Abstract
Basic tendencies to detect and respond to significant events are present in the simplest single cell organisms, and persist throughout all invertebrates and vertebrates. Within vertebrates, the overall brain plan is highly conserved, though differences in size and complexity also exist. The forebrain differs the most between mammals and other vertebrates. The classic notion that the evolution of mammals led to radical changes such that new forebrain structures (limbic system and neocortex) were added has not held up, nor has the idea that so-called limbic areas are primarily involved in emotion. Modern efforts have focused on specific emotion systems, like the fear or defense system, rather than on the search for a general purpose emotion systems. Such studies have found that fear circuits are conserved in mammals, including humans. Animal work has been especially successful in determining how the brain detects and responds to danger. Caution should be exercised when attempting to discuss other aspects of emotion, namely subjective feelings, in animals since there are no scientific ways of verifying and measuring such states except in humans.
Introduction
The topic of emotion and evolution typically brings to mind Darwin’s classic treatise, Emotions in Man and Animals (Darwin, 1872). In this book Darwin sought to extend his theory of natural selection beyond the evolution of physical structures and into the domain of mind and behavior by exploring how emotions too might have evolved. Particularly important to his argument was the fact that certain emotions are expressed similarly in people around the world, including in isolated areas where there had been little contact with the outside world and thus little opportunity for emotional expressions to have been learned and culturally transmitted. This suggested to him that there must be a strong heritable component to emotions in people. Also important was his observation that certain emotions are expressed similarly across species, especially closely related species, further suggesting that these emotions are phylogenetically conserved.
With the rise of experimental brain research in the late 19th century, emotion was one of the key topics that early neuroscientists sought to relate to the brain (see LeDoux, 1987). The assumption was that emotion circuits are conserved across mammalian species, and that it should be possible to understand human emotions by exploring emotional mechanisms in the non-human mammalian brain.
In this chapter, I will first briefly survey the history of ideas about the emotional brain, and especially ideas that have attempted to explain the emotional brain in terms of evolutionary principles. This will lead to a discussion of fear, since this is the emotion that has been studied most thoroughly in terms of brain mechanisms. The chapter will conclude with a reconsideration of what the term emotion refers to, and specifically which aspects of emotion can be studied in animals and which must be studied in humans.
A Brief History of the Emotional Brain: The Rise and Fall of the Limbic System Theory
All organisms, even single cell organisms, must have the capacity to detect and respond to significant stimuli in order to survive. Bacteria, for example, approach nutrients and avoid harmful chemicals (Macnab and Koshland, 1972). With the evolution of multicellular, metazoan organisms with specialized systems, particularly a nervous system, the ability to detect and respond to significant events increases in sophistication (Shepherd, 1983).
Invertebrates, the oldest and largest group of multicellular organisms, exhibit a wide variety of types of nervous systems. However, all vertebrates share a common basic brain plan consisting of three broad zones (hindbrain, midbrain, and forebrain) with conserved basic circuits (Nauta and Karten, 1970; Swanson, 2002; Bulter and Hodos, 2005; Striedter, 2005). In spite of this overall similarity, differences in size and complexity exist. For example, the forebrain differs the most between mammals and reptiles. On the basis of such differences, the classic view of forebrain evolution emerged in the first half of the 20th century (e.g. Smith, 1924; Herrick, 1933; Arien Kappers et al, 1936; Papez, 1937; MacLean, 1949, 1952). According to this view, with the emergence of mammals, the forebrain plan underwent radical changes in which new structures, especially cortical structures, were added. These were layered over and covered the reptilian forebrain, which mainly consisted of the basal ganglia. First came “primitive” cortical regions in early mammals. In these organisms the basic survival functions related to feeding, defense and procreation were taken care of by fairly undifferentiated (weakly laminated) cortical regions (primitive cortex, including the hippocampus and cingulate cortex) and related subcortical areas (such as the amygdala) that were closely tied to the olfactory system. Later mammals added highly novel, laminated cortical regions (neocortex) that made possible enhanced non-olfactory sensory processing and cognitive functions (including learning and memory, reasoning, and planning capacities, and, in humans, language).
The basic principle that equated cognition with evolutionarily new cortex (neocortex) and emotion with older cortex and related subcortical forebrain regions culminated in Paul MacLean’s limbic system theory of emotion (1949, 1952, 1970). The term limbic was first used by the French anatomist Paul Broca as a structural designation for a rim of cortex in the medial wall of the hemisphere. Broca called this rim the limbic lobe (le grande lobe limbique) (limbic is from the Latin word for rim, limbus). MacLean built on the classic findings of comparative anatomists such as Herrick and Papez, and experimental findings from Walter Cannon, Phillip Bard and Henrich Kluver and Paul Bucy (Cannon, 1929; Bard, 1928; Kluver and Bucy, 1937) to transform the limbic lobe into an emotion system, the limbic system. The limbic system was defined anatomically as the primitive medial cortical areas and interconnected subcortical nuclei (including the amygdala and septum).
