Abstract
Context:
Quadriceps dysfunction is a common consequence of knee joint injury and disease, yet its causes remain elusive.
Objective:
To determine the effects of pain on quadriceps strength and activation and to learn if simultaneous pain and knee joint effusion affect the magnitude of quadriceps dysfunction.
Design:
Crossover study.
Setting:
University research laboratory.
Patients or Other Participants:
Fourteen (8 men, 6 women; age = 23.6 ± 4.8 years, height = 170.3 ± 9.16 cm, mass = 72.9 ± 11.84 kg) healthy volunteers.
Intervention(s):
All participants were tested under 4 randomized conditions: normal knee, effused knee, painful knee, and effused and painful knee.
Main Outcome Measure(s):
Quadriceps strength (Nm/kg) and activation (central activation ratio) were assessed after each condition was induced.
Results:
Quadriceps strength and activation were highest under the normal knee condition and differed from the 3 experimental knee conditions (P < .05). No differences were noted among the 3 experimental knee conditions for either variable (P > .05).
Conclusions:
Both pain and effusion led to quadriceps dysfunction, but the interaction of the 2 stimuli did not increase the magnitude of the strength or activation deficits. Therefore, pain and effusion can be considered equally potent in eliciting quadriceps inhibition. Given that pain and effusion accompany numerous knee conditions, the prevalence of quadriceps dysfunction is likely high.
Key Words: arthrogenic muscle inhibition, central activation failure, voluntary activation, muscles
Key Points.
Knee pain and effusion resulted in arthrogenic muscle inhibition and weakness of the quadriceps.
The simultaneous presence of pain and effusion did not increase the magnitude of quadriceps dysfunction.
To reduce arthrogenic muscle inhibition and improve muscle strength, clinicians should employ interventions that target removing both pain and effusion.
Quadriceps weakness is a common consequence of traumatic knee joint injury1,2 and chronic degenerative knee joint conditions.3,4 Arthrogenic muscle inhibition (AMI), a neurologic decline in muscle activation, results in quadriceps weakness and hinders rehabilitation by preventing gains in strength.5 The inability to reverse AMI and restore muscle function can lead to decreased physical abilities,6 biomechanical deficits,7 and possibly reinjury.5 Furthermore, researchers8,9 have suggested that quadriceps weakness resulting from AMI may place patients at risk for developing osteoarthritis in the knee. In light of the substantial influence of quadriceps AMI on these clinically relevant outcomes, we need to improve our understanding of the factors that contribute to this neurologic decline in muscle activity so efforts to target and reverse it can be implemented and gains in strength can be achieved more easily.
Joint injury and disease are accompanied by numerous sequelae (ie, pain, swelling, tissue damage, inflammation), so ascertaining which one ultimately leads to neurologic muscle dysfunction is difficult. Whereas a joint effusion can result in AMI,10–12 the effects of pain are less understood despite many clinicians attributing AMI to pain. Using techniques that introduce knee pain without accompanying injury may provide insights into the role of pain in eliciting AMI.
The degree of knee joint damage may play a role in the quantity of AMI that manifests. Hurley et al13,14 demonstrated that quadriceps AMI, measured using an interpolated-twitch technique, was greater in patients with extensive traumatic knee injury (eg, fractured tibial plateau, ruptured medial collateral ligament, and medial meniscectomy) than patients with isolated joint trauma (ie, isolated anterior cruciate ligament [ACL] rupture). Similarly, patients with more knee joint symptoms (ie, greater number of symptoms and increased severity of symptoms) may present with greater magnitudes of quadriceps inhibition. Recently, investigators15 have suggested that patients with more pain display less quadriceps strength, supporting this tenet. Given that effusion and pain often present simultaneously with joint injuries and diseases, such as ACL injury and osteoarthritis, examining both the isolated and cumulative effects of these sequelae appears warranted to determine if they influence the magnitude of muscle inhibition.
