. It eliminated leukemia granulocytes, and probably LSCs as well, and selected for low immunogenic LSC variants. Alternatively, CTL-secreted IFN-γ established a cytokine environment that facilitated leukemia progression. It is well documented that effector mechanisms of the immune system contribute to tumor control as well as to tumor progression (Schreiber et al., 2011). Whether effector CTLs, in addition to their tumor-protective role, may also enhance tumor growth, is less clear. In addition to IFN-γ, we recently showed that T cells contribute to leukemia progression via the co-stimulatory CD70–CD27 interaction (Schürch et al., 2012).
The expansion of the LSC population clearly depended on IFN-γ, whereas the lytic effect on leukemia cells was independent of IFN-γ and mediated by perforin/granzyme B. The level of IFN-γ produced after adoptive T cell transfer depended on the antigen-load in the leukemia mouse. We used the LCMV-gp33 antigen as leukemia antigen to be able to control for the expression of the antigen. When gp33 was expressed also on nonleukemic host cells, or when the leukemia load was high and gp33-expressing cells were abundant, the transferred cells were restimulated in vivo to produce high amounts of IFN-γ. Similarly, adoptive transfer of a BCR/ABL fusion protein–specific CTL line induced the expansion of LSCs in the humanized CML model, confirming that our findings are not specific for LCMV gp33. In addition, IFN-γ induced cell-cycling and expansion of primary human CD34+ CML stem/progenitor cells, indicating the relevance of this pathway for human disease.
In contrast, in a situation with a low leukemia load resembling minimal residual disease, restimulation of the transferred CTLs did not lead to IFN-γ levels sufficient to induce LSC expansion. This fits well with clinical observations. Although recent studies showed that chimeric antigen receptor-modified T cells are able to eliminate up to 1kg of leukemia mass (Porter et al., 2011), it is generally accepted that immunotherapy is most efficacious in the presence of a low leukemia load (Carlens et al., 2001; Raiola et al., 2003). Therefore, most immunotherapies including alloSCT and donor lymphocyte infusions are applied after induction chemotherapy (Kolb et al., 1990; Kolb et al., 1995; Weiden et al., 1979). Independent of the leukemia load, the expression of the target antigen is crucial for the efficacy of adoptive T cell therapy. Our results reveal that even in a situation with a low leukemia load, the transfer of T cells that target an antigen that is widely expressed in the body leads to restimulation of the transferred T cells and secretion of high amounts IFN-γ. This leads to proliferation of LSCs and precludes the success of the therapy.
Our study now documents that LSCs are immunogenic and can be targeted by specific CTLs, provided that the target antigen is leukemia specific and that the immunotherapy is applied in a situation with a low leukemia load, probably after cytoreduction.
MATERIALS AND METHODS
Mice.
C57BL/6J (BL/6) mice were obtained from RCC Ltd. CD45.1+ mice. p14 TCR transgenic mice (Pircher et al., 1989) specific for the LCMV glycoprotein epitope 33–41 (gp33), H8 transgenic mice (Ehl et al., 1998) ubiquitously expressing LCMV-gp33 under the control of an MHC class I promoter, IFN-γ–deficient mice (Dalton et al., 1993), and β2m/HLA-A2.1 monochain transgenic, H2-Db/β2m double knock-out mice (HLA-A2tg mice; Pascolo et al., 1997) were obtained from the Institute for Laboratory Animals (Zürich, Switzerland). Animal experiments were approved by the local experimental animal committee of the Canton of Bern and performed according to Swiss laws for animal protection.
Patients.
Peripheral blood samples and one BM aspirate from untreated, newly diagnosed CML patients (n = 3; age, 54.6 ± 19.4 yr) were obtained at the University Hospital of Bern, Switzerland, in 2011 and 2012. All CML patients possessed a BCR/ABL translocation as tested by molecular analysis from the blood. Analysis of blood and BM samples was approved by the local ethical committee of the Canton of Bern and all patients gave written, informed consent.
Virus and peptides.
LCMV strain WE was provided by R.M. Zinkernagel (University of Zürich, Zürich, Switzerland) and propagated on L929 fibroblasts as previously described (Matter et al., 2006; Mumprecht et al., 2006). The HLA-A2.1–binding BCR/ABL b3a2-junction peptide (aa 926–934, SSKALQRPV; Yotnda et al., 1998) was purchased from Innovative Peptide Solutions. The MHC-II–specific LCMV glycoprotein peptide p13 (aa 61–80, GLNGPDIYKGVYQFKSVEFD) was purchased from NeoMPS SA. The HIV peptide SL9 (aa 77–85, SLYNTVATL; Brander et al., 1998) was provided by D. Yerly (University of Bern, Bern, Switzerland).