MacLean called the limbic system the paleomammalian brain (since it was said to have emerged with the evolution of early mammals), and contrasted it with the reptilian brain (basal ganglia and brainstem). In more recent mammals the neocortex, also called the neomammalian brain, was said by MacLean to increases in size and complexity at the expense of the limbic system. The decrease of the limbic system reduced the dependence of humans on base emotions, and the increase in the neocortex allowed humans greater control over remaining emotional circuits as well as greater cognitive capacities.
The limbic system concept stimulated much research in the 1950s, 60s, and 70s. However, it has been criticized on a number of grounds, and has been rejected by many scientists (Swanson, 1983; LeDoux, 1987, 1991, 1996; Kotter and Meyer, 1992; Butler and Hodos, 2005). Because the limbic system concept continues to be referred to in some scientific circles (e.g. Panksepp, 1998, 2005) and persists in many lay accounts of the brain, it is worth considering why it is not acceptable.
First, the theory presumes that the neocortex and limbic system are unique mammalian specializations. Neither of these ideas is accepted by contemporary comparative neuroanatomists (Nauta and Karten, 1970; Northcutt and Kaas, 1995; Butler and Hodos, 2005; Striedter, 2005). Birds and reptiles, for example, have been shown to have structures that correspond with both mammalian neocortex and with MacLean’s cortical and subcortical limbic areas (hippocampus, amygdala). Second, MacLean argued the architecture of limbic areas is ill-suited for cognitive processes. However, the hippocampus, viewed by MacLean as the centerpiece of the limbic system and a central structure for emotional functions, is recognized as one of the key areas related to higher cognitive functions, such as declarative or explicit memory (Squire, 1987; Eichenbaum, 2002) and spatial cognition (O’Keefe and Nadel, 1978). Third, efforts to define the system have failed. Connectivity with old cortex is a flawed criterion if old cortex is itself an unjustified notion. Connectivity with the hypothalamus once seemed plausible, since that was a way of distinguishing relevant and irrelevant cortical areas (Issacson, 1982). However, as anatomical techniques improved, areas from the neocortex were also found to be connected with the hypothalamus, as were areas of the spinal cord, potentially extending the limbic system across the entire brain. Finally, and perhaps most important, there is no evidence that the limbic system, however defined, functions as an integrated system in the mediation of emotion. While specific areas of the limbic system contribute to some emotional functions, these areas do not do so because they belong to a limbic system that evolved to perform emotional functions. Indeed, relatively few limbic areas have been shown to contribute to emotional functions. As noted above, the hippocampus, the centerpiece of the limbic system theory of emotion, has been strongly implicated in cognitive functions but the evidence for a role in emotion is far less impressive.
The limbic system theory attempted to explain all emotions within a single anatomical concept. Contemporary researchers are more inclined to focus on tasks designed to study the brain systems of specific emotions. As we will see, this has been a more profitable empirical approach.
Contributions of the Amygdala to Avoidance Conditioning: An Early Approach to Linking Emotional Behavior to the Limbic System
Why, then, has the limbic system concept persisted for so long given that it proved questionable on evolutionary, structural, and functional grounds? The key reason can be summarized in the term “guilt by association.” One limbic area, the amygdala, has consistently been implicated in emotional behavior. Because the amygdala was part of the limbic system concept, its involvement vindicated the whole concept. This does not mean that the amygdala is the only structure involved in emotion, but instead that the amygdala is one area that has been extensively implicated in emotion, in part because of the behavioral tasks that have most often been used to study emotion.
The amygdala was first implicated in emotion through studies of the Kluver-Bucy Syndrome, a set of unusual behaviors observed in monkeys after removal of large areas of the temporal lobe (Kluver and Bucy, 1937). Monkeys with such lesions attempted to eat inappropriate items and copulate with inappropriate partners, and lost their fear of snakes and humans. It was concluded that the animals had psychic blindness, an inability to appreciate the significance or value of visual stimuli. Weiskrantz (1956) attempted to localize the effects within the temporal lobe using a behavioral task where behavior was guided by stimulus value. Specifically, he used an avoidance conditioning paradigm where monkeys learned to use a cue to signal when to perform a behavioral response in order to avoid receiving a painful shock. Such a paradigm was viewed as especially useful in assessing the role of the amygdala in processing threats that lead to fear. Damage targeted to the amygdala disrupted performance, leading Weiskrantz to conclude that the amygdala was responsible for the inability of animals with temporal lobe damage to use stimulus value to guide behavior, and thus that an important function of the amygdala was to ascertain stimulus value. Specifically, Weiskrantz proposed that the amygdala processes the rewarding and punishing consequences of events. However, the data were essentially about aversive or punishing events since avoidance conditioning is a fear-based paradigm.
Subsequently, throughout the 1960s and 1970s, avoidance conditioning paradigms were used to study the contribution of the amygdala to emotion, an especially to fear. The bulk of the evidence was consistent, in general, with the idea that the amygdala is a key structure in avoidance conditioning, and by implication, in processing the value of emotional stimuli. Such findings were treated as evidence in support of the limbic system theory of emotion since the amygdala was part of the limbic system.