Experimental joint-effusion and pain models are safe and effective experimental methods that allow for the isolated examination of their effects on muscle function. The effusion model, whereby sterile saline is injected directly into the knee joint capsule,7 produces a clinically relevant magnitude of the joint effusion that may be present with traumatic injury. Effusion is thought to activate group II afferents responding to stretch or pressure,16–18 which in turn may facilitate group Ib interneurons and result in quadriceps AMI.5 The pain model involves injecting hypertonic saline into the infrapatellar fat pad to produce anteromedial knee pain similar to that described in patients with patellofemoral pain syndrome.19 Pain is considered to initiate AMI through activation of group III and IV afferents that act as nocioceptors to signal damage or potential damage to joint structures.16–18 The firing of these afferents then may lead to facilitation of group Ib interneurons, the flexion reflex, or the gamma loop, ultimately resulting in quadriceps inhibition.20 Thus, these models allow us to create symptoms that are associated with knee injury and have the added benefit of providing a way to examine their effects in isolation.
Therefore, the purpose of our study was to determine the effects of pain on quadriceps strength and activation and to learn if simultaneous pain and knee joint effusion would affect the magnitude of quadriceps dysfunction. We hypothesized that pain alone would result in quadriceps inhibition and that the magnitude of inhibition would be greater when effusion and pain were present simultaneously.
METHODS
Participants
Fifteen healthy individuals originally volunteered to participate, but after receiving a knee-effusion injection, 1 participant fainted and subsequently was removed from the study at the discretion of the investigators. Therefore, 14 healthy participants (8 men, 6 women; age = 23.6 ± 4.8 years, height = 170.3 ± 9.16 cm, mass = 72.9 ± 11.84 kg) were included in the study. Participants were excluded if they had a history of knee injury or surgery, had knee pain at the time of the study, had an allergy to lidocaine, had any orthopaedic or rheumatologic disorder that affected the lower extremity, or reported being pregnant. All participants provided written informed consent, and the study was approved by the Institutional Review Boards of the University of Michigan Medical School. We recorded the age, height, weight, and dominant lower extremity of the participants. The dominant lower extremity was defined as the limb with which the participant would kick a ball.21 Activity level at the time of the study was not documented, but participants were instructed to refrain from any training or physical activity for 24 hours before reporting for testing.
Testing Procedures
We quantified the quadriceps strength and activation of all participants under 4 randomized conditions: normal knee, effused knee, painful knee, and effused and painful knee. For the normal knee condition, we quantified strength and activation without manipulating the knee joint, whereas for the other conditions, we quantified quadriceps strength and activation after the induction of an experimental knee joint effusion (effused knee), experimental knee pain (painful knee), or experimental knee effusion and pain (effused and painful knee). Furthermore, to assess the reliability of our dependent measures between days, baseline or normal knee testing occurred each day before any injections. Each of the 4 conditions was tested on a separate day, and testing sessions were separated by 5 to 7 days. Randomization was completed using an online research randomization Web site (http://www.randomizer.com). The investigators were not blinded to condition, and order allocation was not concealed. Data collection occurred around the same time of day (morning, afternoon, evening) for each condition for every participant.
Quadriceps Strength and Activation Procedures
Participants were seated on an isokinetic dynamometer (Biodex System 3; Biodex Medical Systems, Inc, Shirley, NY) with their hips and knees flexed to 90° and their backs supported. Their dominant lower extremities were secured to the arm of the dynamometer with straps at both the thighs and ankles, and their trunks were fixed to the chair with hook-and-loop straps. Self-adhesive, 5-cm × 9-cm electrodes (Dura-Stick II; Chattanooga Group, Hixson, TN) were placed proximally over the rectus femoris and distally over the vastus medialis to deliver the stimuli for quadriceps activation testing.
On each testing day and before any injections, participants were instructed to perform 3 10-second maximal voluntary isometric contractions (MVICs) for knee extension to familiarize them with the task. During all MVICs, an investigator (M.V.) provided constant spoken encouragement to promote the participant to extend the knee as hard as he or she could. After the practice trials, 1 of the 4 conditions was induced. In the case of the normal knee condition, participants rested for 5 minutes and then performed the remaining MVICs for knee extension.
After the experimental condition was achieved, participants were instructed to perform 3 additional MVICs for knee extension. These contractions were initiated within 2 minutes after the experimental condition was induced. In addition to spoken feedback from the investigators, visual feedback was provided whereby they were encouraged to reach a target line on a computer screen that was set to a torque value 10% above that of their MVICs recorded during the familiarization trials. 22 A 2-minute rest was provided between contractions to minimize the effects of fatigue. The torque signal generated from the dynamometer was exported to a separate data-acquisition unit (MP100; BIOPAC Systems, Inc, Goleta, CA) for real-time data acquisition. The average torque value calculated over the 3 repetitions was normalized to the participant's body mass (kg) and used to quantify quadriceps strength (Nm/kg).