Antibodies.
αH2-Db-PE, αI-A/I-E-PE, αc-kit-PE and -PE-Cy7, αSca-1-perinidin-chlorophyll-protein (PerCP)-Cy5.5 and –allophycocyanin (APC), αLy6G-PE, αGr1-PE and –APC; αCD80-PE, αCD86-APC, αIFN-γ–PE, αIFN-γ-Rα-PE, αIFN-γ-Rβ-PE, Armenian hamster-IgG-PE, αPD-L1-PE, αCD127-PE, and -APC were obtained from eBioscience. CD11c-FITC, αI-A/I-E-APC-Cy7, αc-kit-APC-Alexa750, αCD80-Pacific Blue, mouse-IgG2b-PE, rat-IgG2a-APC, rat-IgG2b-APC-Cy7, Armenian hamster-IgG-Pacific Blue, αCD3ε-biotin, αCD19-biotin, αGr1-biotin, αTer119-biotin, αPD-L2-PE, α-human-IFN-γ-Rα-PE, LEAF-purified anti-FAS-ligand, LEAF-purified Armenian hamster-IgG isotype control, and Annexin V-Pacific Blue were purchased from BioLegend. Annexin V-FITC was obtained from ImmunoTools. 7-Aminoactinomycin D (AAD), αBrdU-APC (3D4), and the corresponding isotype control (MOPC21) were obtained from BD. αCD8 (2.43) was purchased from BioXCell, and IgG from rat serum was purchased from Sigma-Aldrich.
Retroviral particles.
The retroviral vector pMSCV-p210BCR/ABL-IRES-GFP and the packaging vector pIK7 were a gift from J. Schwaller (University of Basel, Basel, Switzerland). Retroviral particles were produced and titrated as previously described (Mumprecht et al., 2009a).
CML model.
CML was induced and monitored as previously described (Mumprecht et al., 2009a,b). In brief, 4 × 106 BM cells of 5-fluorouracil–pretreated BL/6 mice, H8 mice, or HLA-A2tg mice were transduced twice in transplant medium with 105 retroviral particles through spin infection. 105-transduced BM cells were injected i.v. into previously sublethally irradiated BL/6 recipient mice (BL/6 → BL/6, BL/6 CML; irradiation, 4.5 Gy; H8 → BL/6, H8 CML; irradiation, 6.5 Gy) or HLA-A2tg mice (HLA-A2tg → HLA-A2tg, HLA-A2tg CML; irradiation: 6.5 Gy). 5 × 105 transduced BM cells were incubated for 3 d in transplant medium, and GFP expression was analyzed by FACS. For secondary whole BM transplantations, 3 × 106 BM cells from primary CML mice (18–20 d after primary transplantation) were injected i.v. into previously sublethally irradiated (6.5 Gy) secondary recipients.
Lineage depletion and flow cytometry.
BM lineage depletion was performed using biotinylated antibodies against red cell precursors (αTer119), B cells (αCD19), T cells (αCD3ε), and myeloid cells (αGr1), MACS α-biotin beads, and LS columns (Miltenyi Biotec).
Determination of granulocyte counts/microliter was performed using a VetABC animal blood counter (Medical Solution GmbH) in combination with FACS. All samples were analyzed on a BD LSRII and sorting was performed on a BD FACSAria (BD). Data were analyzed using FlowJo software (Tree Star).
Cell cycle analysis.
FACS-sorted, lin−BCR/ABL-GFP+c-kithi were pooled and incubated in 1% PFA/PBS overnight at 4°C. Samples were permeabilized with 0.2% Triton X-100 for 30 min at 4°C and labeled with 5 µg/ml DAPI (Roche).
Generation of p14 and p14xIFN-γ−/− effector CTLs and memory cells.
106 splenocytes from p14 TCR transgenic (p14) mice or p14xIFN-γ−/− mice (all CD45.1+) were adoptively transferred to BL/6 mice. 18 h later, BL/6 mice were infected with 104 plaque-forming units of LCMV-WE, and spleens were harvested 6 d later. For experiments using p14 CD8+ effector T cells exclusively, cells were MACS-purified by αCD8 beads. 5 × 106 p14 CD8+ effector T cells were injected i.v. into recipient mice. For experiments using p14 and p14xIFN-γ−/− CD8+ effector T cells in vivo, cells were FACS-purified for CD8, CD45.1, Vα2, and Vβ8.1. 3–5 × 106–purified effector T cells were injected i.v. into recipient mice. For the isolation of memory p14 cells, spleens of BL/6 mice were harvested 6 wk after infection.
Generation of mature DC from HLA-A2tg mice.