By the mid 1980s, thirty years of research on the brain mechanisms of avoidance had been conducted. While it seemed clear that the amygdala was somehow involved, there was considerable confusion as exactly what its role might be (Sarter and Markowitsch, 1985). There are several explanations likely for this unsettled state of affairs. First, there was little appreciation of the anatomical complexity of the amygdala, a brain region with a dozen or so nuclei, each with subnuclei (Amaral et al, 1992; Pitkanen et al, 1997; LeDoux, 2007). Failure to recognize this anatomical complexity may have led to confusion. Indeed, more recent work has shown that different nuclei and subnuclei have different functions (Repa et al, 2001; LeDoux, 2007). Second, the behavioral complexity of avoidance conditioning itself was not fully appreciated. Avoidance tasks can be constructed in various ways (active, passive, signaled, unsignaled), and each involves the learning of a Pavlovian association and an instrumental association (Cain and LeDoux, 2007; Cain et al., 2010; LeDoux et al., 2009; Choi et al, 2010; Amorapanth et al, 2000; Lazaro-Munoz et al., 2010). In retrospect, failure to separate these components also probably played a role in adding confusion to efforts to understand the brain mechanisms of avoidance.
Contribution of the Amygdala to Fear: Studies of Aversive Pavlovian Conditioning
During the 1960s, researchers began using Pavlovian conditioning to pursue the cellular and molecular mechanisms of learning in invertebrates (e.g. Kandel and Spencer, 1968; Kandel et al., 1986). The success of this approach, together with the fact that avoidance conditioning was stuck in a rut, led mammalian researchers to turn to Pavlovian conditioning as well (Thompson, 1986; Kapp et al, 1979; LeDoux et al, 1984; Tischler and Davis, 1983).
As mentioned already, Pavlovian conditioning is the initial phase of avoidance conditioning. After the subjects rapidly undergo Pavlovian conditioning, they then slowly learn to perform avoidance responses using the CS as a warning signal. Indeed, the emotional learning that occurs in avoidance conditioning occurs during the Pavlovian phase. Pavlovian conditioning is thus a more direct means of studying emotional processing. Perhaps Pavlovian conditioning would be easier to understand.
In Pavlovian fear conditioning, the subject receives a neutral conditioned stimulus (CS), usually a tone, followed by an aversive unconditioned stimulus (US), typically footshock. After one or at most a few pairings, the CS comes to elicit innate emotional responses that naturally occur in the presence of threatening stimuli, such as predators. For example, after conditioning a CS elicits defensive freezing behavior and associated autonomic and endocrine responses that support the behavior (Blanchard and Blanchard, 1969; Bolles and Fanselow, 1980; LeDoux et al, 1984). The subject does not have to learn to perform these responses. The responses are innate. What is learned is an association that allows a novel stimulus, a warning of danger, to elicit the defensive responses in anticipation of the actual danger.
With the simpler approach provided by fear conditioning, as opposed to avoidance, much progress was made in mapping the circuitry, including the regions in the brain where the CS and US converge to form the associations and the regions involved in the control of emotional responses by the CS in animals (see LeDoux, 2000; Maren, 2001, 2005; Rodrigues et al, 2004; Johansen et al, in review; Davis, 1992; Davis et al., 1997; Fanselow and Poulos, 2005; Pape and Pare, 2010) and humans (Phelps and LeDoux, 2005; Phelps, 2006; Sehlmeyer et al., 2009; Kim et al., 2011; Whalen and Phelps, 2009). In brief, CS and US convergence occur in the lateral nucleus of the amygdala (LA), and specifically in the dorsal subregion of the LA. This convergence leads to synaptic plasticity and the formation of a CS-US association. Damage to LA, inactivation of LA, or manipulation of a variety of molecular pathways in LA prevents fear conditioning. A second important region is central nucleus of the amygdala (CE). Manipulations of the region also disrupts conditioning. LA and CE are connected directly and by way of various intra-amygdala pathways. Once the CS-US association is formed, later exposure to the CS results in the retrieval of the learned association formed by CS-US convergence during conditioning. Information then flows from LA to CE, which then connects to hypothalamic and brainstem areas that control behavioral, autonomic, and hormonal responses that help the organism cope with the threat. Plasticity also occurs in CE, and in CS processing regions and motor control regions. This simplified description omits many details.
Much has been learned about the molecular mechanisms in LA that make fear conditioning possible (Blair et al, 2001; Schafe et al, 2001; Rodrigues et al, 2004; Fanselow and Poulos, 2005; Maren, 2001, 2005; Pape and Pare, 2010; Sah et al, 2008; Johansen et al, in review). In brief, the CS input synapses undergo plasticity when the LA neurons they connect with are depolarized by the shock US. As a result, the ability of the CS to activate the LA cell is potentiated. Plasticity is triggered when the depolarizing US allows calcium to flow into the cell via NMDA receptors and voltage sensitive calcium channels. The elevated calcium activate a number of protein kinases that ultimately lead to phosphoylation of transcription factors such as CREB that lead to gene expression and protein synthesis. The newly synthesized proteins then stabilize the synaptic potentiation, allowing the CS to strongly activate the LA cell for over long periods of time. It is particularly interesting that many of the molecular changes that underlie fear conditioning in mammals have also been show to be important for Palovian conditioning in invertebrates, showing the conserved nature of the molecular mechanisms of learning and memory.