During the performance of the MVICs, we also assessed quadriceps activation, which is a measure used to quantify AMI. Using the burst-superimposition technique, we delivered a supramaximal electrical stimulus (GRASS S88 and SIU8T; Astro-Med, Inc, West Warwick, RI) with a train of 100 pulses per second, pulse duration of 600 milliseconds, train duration of 100 milliseconds, and maximal voltage of 130 V23,24 to the participants while they performed the previously described MVICs for knee extension. The central activation ratio (CAR) was calculated for each repetition using the following equation:
where MVIC torque was the peak torque recorded before the delivery of the electrical stimulus, and superimposed-burst torque was the maximal torque value elicited via the electrical stimulus. A CAR equal to 1.0 represents maximal voluntary activation, but a CAR equal to 0.95 represents complete or normal activation.25 The average CAR over the 3 repetitions was used to quantify quadriceps AMI.
Experimental Knee-Effusion Procedures
To induce the experimental knee-joint effusion, the area superolateral to the patella of the dominant lower extremity was cleaned with alcohol and povidone-iodine. All participants' lower extremities were placed in extension while they lay supine in the dynamometer chair, which was fully reclined. We used a sterile syringe with a 25-gauge, 1.5-inch needle to inject 3 mL of 1% lidocaine subcutaneously to anesthetize the skin. After the lidocaine was released from the syringe, the needle was guided into the knee joint capsule, a 60-mL syringe was attached, and 60 mL of sterile saline was injected into the subcapsular synovial cavity.7,26 After the injection, we performed a sweep test to confirm the saline was in the knee joint capsule. All injections were performed by the same investigator (B.D.), a certified physician assistant.
Experimental Knee-Pain Procedures
To induce experimental knee pain, participants were positioned as described for the knee-effusion injection. An area inferior and medial to the patella was cleaned with alcohol and povidone-iodine. We used a sterile syringe with a 25-gauge, 1.5-inch needle to inject 0.3 mL of 5% hypertonic saline into the medial infrapatellar fat pad. After being injected with the hypertonic saline, participants were instructed verbally to rate their pain on a scale of 0 (no pain) to 10 (worst pain). Participants who rated their pain as 5 or more did not receive a second injection of hypertonic saline. Participants who rated their pain as less than 5 on the scale were injected with another 0.3-mL bolus of 5% hypertonic saline. Nine of 14 volunteers required the second injection of hypertonic saline. The described procedures were similar to those used in previous investigations.19,27,28 Participants were not informed in advance of the criterion used to determine the need for administration of a second injection.
For the effused and painful condition, we followed the procedures described for both conditions. The injection for effusion always preceded the injection for pain because the pain resolved more quickly than the effusion.
Pain Ratings
After each experimental condition, participants were instructed to complete a short-form McGill Pain Questionnaire. The visual analog scale (10-cm line) included on this form was used to estimate the overall intensity of pain participants experienced in their knees due to the injection or injections. The pain rating was taken approximately 1 minute after the injection, which was before the completion of the quadriceps strength and activation assessment.
Statistical Analysis
We used 2 separate 1 × 4 repeated-measures analyses of variance to compare quadriceps strength and activation across the 4 conditions. Similarly, we used a repeated-measures analysis of variance to compare the pain ratings across the 4 conditions. Bonferroni multiple-comparisons procedures were employed post hoc. The α level was set at equal to or less than .05 for all tests. Effect sizes (95% confidence intervals [CIs]) were quantified for each condition between the normal knee condition and the experimental knee condition (effused, painful, or effused and painful) using the Cohen d ([group mean normal − group mean at experimental condition]/the pooled standard deviation). To establish between-sessions reliability of our dependent measurements, intraclass correlation coefficients (ICC) (2,1) ± standard error of the mean were calculated using the torque and CAR data recorded at each session before the delivery of any injections.
RESULTS
Effect sizes and their 95% CIs for quadriceps strength and activation are presented in Table 1. The between-sessions reliability for our measurements was high (MVIC = 0.924 ± 0.192; CAR = 0.91 ± 0.027), suggesting that our comparison across days can be considered with confidence.