BM was flushed from femurs and tibias of HLA-A2tg mice using RPMI 1640 medium (Sigma-Aldrich) containing 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine. Erythrocytes were removed using RBC lysis buffer (QIAGEN). 2–3 × 106 BM cells were cultured in RPMI 10% FCS supplemented with 50 µM 2-mercaptothanol and 10% (vol/vol) supernatant from a GM-CSF hybridoma cell line in nontissue culture–treated Petri dishes at 37°C and 5% CO2. After 6 and 8 d, half of the medium was removed and fresh medium was added. After 10 d, cells were transferred to tissue culture Petri dishes and 104 U/ml recombinant mouse (rm)-TNF (Prospec) was added to cultures. 2 d later, mature DC were harvested from the supernatant and analyzed by flow cytometry for expression of CD11c, MHC class II, CD80, and CD86.
Generation of HLA-A2–specific CD8+ effector cells.
To induce an optimal CTL response in HLA-A2tg mice, the coexpression of both CTL and CD4 helper peptides on DCs was necessary (Bennett et al., 1997). Therefore, mature DCs from HLA-A2tg mice were pulsed with the CTL peptide b3a2 (10−5 M) in combination with the CD4 helper peptide p13 (10−6 M) in RPMI 2% FCS for 90 min at 37°C and 5% CO2. DCs were washed twice and naive HLA-A2tg mice were vaccinated i.v. with 106 DCs at days 0, 2, 10, and 12 (Ludewig et al., 2000). At day 14, spleens from HLA-A2tg mice were harvested and CD8+ T cells were purified by MACS, and then cultured in IMDM 10% FCS in the presence of 50 U/ml rm-IL-2 (Prospec) and 0.01% 2-mercaptoethanol. Mature DCs from HLA-A2tg mice were pulsed as with the CTL peptide b3a2 (10−5 M) only and added to CD8+ T cell cultures at a ratio of 1:5 to 1:10 at the beginning of culture. Fresh DCs were added weekly. Cultures were maintained at 37°C and 5% CO2 for 3 wk. 2–3 × 106 MACS-purified CD8+ T cells were injected into HLA-A2tg CML mice.
Proliferation assay.
3 × 103 FACS-sorted LSCs were irradiated (10 Gy) and co-cultured with 1.5 × 104 naive or memory p14 CD8+ T cells in 96-well V-bottom plates (Corning) for 4 d. For the last 18 h of culture, 0.5 µCi of 3H-thymidine (PerkinElmer) was added to each well. Next, cells were collected with a Packard Filtermate 196-Harvester (Packard Bioscience) on UniFilter-96, GF/C plates (PerkinElmer). Microscint-0 (PerkinElmer) was added, and the incorporation of radioactivity was detected on a TopCount microplate scintillation counter (PerkinElmer).
Colony-forming assays.
5 × 103 MACS-purified lineage-negative cells from CML mice were plated into MethoCult M3134 medium (STEMCELL Technologies) supplemented with 15% FCS, 20% BIT (50 mg/ml BSA in IMDM, 1.44 U/ml rh-insulin [Actrapid; Novo Nordisk], and 250 ng/ml human holo transferrin [Prospec]), 100 µM 2-mercaptoethanol, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine, and 50 ng/ml rm-SCF, 10 ng/ml rm-IL-3, 10 ng/ml rh-IL-6, and 50 ng/ml rm-Flt3-ligand (all from Prospec). BCR/ABL-GFP+ colonies were enumerated after 7 d (≥30 cells/colony) on a DMIL inverted microscope (Leica) equipped with an Intensilight C-HGFI unit (Nikon).
2 × 103 FACS-purified CD34+ stem/progenitor cells from peripheral blood and BM of CML patients were plated into MethoCult H4435 Enriched medium (STEMCELL Technologies) in the presence or absence of 500 U/ml rh-IFN-γ (Prospec). Colonies and cells were enumerated after 14 d (≥30 cells/colony).
In vitro LSC killing assay.
104 FACS-sorted H8 or BL/6 LSCs were co-cultured overnight in 96-well V-bottom plates (Corning) with titrated numbers of naive cells or p14 effector cells (either total splenocytes or MACS-purified CD8+ T cells). On the next day, the contents of wells were resuspended and colony assays were performed.