The advances made in understanding Pavlovian fear conditioning made it possible to revisit the neural system of avoidance and related aversive instrumental behaviors (Cain and LeDoux, 2007; Cain et al., 2010; LeDoux et al., 2009; Choi et al, 2010; Amorapanth et al, 2000; Lazaro-Munoz et al., 2010). This work showed that as in fear conditioning, the LA is essential for forming the CS-US association. But in contrast to fear conditioning, in avoidance information flows from the LA to the basal amygdala (not to the CE). Extrapolating from appetitive conditioning finding (Everitt et al, 1989, 1999; Cardinal et al, 2002), it has been proposed that connections from the basal amygdala to the ventral striatum allow the CS-US association to control aversively motivated instrumental behavior.
Avoidance responses are not emotional responses per se. They are simply responses. An animal can learn to avoid harm by running, climbing, pressing, swimming, or even remaining stationary. The animal learns to do what it needs to do to attain safety. But the same responses could be used to obtain food if the animal is hungry and those responses are a way to gain access to food. In contrast, in Pavlovian fear conditioning the CS elicits specific emotional responses, fear or defense responses. Researchers were much more inclined to discuss Pavlovian conditioning results specifically in terms of fear/defense circuits.
Comparative Observations
Amygdala areas have been implicated in fear conditioning in a variety of mammals, including rats, mice, rabbits, and monkeys (see LeDoux, 1996, 2000; Johansen et al, in review; LeDoux, 2000; Maren, 2001, 2005; Rodrigues et al, 2004; Davis, 1992; Davis et al., 1997; Fanselow and Poulos, 2005; Pape and Pare, 2010). This suggests strong conservation of the circuitry within mammals, including humans. Indeed, a large body of work implicates the human amygdala in fear conditioning and in instrumental responses like avoidance (Phelps and LeDoux, 2005; Phelps, 2006; Whalen et al., 2004; Dolan and Vuilleumier, 2003; Whalen and Phelps, 2009; Delgado et al., 2009; Labar, 2003; Ousdal et al., 2008; Gianaros et al., 2008; Damasio, 1994; Bechara et al., 1995). Thus, damage to the amygdala in humans prevents fear conditioning from occurring and functional imaging studies show that activity increases in the amygdala during fear conditioning. Additionally, a number of studies have found amygdala activation in response to angry or fearful faces, considered to be unconditioned threat stimuli (Adolphs, 2008). Thus, findings involving both lesion studies and functional imaging suggest strong correspondence with the animal literature, at least at a gross anatomical level. Techniques available for studying the human brain do not allow precise localization of specific nuclei, though some progress is being made in this regard (Davis et al., 2011; Bach et al., 2011).
An important question concerns the nature of the amygdala in non-mammals and the role of the homologous structure in fear conditioning. According to classic view, areas such as the amygdala, being paleomammalian structures, should not exist in reptiles. However, in the 1970s, Cohen (1975) claimed to have indentified the amygdala in avian species and found that lesions of this regions disrupted of Pavlovian fear conditioning in pigeons. More recently, there has been much debate about what constitutes the amygdala, and specifically individual amygdala nuclei, in reptiles and birds (Karten, 1997; Martinez-Garcia et al., 2002; Lanuza et al., 1998; Moreno and Gonzalez, 2007; Bruce and Neary, 1995). Using connectivity patterns established in mammals, areas believed to be homologous to the lateral and central nucleus have been identified in lizards (Martinez-Garcia et al., 2002; Lanuza et al., 1998). When threatened, these animals undergo tonic immobility, and damage to the CE homologue interferes with this defensive response (Davies et al., 2002).
Much more work is needed to resolve what constitutes the amygdala in non-mammalian vertebrates and to determine whether the functions of the amygdala known to exist in mammals have some relation to the function of the homologous regions in the vertebrate ancestors of mammals.
Amygdala Contributions to Other Emotions
While the contribution of the amygdala to fear has been most thoroughly studied, it is clear that the amygdala contributes to other emotional states as well. A relatively large body of research has focused on the role of the amygdala in processing of rewards and the use of rewards to motivate and reinforce behavior (Cardinal et al., 2002; Everitt et al., 1999; Holland and Gallagher, 2004; Murray, 2007; Salzman et al., 2007; Nishijo et al., 2008). As with aversive conditioning, the lateral, basal, and central amygdala have been implicated in different aspects of reward learning and motivation, as well as drug addiction. The amygdala has also been implicated in emotional states associated with aggressive, maternal, sexual, and ingestive (eating and drinking) behaviors (Bahar et al., 2003; Galaverna et al., 1993; Miczek et al., 2007; Pfaff, 2005; Siegel and Edinger, 1981). Less is known about the detailed circuitry involved in these emotional states than is known about fear.