Table 1. .
Effect Sizes (95% Confidence Intervals) for Quadriceps Strength and Activation Between the Normal Knee Condition and Each Experimental Knee Condition
| Measure |
Knee Condition |
||
| Effused |
Painful |
Effused and Painful |
|
| Torque | 0.49 (−0.28, 1.22) | 0.46 (−0.30, 1.20) | 0.71 (−0.05, 1.47) |
| Central activation ratio | 0.64 (−0.29, 1.21) | 0.48 (−0.14, 1.38) | 0.73 (−0.06, 1.47) |
| Pain rating | −1.32 (−2.09, −0.47) | −3.05 (−4.03, −1.89) | −2.92 (−3.89, −1.79) |
Strength and Activation
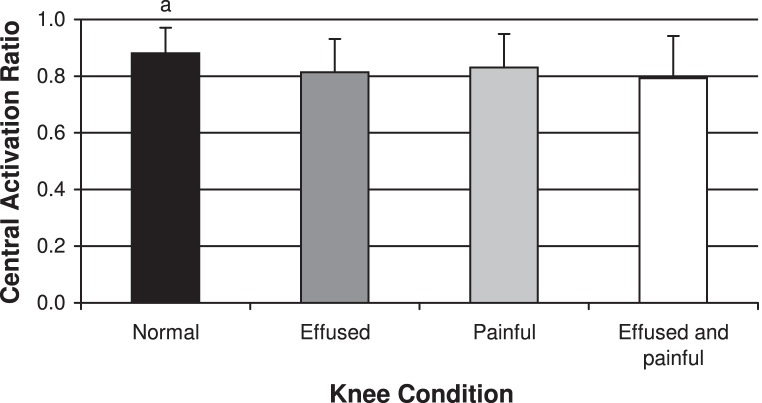
We noted differences between conditions for both quadriceps strength (F3,39 = 7.56, P < .001) and activation (F3,39 = 6.21, P = .001; Figures 1 and 2). The quadriceps strength recorded during the normal knee condition (2.49 ± 0.70 Nm/kg) differed from the other 3 knee conditions (effused: 2.16 ± 0.69 Nm/kg, P = .04; painful: 2.15 ± 0.71, P = .01; effused and painful: 1.96 ± 0.77, P = .009) and was greatest under the normal knee condition. Similarly, the CAR was highest under the normal knee condition (0.88 ± 0.09) and differed from the 3 experimental knee conditions (effused: 0.81 ± 0.11, P = .01; painful: 0.83 ± 0.11, P = .03; effused and painful: 0.79 ± 0.11, P = .02). We did not note differences among the 3 experimental knee conditions for either quadriceps strength or activation (P > .05).
Figure 1. .
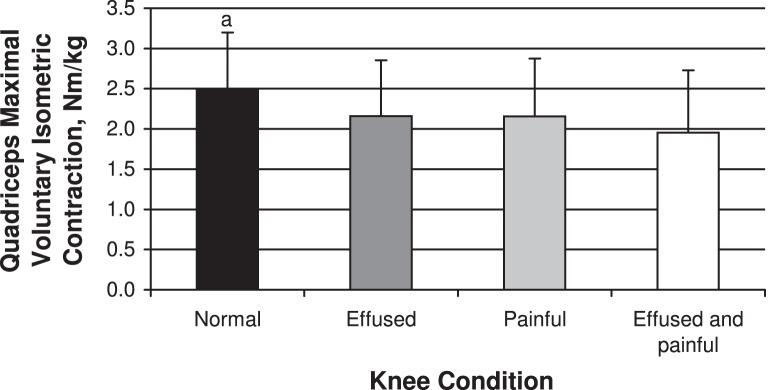
Quadriceps peak torque for each knee condition. a Indicates difference from the other 3 knee conditions (P < .05).
Figure 2. .
Quadriceps central activation ratio (CAR) for each knee conditions. a Indicates difference from the other 3 knee conditions (P < .05).
Pain
We found differences between conditions for intensity of pain (F3,39 = 35.16, P < .001; Table 2). The intensity of pain was lower under the normal knee condition than under the 3 experimental knee conditions (effused: P = .02; painful: P < .001; effused and painful: P < .001). The intensity of pain was greater during the painful condition than the effused condition (P = .005) but not the effused and painful condition (P = .98). In addition, the intensity of pain was lower during the effused condition than the effused and painful condition (P = .001).