In vitro BCR/ABL-GFP+ leukemia cell killing assay.
p14 or p14xIFN-γ−/− CTLs were generated as described in Generation of p14 and p14xIFN-γ−/− effector CTLs and memory cells and were isolated by MACS from spleens and lymph nodes. CTLs were preincubated for 20 min with or without 10 µg/ml anti–FAS-ligand (MFL4; BioLegend), in the presence or absence of titrated concentrations of the granzyme B inhibitor Z-AAD-CMK (1, 10, and 100 mM; Enzo Life Sciences). Armenian hamster IgG (HTK888; BioLegend) and water served as negative controls, respectively. Bulk BM cells from H8 CML mice (containing ∼20% of BCR/ABL-GFP+ cells) were either incubated alone or were co-cultured with CTLs at a ratio of 5:1 in the presence or absence of the indicated compounds in 24-well tissue culture plates. After 4 h, cells were analyzed by FACS and the killing of BCR/ABL-GFP+ cells was calculated in relation to control conditions.
In vitro liquid culture of primary human CD34+ CML stem/progenitor cells.
104 FACS-purified CD34+ stem/progenitor cells from the peripheral blood and BM of CML patients were cultured in StemSpan SFEM medium (Stem Cell Technologies) supplemented with human cytokines (StemSpan CC100; Stem Cell Technologies) in the presence or absence of 500 U/ml rh-IFN-γ in 96-well plates at 37°C and 5% CO2 in sextuplicates. 10 µM BrdU (BD) was added to culture for the last 4 h of incubation. Numbers of viable cells were determined by trypan blue staining and BrdU staining was performed according to the manufacturer’s instructions (BrdU Flow kit; BD) after 7 d of culture.
IFN-γ treatment of CML mice.
CML mice were treated with 2.5 × 105 U of rm-IFN-γ (Prospec) i.p. twice daily on two consecutive days, 16–18 d after primary transplantation or 18 h after secondary transplantation (a total of 106 U per animal).
CD8+ T cell depletion in vivo.
Depletion of CD8+ T cells was performed by two injections of 75 µg αCD8 mAb (clone 2.43; BioXCell) at days 10 and 11 after transplantation.
IFN-γ determination in mouse serum.
IFN-γ protein levels were measured using a multiplexed particle-based flow cytometry cytokine assay (R&D Systems) according to the manufacturer’s instructions.
Analysis of proliferation in vivo using BrdU.
BL/6 CML mice received BrdU (Sigma; 0.8mg/ml in drinking water and 1mg i.p./day) on 2 consecutive days (days 17 and 18 after primary transplantation). BrdU staining was performed according to the manufacturer’s instructions (BrdU Flow kit; BD).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software). Data are represented as mean ± SEM. Data were analyzed using one-way ANOVA and Tukey’s multiple comparison test or Dunnet’s test, Student’s t test (two-tailed), one-sample t test, or two-way ANOVA and Bonferroni post-hoc test (p-value shows interaction). Significance of differences in Kaplan-Meier survival curves was determined using the log-rank test (two-tailed). P < 0.05 was considered significant.
Acknowledgments
We thank Anne-Laure Huguenin for excellent technical assistance and Sabine Höpner for critically reading the manuscript.
This work was supported by grants from the Swiss National Science Foundation, the Swiss Cancer League, the Cancer League of the Canton of Bern, the Werner und Hedy Berger-Janser-Stiftung (A.F. Ochsenbein), a Swiss MD-PhD scholarship, the Gertrud Hagmann-Stiftung für Malignomforschung, the SwissLife Jubiläumsstiftung (C. Schürch), the Sassella Foundation (C. Riether), the Ehmann Stiftung, the Fondazione per la ricerca sulla trasfusione e sui trapianti, the Olga Mayenfisch Stiftung (C. Schürch and C. Riether), and the Novartis Stiftung für medizinisch-biologische Forschung (A.F. Ochsenbein and C. Riether).
The authors have declared that no competing financial interests exist.
C. Schürch and C. Riether designed and performed experiments, analyzed data and wrote the manuscript. M.A. Amrein performed experiments and analyzed data. A.F. Ochsenbein designed experiments, wrote the manuscript, and supervised the project.