Emotional Evolution in Perspective
There is no shortage of theories that have speculated about the relation of emotion circuits to brain evolution. In the tradition of Darwin, basic emotions theorists have proposed that certain emotions are innate, in part because they are expressed the same in people around the world (Tomkins, 1962; Ekman, 1977, 1992; Izard, 1971, 1992; Plutchik, 1980; Buck, 1981). These innate emotions are said to be mediated by affect programs in the brain. An affect program, in effect, is psychological description of a dedicated neural circuit. Some neuroscientist have adopted the basic emotions idea, and have proposed specific circuits for different basic emotions (Panksepp, 1980, 1998, 2005), though the basic emotions discussed do not completely correspond with those proposed in the psychological theories.
The above discussion of the amygdala and its role in fear and defense might be construed as a mini-version of basic emotions theory, a version focused on one basic emotion. However, there is a fundamental difference between the approach I take and the approach of basic emotions theorists.
The goal of basic emotions theories is to understand subjective states of conscious experience that humans label with emotion words (fear, love, sadness, joy, etc). Their goal is to understand “feelings.” This is also true of brain science theories of emotion focused on basic emotions. Panksepp (1980, 1998, 2005), for example, searches for brain systems in animals that underlie feelings in the animals as a way of understanding the brain systems that underlie human feelings. Vocalizations that result from tickling a rat are ways of indexing joyful or pleasurable feelings in the rat brain, and freezing, flight and fight behaviors are markers of fearful feelings.
The approach I take is quite different (LeDoux, 1984, 1996, 2002, 2008). I use emotional behavior as a means of indexing circuits that have evolved to allow organisms to deal with challenges and opportunities in their environments. I make no assumption about what an animal is feeling, since I believe it is not possible to scientifically measure, and thus not possible to research, feelings in animals other than humans. I do not deny that other animals may have feelings. I simply question whether these can be studied using scientific methods. Beyond this methodological barrier, I am also critical of attempts to equate feelings in humans and other animals for other reasons. First, most studies that have explored conscious experience in humans have found that when information (including emotional information) reaches awareness the dorsolateral prefrontal cortex is active, and if information is experimentally prevented from reaching awareness this area is not active (for summary see LeDoux, 2008). The dorsolateral granular prefrontal cortex is a unique primate specialization (Preuss, 1995; Wise, 2008) and has features in the human brain that are lacking in other primates (Semendeferi et al., 2011). If human conscious experience depends on these unique features of brain organization, we should be cautious about attributing the kinds of mental states made possible by these features to animals that lack the feature or the brain region. Second, language is a unique human capacity, and conscious experience, including emotional experience, is influenced by language. The once disputed idea that language, and the cognitive processes required to support language functions, add complexity to human experience, has regained respect (Lakoff, 1987; Lucy, 1997). In the absence of language experience cannot be partitioned in the same way-- English speakers can partition fear and anxiety into more than 30 categories (Marks, 1987). The diversity with which non-verbal organisms can conceptualize the world and their experiences in it is thus likely constricted by the absence of language.
Conclusion
In sum, basic tendencies to detect and respond to significant events are present in the simplest single cell organisms, and persist throughout all invertebrates and vertebrates. Within vertebrates, the overall brain plan is highly conserved, though differences in size and complexity also exist. The forebrain differs the most between mammals and other vertebrates, though the old notion that the evolution of mammals led to radical changes such that new forebrain structures were added has not held up. Thus, the idea that mammalian evolution is characterized by the addition of a limbic system (devoted to emotion) and a neocortex (devoted to cognition) is flawed. Modern efforts to understand the brain mechanisms of emotion have made more progress by focusing on specific emotion systems, like the fear or defense system, rather than on efforts to find a single brain system devoted to emotion. Also, progress has been made in animal studies by focusing on emotion in terms of brain circuits that contribute to behaviors related to survival functions. Efforts to use scientific methods identify circuits in animals that might correspond to circuits in the human brain that are responsible for conscious feelings are not likely to succeed since we have no way of scientifically measuring feelings in animals. Conscious feelings are an important topic, but one that is best pursued through studies of humans.
Fig 1. Pavlovian Fear and Avoidance Conditioning Circuits.