Table 2. .
Overall Pain Rating for Each Conditiona
| Knee Condition |
Mean ± SD |
| Normal | 0.00 ± 0.00 |
| Effused | 2.17 ± 2.33b |
| Painful | 5.22 ± 2.42c |
| Effused and painful | 5.46 ± 2.64c |
0 = no pain, 10 = worst pain.
Indicates different from the normal knee condition only.
Indicates different from the normal and effused knee conditions.
DISCUSSION
We used experimental knee pain and effusion models to examine the effects that pain and effusion may have on quadriceps muscle strength and activation. Both the effused knee and painful knee groups demonstrated quadriceps muscle dysfunction, but the amounts of quadriceps activation and strength deficits were not magnified when these 2 stimuli were present simultaneously.
Reports are conflicting about whether the presence of pain results in quadriceps dysfunction. Shakespeare29 noted that quadriceps inhibition can occur in the absence of perceived pain in patients after meniscectomy, whereas Arvidsson et al30 found that reducing pain via epidural injections of lidocaine can increase quadriceps electromyographic activity in patients after ACL reconstruction. Similarly, pain has been shown to be both related31 and unrelated32 to quadriceps strength and activation in patients undergoing total knee arthroplasty. Our study was different from those earlier reports because we examined a cause-and-effect relationship between pain and quadriceps activation and strength. We showed that moderate amounts of pain created a small magnitude of quadriceps AMI (5.7% change from the normal knee condition) and also resulted in a decline in quadriceps strength (13.7% change from the normal knee condition). Henriksen et al15 noted decrements in isometric and isokinetic knee-extension strength ranging from 5% to 15% after the induction of experimental knee pain, which was consistent with our data. Considering that pain accompanies numerous knee joint injuries and conditions, the prevalence of AMI and quadriceps strength deficits with joint trauma is likely high. Our finding that the magnitude of inhibition resulting from pain was not large agrees with data suggesting that pain contributes to a small but substantial portion of the AMI present after total knee arthroplasty.31 Notably, the relationship between pain and AMI may be mediated by the severity of the pain experienced, but that connection requires future study. Knee-extension strength has been positively correlated with pain intensity, so we would hypothesize a similar relation between AMI and pain.15
Contrary to our hypothesis, the interaction of pain and effusion did not result in different magnitudes of AMI (change from the normal knee condition was 5.7% in the painful condition, 7.6% in the effused condition, and 10% in the effused and painful condition) or quadriceps strength declines (change from the normal knee condition was 13.7% in the painful condition, 13.6% in the effused condition, and 21.8% in the effused and painful condition), suggesting that the 2 stimuli did not have an additive effect. Given that severity of injury can influence the degree of AMI,13 we expected that a more noxious stimuli provided to the knee would increase quadriceps AMI and decrease quadriceps strength. Although the pain and effusion in our study were both experimentally induced and may account for the lack of difference, the degree of pain (5/10 on a visual analog scale) and the size of the effusion (60-mL joint effusion) were moderate. Thus, we suggest our findings have meaning for clinical populations with knee injury. The lack of difference between the effused and painful condition and each of the other 2 conditions also could be attributed to a lack of statistical power. However, the effects were small when we examined effect sizes for comparisons between the effused and painful condition and the painful condition (CAR = 0.16, MVIC = 0.27) and the effused and painful condition and the effused condition (CAR = 0.28, MVIC = 0.27). Therefore, based on our results, we suggest that pain and effusion have no additive effect on quadriceps strength and activation. Unexpectedly, our results indicated that our participants experienced some pain during the effused condition, but this level of pain was less than that during the painful and the effused and painful conditions. Researchers33 using the effusion model have indicated that the effusion is painless, and we were anticipating similar outcomes. Group III and IV afferents (nocioceptors) have been found to respond to local mechanical stimulation of the joint, and their stimulation may have contributed to this outcome.34 Thus, the presence of pain in both the effused and painful condition and the effused condition may help explain why quadriceps strength and activation did not differ between these groups.