Footnotes
Abbreviations used:
- AAD
- aminoactinomycin D
- alloSCT
- allogeneic stem cell transplantation
- BCR/ABL
- break point cluster region/Abelson murine leukemia viral oncogene homolog 1
- CML
- chronic myeloid leukemia
- CMP
- common myeloid progenitor
- DLI
- donor lymphocyte infusion
- GMP
- granulocyte-macrophage progenitor
- HSC
- hematopoietic stem cell
- LCMV
- lymphocytic choriomeningitis virus
- LMPP
- leukemia MPP
- LSC
- leukemia stem cell
- LSK
- lin−Sca-1+c-kithi
- MPP
- multipotent progenitor cell
- rh
- recombinant human
- rm
- recombinant murine
- TKI
- tyrosine kinase inhibitor
References
- Baccarani M., Saglio G., Goldman J., Hochhaus A., Simonsson B., Appelbaum F., Apperley J., Cervantes F., Cortes J., Deininger M., et al. ; European LeukemiaNet 2006. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 108:1809–1820 10.1182/blood-2006-02-005686 [DOI] [PubMed] [Google Scholar]
- Baldridge M.T., King K.Y., Boles N.C., Weksberg D.C., Goodell M.A. 2010. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 465:793–797 10.1038/nature09135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellodi C., Lidonnici M.R., Hamilton A., Helgason G.V., Soliera A.R., Ronchetti M., Galavotti S., Young K.W., Selmi T., Yacobi R., et al. 2009. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J. Clin. Invest. 119:1109–1123 10.1172/JCI35660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev N.N., Brown D.E., Diaz A.I., Rae A., Jarra W., Thompson J., Langhorne J., Potocnik A.J. 2010. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat. Immunol. 11:477–485 10.1038/ni.1869 [DOI] [PubMed] [Google Scholar]
- Bennett S.R., Carbone F.R., Karamalis F., Miller J.F., Heath W.R. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65–70 10.1084/jem.186.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D., Czechowicz A., Ooi A.G., Rossi D.J., Bryder D., Weissman I.L. 2009. Niche recycling through division-independent egress of hematopoietic stem cells. J. Exp. Med. 206:2837–2850 10.1084/jem.20090778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchia M., Korontsvit T., Xu Q., Mackinnon S., Yang S.Y., Sette A., Scheinberg D.A. 1996. Specific human cellular immunity to bcr-abl oncogene-derived peptides. Blood. 87:3587–3592 [PubMed] [Google Scholar]
- Brander C., Hartman K.E., Trocha A.K., Jones N.G., Johnson R.P., Korber B., Wentworth P., Buchbinder S.P., Wolinsky S., Walker B.D., Kalams S.A. 1998. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. J. Clin. Invest. 101:2559–2566 10.1172/JCI2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt N.M., Rojas J.M., Wang L., Christmas S.E., Abu-Eisha H.M., Clark R.E. 2005. Circulating bcr-abl-specific CD8+ T cells in chronic myeloid leukemia patients and healthy subjects. Haematologica. 90:1315–1323 [PubMed] [Google Scholar]
- Capron C., Lacout C., Lécluse Y., Jalbert V., Chagraoui H., Charrier S., Galy A., Bennaceur-Griscelli A., Cramer-Bordé E., Vainchenker W. 2010. A major role of TGF-beta1 in the homing capacities of murine hematopoietic stem cell/progenitors. Blood. 116:1244–1253 10.1182/blood-2009-05-221093 [DOI] [PubMed] [Google Scholar]
- Carlens S., Remberger M., Aschan J., Ringdén O. 2001. The role of disease stage in the response to donor lymphocyte infusions as treatment for leukemic relapse. Biol. Blood Marrow Transplant. 7:31–38 10.1053/bbmt.2001.v7.pm11215696 [DOI] [PubMed] [Google Scholar]
- Clark R.E., Dodi I.A., Hill S.C., Lill J.R., Aubert G., Macintyre A.R., Rojas J., Bourdon A., Bonner P.L., Wang L., et al. 2001. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 98:2887–2893 10.1182/blood.V98.10.2887 [DOI] [PubMed] [Google Scholar]
- Colvin G.A., Lambert J.F., Dooner M.S., Cerny J., Quesenberry P.J. 2007. Murine allogeneic in vivo stem cell homing(,). J. Cell. Physiol. 211:386–391 10.1002/jcp.20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.V., Evely R.S., Oakhill A., Pamphilon D.H., Goulden N.J., Blair A. 2004. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 104:2919–2925 10.1182/blood-2004-03-0901 [DOI] [PubMed] [Google Scholar]
- Dalton D.K., Pitts-Meek S., Keshav S., Figari I.S., Bradley A., Stewart T.A. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 259:1739–1742 10.1126/science.8456300 [DOI] [PubMed] [Google Scholar]