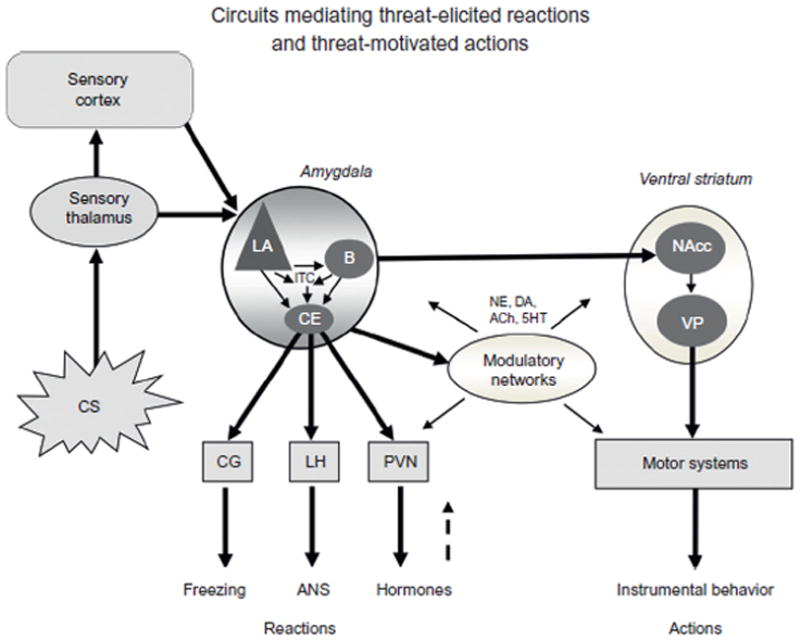
Fear is the emotion most thoroughly understood in the brain. Much of the progress made has involved studies of Pavlovian fear conditioning in rats. During conditioning the conditioned stimulus (CS), usually a tone, and the unconditioned stimulus (US), usually a footschock, converge in the lateral nuclecus of the amygdala (LA) to induce synaptic plasticity of the CS inputs (CS–US convergence not shown). The CS is then able to flow through amygdala circuits to the central nucleus (CE) to control the expression of hard-wired, automatic, defensive reactions (freezing behavior, autonomin nervous system, ANS, activity, and hormonal release). CE outputs also activate networks in that control the release of neuromodulators, such as norepinephrine (NE), dopamine (DA), acetylcholine (ACh), and serotonin (5HT) throughtout the brain. These, like hormonal feedback, help add intensity to and prolong the duration of the aroused state. In addition to these various automatic responses controlled by CE, the LA also sends information, via the basal nucleus (B) to the ventral striatum, especially the nucleus accumbens. The latter connections are likely to be involved in the invigoration of goal-directed behaviors that allow the organism to act in certain ways on the basis of past instrumental learning or on-the-spot decisions about how to cope with the threat. Other abbreviations: ITC, intercalated nuclei of the amydala; CG, central gray; LH, lateral hypothalamus; PVN, paraventricular nucleus of the hypothalamus; VP, ventral pallidum.
References
- Adolphs R. Fear, faces, and the human amygdala. Current Opinion in Neurobiology. 2008;18:166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss, Inc; 1992. pp. 1–66. [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nature Neuroscience. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- Ariëns Kappers CU, Huber CG, Crosby EC. The Comparative Anatomy of the Nervous System of Vertebrates, Including Man. New York: Macmillan Company; 1936. [Google Scholar]
- Bach DR, Talmi D, Hurlemann R, Patin A, Dolan RJ. Automatic relevance detection in the absence of a functional amygdala. Neuropsychologia. 2011;49:1302–1305. doi: 10.1016/j.neuropsychologia.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. European Journal of Neuroscience. 2003;17:1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. American Journal of Physiology. 1928;84:490–515. [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn & Memory. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behavioral and Brain Sciences. 1980;3:291–323. [Google Scholar]
- Bruce LL, Neary TJ. The limbic system of tetrapods: a comparative analysis of cortical and amygdalar populations. Brain, Behavior and Evolution. 1995;46:224–234. doi: 10.1159/000113276. [DOI] [PubMed] [Google Scholar]
- Buck R. The evolution and development of emotion expression and communication. In: Brehm SS, Kassin SM, Gibbons FX, editors. Developmental Social Psychology. New York: Oxford Univ. Press; 1981. pp. 127–151. [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. 2. Hoboken: John Wiley & Sons, Inc; 2005. [Google Scholar]
- Cain CK, Choi JS, LeDoux JE. Active Avoidance and Escape Learning. In: Koob G, Thompson R, Le Moal M, editors. Encyclopedia of Behavioral Neuroscience. New York: Elsevier; 2010. [Google Scholar]
- Cain CK, LeDoux JE. Escape from fear: a detailed behavioral analysis of two atypical responses reinforced by CS termination. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:451–463. doi: 10.1037/0097-7403.33.4.451. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Bodily changes in pain, hunger, fear, and rage. Vol. 2. New York: Appleton; 1929. [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Choi JS, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn & Memory. 2010;17:139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DH. Involvement of the avian amygdalar homologue (archistriatum posterior and mediale) in defensively conditioned heart rate change. Journal of Comparative Neurology. 1975;160:13–35. doi: 10.1002/cne.901600103. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descarte’s error: Emotion, reason, and the human brain. New York: Gosset/Putnam; 1994. [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. London: Fontana Press; 1872. [Google Scholar]
- Davies DC, Martinez-Garcia F, Lanuza E, Novejarque A. Striato-amygdaloid transition area lesions reduce the duration of tonic immobility in the lizard Podarcis hispanica. Brain Research Bulletin. 2002;57:537–541. doi: 10.1016/s0361-9230(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cerebral Cortex. 2011;20:612–621. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, LeDoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Frontiers in Behavioral Neuroscience. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Annals of the New York Academy of Sciences. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The cognitive neuroscience of memory. New York: Oxford University Press; 2002. [Google Scholar]