Although not a main purpose of our study, knee joint effusion led to declines in quadriceps strength and activation. This result was not novel; many investigators10–12,33 have noted that effusion leads to AMI. However, AMI in these previous investigations was quantified using the H reflex. We quantified AMI in our study using the CAR, which was recorded while volunteers performed muscle contraction, rather than the H reflex, which is measured under static conditions. Thus, the finding that effusion leads to quadriceps inhibition during a quadriceps contraction is worth highlighting.
Our participants presented with an average CAR of 0.89, which is lower than the 0.95 considered to be complete activation for healthy adults.25 Therefore, before experimentally altering their knees, some of our participants would be considered to have incomplete quadriceps activation. The reason behind this is unclear, but it could be due to not truly completing a maximal quadriceps isometric contraction during testing. We encouraged maximal contraction by providing both visual and oral feedback during testing and thus had to assume participants were completing the MVICs to the best of their abilities. Given that we used a crossover design and all participants completed all conditions, we suggest our results comparing the conditions are not hindered. Although not reported as part of this study, we collected data for each participant in a normal knee condition each day before inducing any experimental condition and found that the baselines were not different from each other (P = .96 for quadriceps CAR). Thus, whereas the magnitude of CAR of the normal knee was lower than expected for healthy adults, we are confident that the change resulting from the effusion or pain or both models was accurate.
The computed effect sizes listed in Table 1 suggest that the injections resulting in pain or effusion or both led to small to moderate changes in quadriceps torque and CAR when compared with the normal knee condition. These findings led us to conclude that pain or effusion or both resulted in statistical differences and potentially clinically meaningful differences in our dependent measures. However, the wide 95% CIs suggest that we cannot rule out a large increase or decrease in torque or CAR due to the injections. This wide variation likely can be attributed to our small sample size. In future investigations, using a larger sample may be necessary to definitively conclude the effects of pain or effusion or both on quadriceps torque and the CAR.
A limitation of our study was that pain was only quantified directly after the experimental conditions were induced. Pain levels likely decreased from when the pain rating was quantified to the time when the last MVIC took place. Researchers19 using methods to induce pain similar to those we described found knee pain peaked (5.8/11 on a visual analog scale) approximately 3 minutes after injection of hypertonic saline and declined (2/11 on a visual analog scale) approximately 10 minutes postinjection. Given that testing took about 10 minutes to complete from the time of the injection to the last MVIC, the pain rating likely had declined by the time we recorded the last MVIC or CAR, which may have influenced our results. However, our findings still illustrate that the pain produced by the model employed resulted in quadriceps weakness and activation failure despite a possible decline in the magnitude of pain throughout testing. Further research is needed to determine if the magnitude of pain influences the magnitude of deficits in quadriceps strength and activation.
CONCLUSIONS
Knee pain and effusion resulted in quadriceps AMI and weakness. However, the simultaneous presentation of pain and effusion did not appear to increase the magnitude of quadriceps dysfunction. Based on our results, we conclude that clinicians who want to reduce AMI and improve muscle strength should employ interventions that target the removal of both pain and effusion.
REFERENCES
- 1.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3):405–424. doi: 10.1016/j.csm.2008.02.001. vii–ix. [DOI] [PubMed] [Google Scholar]
- 2.Hurley MV. Muscle dysfunction and effective rehabilitation of knee osteoarthritis: what we know and what we need to find out. Arthritis Rheum. 2003;49(3):444–452. doi: 10.1002/art.11053. [DOI] [PubMed] [Google Scholar]
- 3.Palmieri-Smith RM, Thomas AC, Karvonen-Gutierrez C, Sowers MF. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89(7):541–548. doi: 10.1097/PHM.0b013e3181ddd5c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56(11):641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9(2):135–159. [Google Scholar]
- 6.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52(4):258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmieri-Smith RM, Kreinbrink J, Ashton-Miller JA, Wojtys EM. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35(8):1269–1275. doi: 10.1177/0363546506296417. [DOI] [PubMed] [Google Scholar]