- Deininger M., Lehmann T., Krahl R., Hennig E., Müller C., Niederwieser D. 2000. No evidence for persistence of BCR-ABL-positive cells in patients in molecular remission after conventional allogenic transplantation for chronic myeloid leukemia. Blood. 96:779–780 [PubMed] [Google Scholar]
- Druker B.J., Sawyers C.L., Kantarjian H., Resta D.J., Reese S.F., Ford J.M., Capdeville R., Talpaz M. 2001a. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344:1038–1042 10.1056/NEJM200104053441402 [DOI] [PubMed] [Google Scholar]
- Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Sawyers C.L. 2001b. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344:1031–1037 10.1056/NEJM200104053441401 [DOI] [PubMed] [Google Scholar]
- Druker B.J., O’Brien S.G., Cortes J., Radich J. 2002. Chronic myelogenous leukemia. Hematology (Am Soc Hematol Educ Program). 1:111–135 10.1182/asheducation-2002.1.111 [DOI] [PubMed] [Google Scholar]
- Ehl S., Hombach J., Aichele P., Rülicke T., Odermatt B., Hengartner H., Zinkernagel R., Pircher H. 1998. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J. Exp. Med. 187:763–774 10.1084/jem.187.5.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers M.A., Offner S., Blanco-Bose W.E., Waibler Z., Kalinke U., Duchosal M.A., Trumpp A. 2009. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 458:904–908 10.1038/nature07815 [DOI] [PubMed] [Google Scholar]
- Faderl S., Talpaz M., Estrov Z., O’Brien S., Kurzrock R., Kantarjian H.M. 1999. The biology of chronic myeloid leukemia. N. Engl. J. Med. 341:164–172 10.1056/NEJM199907153410306 [DOI] [PubMed] [Google Scholar]
- Farrar M.A., Schreiber R.D. 1993. The molecular cell biology of interferon-gamma and its receptor. Annu. Rev. Immunol. 11:571–611 10.1146/annurev.iy.11.040193.003035 [DOI] [PubMed] [Google Scholar]
- Gale R.P., Horowitz M.M., Ash R.C., Champlin R.E., Goldman J.M., Rimm A.A., Ringdén O., Stone J.A., Bortin M.M. 1994. Identical-twin bone marrow transplants for leukemia. Ann. Intern. Med. 120:646–652 [DOI] [PubMed] [Google Scholar]
- Guzman M.L., Li X., Corbett C.A., Rossi R.M., Bushnell T., Liesveld J.L., Hébert J., Young F., Jordan C.T. 2007. Rapid and selective death of leukemia stem and progenitor cells induced by the compound 4-benzyl, 2-methyl, 1,2,4-thiadiazolidine, 3,5 dione (TDZD-8). Blood. 110:4436–4444 10.1182/blood-2007-05-088815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M.M., Gale R.P., Sondel P.M., Goldman J.M., Kersey J., Kolb H.J., Rimm A.A., Ringdén O., Rozman C., Speck B., et al. 1990. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 75:555–562 [PubMed] [Google Scholar]
- Hughes T.P., Kaeda J., Branford S., Rudzki Z., Hochhaus A., Hensley M.L., Gathmann I., Bolton A.E., van Hoomissen I.C., Goldman J.M., Radich J.P.; International Randomised Study of Interferon versus STI571 (IRIS) Study Group 2003. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 349:1423–1432 10.1056/NEJMoa030513 [DOI] [PubMed] [Google Scholar]
- Ito K., Bernardi R., Morotti A., Matsuoka S., Saglio G., Ikeda Y., Rosenblatt J., Avigan D.E., Teruya-Feldstein J., Pandolfi P.P. 2008. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 453:1072–1078 10.1038/nature07016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Hope K.J., Zhai Q., Smadja-Joffe F., Dick J.E. 2006. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 12:1167–1174 10.1038/nm1483 [DOI] [PubMed] [Google Scholar]
- Kavalerchik E., Goff D., Jamieson C.H. 2008. Chronic myeloid leukemia stem cells. J. Clin. Oncol. 26:2911–2915 10.1200/JCO.2008.17.5745 [DOI] [PubMed] [Google Scholar]
- Kiel M.J., Morrison S.J. 2008. Uncertainty in the niches that maintain haematopoietic stem cells. Nat. Rev. Immunol. 8:290–301 10.1038/nri2279 [DOI] [PubMed] [Google Scholar]
- Kolb H.J., Mittermüller J., Clemm C., Holler E., Ledderose G., Brehm G., Heim M., Wilmanns W. 1990. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 76:2462–2465 [PubMed] [Google Scholar]
- Kolb H.J., Schattenberg A., Goldman J.M., Hertenstein B., Jacobsen N., Arcese W., Ljungman P., Ferrant A., Verdonck L., Niederwieser D., et al. ; European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia 1995. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 86:2041–2050 [PubMed] [Google Scholar]
- Krause D.S., Lazarides K., von Andrian U.H., Van Etten R.A. 2006. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat. Med. 12:1175–1180 10.1038/nm1489 [DOI] [PubMed] [Google Scholar]