- Ekman P. Biological and cultural contributions to body and facial movement. In: Blacking J, editor. The Anthropology of the Body. London: Academic Press; 1977. pp. 39–84. [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus- reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Galaverna OG, Seeley RJ, Berridge KC, Grill HJ, Epstein AN, Schulkin J. Lesions of the central nucleus of the amygdala. I: Effects on taste reactivity, taste aversion learning and sodium appetite. Behavioural Brain Research. 1993;59:11–17. doi: 10.1016/0166-4328(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick CJ. The functions of the olfactory parts of the cerebral cortex. Proceedings of the National Academy of Sciences, U S A. 1933;19:7–14. doi: 10.1073/pnas.19.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Isaacson RL. The Limbic System. New York: Plenum Press; 1982. [Google Scholar]
- Izard CE. The Face of Emotion. New York: Appleton-Century-Crofts; 1971. [Google Scholar]
- Izard CE. Basic emotions, relations among emotions, and emotion-cognition relations. Psychological Review. 1992;99:561–565. doi: 10.1037/0033-295x.99.3.561. [DOI] [PubMed] [Google Scholar]
- Johansen J, Cain C, Ostroff L, LeDoux JE. Neural Mechanisms of Fear Learning and Memory. Cell. doi: 10.1016/j.cell.2011.10.009. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Klein M, Castellucci VF, Schacher S, Goelet P. Some principles emerging from the study of short- and long-term memory. Neuroscience Research. 1986;3:498–520. doi: 10.1016/0168-0102(86)90050-7. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA. Cellular neurophysiological approaches to the study of learning. Physiological Reviews. 1968;48:65–134. doi: 10.1152/physrev.1968.48.1.65. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiology & Behavior. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Evolutionary developmental biology meets the brain: the origins of mammalian cortex. Proceedings of the National Academy of Sciences, U S A. 1997;94:2800–2804. doi: 10.1073/pnas.94.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. Behavioural Brain Research. 2011. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. American Journal of Physiology. 1937;119:352–353. [Google Scholar]
- Kotter R, Meyer N. The limbic system: a review of its empirical foundation. Behavioural Brain Research. 1992;52:105–127. doi: 10.1016/s0166-4328(05)80221-9. [DOI] [PubMed] [Google Scholar]
- LaBar KS. Emotional memory functions of the human amygdala. Current Neurology and Neuroscience Reports. 2003;3:363–364. doi: 10.1007/s11910-003-0015-z. [DOI] [PubMed] [Google Scholar]
- Lakoff G. Women, Fire, and Dangerous Things: What Categories Reveal about the mind. Chicago: The University of Chicago Press; 1987. [Google Scholar]
- Lanuza E, Belekhova M, Martinez-Marcos A, Font C, Martinez-Garcia F. Identification of the reptilian basolateral amygdala: an anatomical investigation of the afferents to the posterior dorsal ventricular ridge of the lizard Podarcis hispanica. European Journal of Neuroscience. 1998;10:3517–3534. doi: 10.1046/j.1460-9568.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- Lazaro-Munoz G, LeDoux JE, Cain CK. Sidman instrumental avoidance initially depends on lateral and Basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biological Psychiatry. 2010;67:1120–1127. doi: 10.1016/j.biopsych.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Cognition and emotion: processing functions and brain systems. In: Gazzaniga MS, editor. Handbook of Cognitive Neuroscience. New York: Plenum Publishing Corp; 1984. pp. 357–368. [Google Scholar]
- LeDoux JE. Emotion. In: Plum F, editor. Handbook of Physiology. 1: The Nervous System. Vol V, Higher Functions of the Brain. Bethesda: American Physiological Society; 1987. pp. 419–460. [Google Scholar]
- LeDoux JE. Emotion and the limbic system concept. Concepts in Neuroscience. 1991;2:169–199. [Google Scholar]
- LeDoux JE. The Emotional Brain. New York: Simon and Schuster; 1996. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Synaptic Self: How our brains become who we are. New York: Viking; 2002. [Google Scholar]
- LeDoux J. The amygdala. Current Biology. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional colouration of consciousness: how feelings come about. In: Weiskrantz L, Davies M, editors. Frontiers of Consciousness: Chichele Lectures. Oxford: Oxford University Press; 2008. pp. 69–130. [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. Journal Neuroscience. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Schiller D, Cain C. Emotional Reaction and Action: From Threat Processing to Goal-Directed Behavior. In: Gazzaniga MS, editor. The Cognitive Neurosciences. 4. Cambridge: MIT Press; 2009. pp. 905–924. [Google Scholar]
- Lucy JA. Linguistic Relativity. Annual Review of Anthropology. 1997;26:291–312. [Google Scholar]
- MacLean PD. Psychosomatic disease and the “visceral brain”: recent developments bearing on the Papez theory of emotion. Psychosomatic Medicine. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain) Electroencephalography and Clinical Neurophysiology. 1952;4:407–418. doi: 10.1016/0013-4694(52)90073-4. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain, emotion and scientific bias. In: Schmitt FO, editor. The Neurosciences: Second Study Program. New York: Rockefeller University Press; 1970. pp. 336–349. [Google Scholar]