- 8.Suter E, Herzog W. Does muscle inhibition after knee injury increase the risk of osteoarthritis? Exerc Sport Sci Rev. 2000;28(1):15–18. [PubMed] [Google Scholar]
- 9.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 10.Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65(4):171–177. [PubMed] [Google Scholar]
- 11.Hopkins JT. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001;33(1):123–126. doi: 10.1097/00005768-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri RM, Ingersoll CD, Edwards JE et al. Arthrogenic muscle inhibition is not present in the limb contralateral to a simulated knee joint effusion. Am J Phys Med Rehabil. 2003;82(12):910–916. doi: 10.1097/01.PHM.0000098045.04883.02. [DOI] [PubMed] [Google Scholar]
- 13.Hurley MV, Jones DW, Newham DJ. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Lond) 1994;86(3):305–310. doi: 10.1042/cs0860305. [DOI] [PubMed] [Google Scholar]
- 14.Hurley MV, Jones DW, Wilson D, Newham DJ. Rehabilitation of quadriceps inhibition due to isolated rupture of the anterior cruciate ligament. J Orthop Rheumatol. 1992;5:145–154. [Google Scholar]
- 15.Henriksen M, Rosager S, Aaboe J, Graven-Nielsen T, Bliddal H. Experimental knee pain reduces muscle strength. J Pain. 2011;12(4):460–467. doi: 10.1016/j.jpain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Halata Z, Rettig T, Schulze W. The ultrastructure of sensory nerve endings in the human knee joint capsule. Anat Embryol (Berl) 1985;172(3):265–275. doi: 10.1007/BF00318974. [DOI] [PubMed] [Google Scholar]
- 17.Heppelmann B. Anatomy and histology of joint innervation. J Peripher Nerv Syst. 1997;2(1):5–16. [PubMed] [Google Scholar]
- 18.Grigg P. Properties of sensory neurons innervating synovial joints. Cells Tissues Organs. 2001;169(3):218–225. doi: 10.1159/000047885. [DOI] [PubMed] [Google Scholar]
- 19.Bennell K, Hodges P, Mellor R, Bexander C, Souvlis T. The nature of anterior knee pain following injection of hypertonic saline into the infrapatellar fat pad. J Orthop Res. 2004;22(1):116–121. doi: 10.1016/S0736-0266(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 20.Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40(3):250–266. doi: 10.1016/j.semarthrit.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Peters M. Footedness: asymmetries in foot preference and skill and neuropsychological assessment of foot movement. Psychol Bull. 1988;103(2):179–192. doi: 10.1037/0033-2909.103.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan C, Williams GN. Evoked tetanic torque and activation level explain strength differences by side. Eur J Appl Physiol. 2009;106(5):769–774. doi: 10.1007/s00421-009-1057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22(1):110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83(4):359–365. [PubMed] [Google Scholar]
- 25.Farquhar SJ, Chmielewski TL, Snyder-Mackler L. Accuracy of predicting maximal quadriceps force from submaximal effort contractions after anterior cruciate ligament injury. Muscle Nerve. 2005;32(4):500–505. doi: 10.1002/mus.20366. [DOI] [PubMed] [Google Scholar]
- 26.Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84(9):1522–1527. doi: 10.2106/00004623-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Bennell K, Wee E, Crossley K, Stillman B, Hodges P. Effects of experimentally-induced anterior knee pain on knee joint position sense in healthy individuals. J Orthop Res. 2005;23(1):46–53. doi: 10.1016/j.orthres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Bennell KL, Hinman RS. Effect of experimentally induced knee pain on standing balance in healthy older individuals. Rheumatology (Oxford) 2005;44(3):378–381. doi: 10.1093/rheumatology/keh493. [DOI] [PubMed] [Google Scholar]
- 29.Shakespeare DT. Reflex inhibition of the quadriceps after meniscectomy: lack of association with pain. Clin Physiol. 1985;5(2):137–144. doi: 10.1111/j.1475-097x.1985.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 30.Arvidsson I, Eriksson E, Knutsson E, Arner S. Reduction of pain inhibition on voluntary muscle activation by epidural analgesia. Orthopedics. 1986;9(10):1415–1419. doi: 10.3928/0147-7447-19861001-13. [DOI] [PubMed] [Google Scholar]
- 31.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21(5):775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 32.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty: the contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87(5):1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins JT, Ingersoll CD, Edwards JE, Klootwyk TE. Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis following knee joint effusion. J Athl Train. 2002;37(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- 34.Schaible HG, Schmidt RF. Activation of groups III and IV sensory units in medial articular nerve by local mechanical stimulation of knee joint. J Neurophysiol. 1983;49(1):35–44. doi: 10.1152/jn.1983.49.1.35. [DOI] [PubMed] [Google Scholar]