- Kurz K., Gluhcheva Y., Zvetkova E., Konwalinka G., Fuchs D. 2010. Interferon-gamma-mediated pathways are induced in human CD34(+) haematopoietic stem cells. Immunobiology. 215:452–457 10.1016/j.imbio.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Lapidot T., Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J., Minden M., Paterson B., Caligiuri M.A., Dick J.E. 1994. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 367:645–648 10.1038/367645a0 [DOI] [PubMed] [Google Scholar]
- Li L., Wang L., Li L., Wang Z., Ho Y., McDonald T., Holyoake T.L., Chen W., Bhatia R. 2012. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 21:266–281 10.1016/j.ccr.2011.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig B., Ochsenbein A.F., Odermatt B., Paulin D., Hengartner H., Zinkernagel R.M. 2000. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J. Exp. Med. 191:795–804 10.1084/jem.191.5.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara K.C., Oduro K., Martin O., Jones D.D., McLaughlin M., Choi K., Borjesson D.L., Winslow G.M. 2011. Infection-induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN-γ signaling. J. Immunol. 186:1032–1043 10.4049/jimmunol.1001893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R., Chao M.P., Alizadeh A.A., Pang W.W., Jaiswal S., Gibbs K.D., Jr, van Rooijen N., Weissman I.L. 2009. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 138:286–299 10.1016/j.cell.2009.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W., Huff C.A., Wang Q., Malehorn M.T., Barber J., Tanhehco Y., Smith B.D., Civin C.I., Jones R.J. 2004. Characterization of clonogenic multiple myeloma cells. Blood. 103:2332–2336 10.1182/blood-2003-09-3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter M., Odermatt B., Yagita H., Nuoffer J.M., Ochsenbein A.F. 2006. Elimination of chronic viral infection by blocking CD27 signaling. J. Exp. Med. 203:2145–2155 10.1084/jem.20060651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means R.T., Jr 1995. Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cells. 13:32–37 10.1002/stem.5530130105 [DOI] [PubMed] [Google Scholar]
- Metcalf D. 2008. Hematopoietic cytokines. Blood. 111:485–491 10.1182/blood-2007-03-079681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molldrem J.J., Lee P.P., Wang C., Felio K., Kantarjian H.M., Champlin R.E., Davis M.M. 2000. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat. Med. 6:1018–1023 10.1038/79526 [DOI] [PubMed] [Google Scholar]
- Mumprecht S., Matter M., Pavelic V., Ochsenbein A.F. 2006. Imatinib mesylate selectively impairs expansion of memory cytotoxic T cells without affecting the control of primary viral infections. Blood. 108:3406–3413 10.1182/blood-2006-04-018705 [DOI] [PubMed] [Google Scholar]
- Mumprecht S., Claus C., Schürch C., Pavelic V., Matter M.S., Ochsenbein A.F. 2009a. Defective homing and impaired induction of cytotoxic T cells by BCR/ABL-expressing dendritic cells. Blood. 113:4681–4689 10.1182/blood-2008-05-156471 [DOI] [PubMed] [Google Scholar]
- Mumprecht S., Schürch C., Schwaller J., Solenthaler M., Ochsenbein A.F. 2009b. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 114:1528–1536 10.1182/blood-2008-09-179697 [DOI] [PubMed] [Google Scholar]
- Mumprecht S., Schürch C., Scherrer S., Claus C., Ochsenbein A.F. 2010. Chronic myelogenous leukemia maintains specific CD8(+) T cells through IL-7 signaling. Eur. J. Immunol. 40:2720–2730 10.1002/eji.201040404 [DOI] [PubMed] [Google Scholar]
- Neering S.J., Bushnell T., Sozer S., Ashton J., Rossi R.M., Wang P.Y., Bell D.R., Heinrich D., Bottaro A., Jordan C.T. 2007. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood. 110:2578–2585 10.1182/blood-2007-02-073031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neviani P., Santhanam R., Oaks J.J., Eiring A.M., Notari M., Blaser B.W., Liu S., Trotta R., Muthusamy N., Gambacorti-Passerini C., et al. 2007. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J. Clin. Invest. 117:2408–2421 10.1172/JCI31095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo S., Bervas N., Ure J.M., Smith A.G., Lemonnier F.A., Pérarnau B. 1997. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J. Exp. Med. 185:2043–2051 10.1084/jem.185.12.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R.M. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 342:559–561 10.1038/342559a0 [DOI] [PubMed] [Google Scholar]
- Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. 2011. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365:725–733 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiola A.M., Van Lint M.T., Valbonesi M., Lamparelli T., Gualandi F., Occhini D., Bregante S., di Grazia C., Dominietto A., Soracco M., et al. 2003. Factors predicting response and graft-versus-host disease after donor lymphocyte infusions: a study on 593 infusions. Bone Marrow Transplant. 31:687–693 10.1038/sj.bmt.1703883 [DOI] [PubMed] [Google Scholar]
- Savona M., Talpaz M. 2008. Getting to the stem of chronic myeloid leukaemia. Nat. Rev. Cancer. 8:341–350 10.1038/nrc2368 [DOI] [PubMed] [Google Scholar]
- Sawyers C.L. 1999. Chronic myeloid leukemia. N. Engl. J. Med. 340:1330–1340 10.1056/NEJM199904293401706 [DOI] [PubMed] [Google Scholar]
- Schreiber R.D., Old L.J., Smyth M.J. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 331:1565–1570 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- Schürch C., Riether C., Matter M.S., Tzankov A., Ochsenbein A.F. 2012. CD27 signaling on chronic myelogenous leukemia stem cells activates Wnt target genes and promotes disease progression. J. Clin. Invest. 122:624–638 10.1172/JCI45977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehn L.H., Alyea E.P., Weller E., Canning C., Lee S., Ritz J., Antin J.H., Soiffer R.J. 1999. Comparative outcomes of T-cell-depleted and non-T-cell-depleted allogeneic bone marrow transplantation for chronic myelogenous leukemia: impact of donor lymphocyte infusion. J. Clin. Oncol. 17:561–568 [DOI] [PubMed] [Google Scholar]
- Selleri C., Maciejewski J.P. 2000. The role of FAS-mediated apoptosis in chronic myelogenous leukemia. Leuk. Lymphoma. 37:283–297 [DOI] [PubMed] [Google Scholar]
- Thomas E.D., Sanders J.E., Flournoy N., Johnson F.L., Buckner C.D., Clift R.A., Fefer A., Goodell B.W., Storb R., Weiden P.L. 1979. Marrow transplantation for patients with acute lymphoblastic leukemia in remission. Blood. 54:468–476 [PubMed] [Google Scholar]
- Van Driessche A., Gao L., Stauss H.J., Ponsaerts P., Van Bockstaele D.R., Berneman Z.N., Van Tendeloo V.F. 2005. Antigen-specific cellular immunotherapy of leukemia. Leukemia. 19:1863–1871 10.1038/sj.leu.2403930 [DOI] [PubMed] [Google Scholar]
- Wang R., Charoenvit Y., Corradin G., Porrozzi R., Hunter R.L., Glenn G., Alving C.R., Church P., Hoffman S.L. 1995. Induction of protective polyclonal antibodies by immunization with Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J. Immunol. 155:1637. [PubMed] [Google Scholar]
- Wang Y., Krivtsov A.V., Sinha A.U., North T.E., Goessling W., Feng Z., Zon L.I., Armstrong S.A. 2010. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 327:1650–1653 10.1126/science.1186624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden P.L., Flournoy N., Thomas E.D., Prentice R., Fefer A., Buckner C.D., Storb R. 1979. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 300:1068–1073 10.1056/NEJM197905103001902 [DOI] [PubMed] [Google Scholar]
- Weiss G., Goodnough L.T. 2005. Anemia of chronic disease. N. Engl. J. Med. 352:1011–1023 10.1056/NEJMra041809 [DOI] [PubMed] [Google Scholar]
- Wilson A., Oser G.M., Jaworski M., Blanco-Bose W.E., Laurenti E., Adolphe C., Essers M.A., Macdonald H.R., Trumpp A. 2007. Dormant and self-renewing hematopoietic stem cells and their niches. Ann. N. Y. Acad. Sci. 1106:64–75 10.1196/annals.1392.021 [DOI] [PubMed] [Google Scholar]
- Xie Y., Yin T., Wiegraebe W., He X.C., Miller D., Stark D., Perko K., Alexander R., Schwartz J., Grindley J.C., et al. 2009. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 457:97–101 10.1038/nature07639 [DOI] [PubMed] [Google Scholar]
- Yotnda P., Firat H., Garcia-Pons F., Garcia Z., Gourru G., Vernant J.P., Lemonnier F.A., Leblond V., Langlade-Demoyen P. 1998. Cytotoxic T cell response against the chimeric p210 BCR-ABL protein in patients with chronic myelogenous leukemia. J. Clin. Invest. 101:2290–2296 10.1172/JCI488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N.S. 2006. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology (Am Soc Hematol Educ Program). 1:72–77 10.1182/asheducation-2006.1.72 [DOI] [PubMed] [Google Scholar]
- Zhao X., Ren G., Liang L., Ai P.Z., Zheng B., Tischfield J.A., Shi Y., Shao C. 2010. Brief report: interferon-gamma induces expansion of Lin(-)Sca-1(+)C-Kit(+) Cells. Stem Cells. 28:122–126 [DOI] [PubMed] [Google Scholar]
- Zou W., Chen L. 2008. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 8:467–477 10.1038/nri2326 [DOI] [PubMed] [Google Scholar]