- Macnab RM, Koshland DE., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proceedings of the National Academy of Sciences, U S A. 1972;69:2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Marks I. Fears, Phobias, and Rituals: Panic, Anxiety and Their Disorders. New York: Oxford University Press; 1987. [Google Scholar]
- Martinez-Garcia F, Martinez-Marcos A, Lanuza E. The pallial amygdala of amniote vertebrates: evolution of the concept, evolution of the structure. Brain Research Bulletin. 2002;57:463–469. doi: 10.1016/s0361-9230(01)00665-7. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. Journal of Neuroscience. 2007;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno N, Gonzalez A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. Journal of Anatomy. 2007;211:151–163. doi: 10.1111/j.1469-7580.2007.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Karten HJ. A general profile of the vertebrate brain, with sidelights on the ancestry of cerebral cortex. In: Schmitt FO, editor. The Neurosciences: Second Study Program. New York: The Rockefeller University Press; 1970. pp. 7–26. [Google Scholar]
- Nishijo H, Hori E, Tazumi T, Ono T. Neural correlates to both emotion and cognitive functions in the monkey amygdala. Behavioural Brain Research. 2008;188:14–23. doi: 10.1016/j.bbr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Kaas JH. The emergence and evolution of mammalian neocortex. Trends in Neurosciences. 1995;18:373–379. doi: 10.1016/0166-2236(95)93932-n. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, Andreassen OA. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience. 2008;156:450–455. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Hypothalamic integration of behavior: rewards, punishments, and related psychological processes. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus. Vol. 3, Behavioral Studies of the Hypothalamus. New York: Marcel Dekker; 1980. pp. 289–431. [Google Scholar]
- Panksepp J. Affective Neuroscience. New York: Oxford U. Press; 1998. [Google Scholar]
- Panksepp J. Affective consciousness: Core emotional feelings in animals and humans. Consciousness and Cognition. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of Neurology and Psychiatry. 1937;79:217–224. [Google Scholar]
- Pfaff D. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. Journal of Endocrinology. 2005;184:447–453. doi: 10.1677/joe.1.05897. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neuroscience. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Plutchik R. Emotion: A Psychoevolutionary Synthesis. New York: Harper & Row; 1980. [Google Scholar]
- Preuss TM. Do rats have a prefrontal cortex? The Rose-Woolsey-Akerty Program Reconsidered. J Cog Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neuroscience. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF, Luthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Annals of the New York Academy of Sciences. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. Annals of the New York Academy of Sciences. 2007;1121:336–354. doi: 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter MF, Markowitsch HJ. Involvement of the amygdala in learning and memory: a critical review, with emphasis on anatomical relations. Behavioral Neuroscience. 1985;99:342–380. doi: 10.1037//0735-7044.99.2.342. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends in Neuroscience. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, Travis K, Buckwalter J. Spatial Organization of neurons in the frontal pole sets humans apart from great apes. Cerebral Cortex. 2010;21:1485–97. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- Shepherd G. Neurobiology. New York: Oxford; 1983. [Google Scholar]
- Siegel A, Edinger H. Neural control of aggression and rage behavior. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus, Vol. 3, Behavioral Studies of the Hypothalamus. New York: Marcel Dekker; 1981. pp. 203–240. [Google Scholar]
- Smith GE. The Evolution of Man. Oxford University Press; Oxford: 1924. [Google Scholar]
- Squire L. Memory and Brain. New York: Oxford; 1987. [Google Scholar]
- Swanson LW. The hippocampus and the concept of the limbic system. In: Seifert W, editor. Neurobiology of the Hippocampus. London: Academic Press; 1983. pp. 3–19. [Google Scholar]
- Swanson LW. Brain Architecture: Understanding the Basic Plan. Oxford: Oxford University Press; 2002. [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Tischler MD, Davis M. A visual pathway that mediates fear-conditioned enhancement of acoustic startle. Brain Research. 1983;276:55–71. doi: 10.1016/0006-8993(83)90548-6. [DOI] [PubMed] [Google Scholar]
- Tomkins SS. Affect, Imagery, Consciousness. New York: Springer; 1962. [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Weiss S. Forward frontal fields: phylogeny and fundamental function. Trends in Neuroscience. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA. The human amgydala. New York: Guilford Press; 2009. [Google Scholar]


