Abstract
Context:
Ankle sprains are common in athletes, with functional ankle instability (FAI) developing in approximately half of cases. The relationship between laxity and FAI has been inconclusive, suggesting that instability may be caused by insufficient sensorimotor function and dynamic restraint. Research has suggested that deafferentation of peripheral mechanoreceptors potentially causes FAI; however, direct evidence confirming peripheral sensory deficits has been elusive because previous investigators relied upon subjective proprioceptive tests.
Objective:
To develop a method for simultaneously recording peripheral sensory traffic, joint forces, and laxity and to quantify differences between healthy ankles and those with reported instability.
Design:
Case-control study.
Setting:
University laboratory.
Patients or Other Participants:
A total of 29 participants (age = 20.9 ± 2.2 years, height = 173.1 ± 8.9 cm, mass = 74.5 ± 12.7 kg) stratified as having healthy (HA, n = 19) or unstable ankles (UA, n = 10).
Intervention(s):
Sensory traffic from muscle spindle afferents in the peroneal nerve was recorded with microneurography while anterior (AP) and inversion (IE) stress was applied to ligamentous structures using an ankle arthrometer under test and sham conditions.
Main Outcome Measure(s):
Laxity (millimeters or degrees) and amplitude of sensory traffic (percentage) were determined at 0, 30, 60, 90, and 125 N of AP force and at 0, 1, 2, 3, and 4 Nm of IE torque. Two-factor repeated-measures analyses of variance were used to determine differences between groups and conditions.
Results:
No differences in laxity were observed between groups (P > .05). Afferent traffic increased with increased force and torque in test trials (P < .001). The UA group displayed decreased afferent activity at 30 N of AP force compared with the HA group (HA: 30.2% ± 9.9%, UA: 17.1% ± 16.1%, P < .05).
Conclusions:
The amplitude of sensory traffic increased simultaneously with greater ankle motion and loading, providing evidence of the integrated role of capsuloligamentous and musculotendinous mechanoreceptors in maintaining joint sensation. Unstable ankles demonstrated diminished afferent traffic at low levels of force, suggesting the early detection of joint loading may be compromised.
Key Words: functional ankle instability, microneurography, ankle arthrometry
Key Points.
Sensory traffic amplitude from muscle spindles increased during ligamentous loading of the ankle joint.
Sensory traffic amplitude in functionally unstable ankles did not increase at low levels of anterior force when compared with healthy ankles.
During inversion loading, functionally unstable ankles reached their peak sensory traffic amplitude earlier than did healthy ankles.
Ankle sprains are among the most common unintentional injuries related to sport and physical activity, accounting for approximately 15% of all collegiate sports injuries and approximately 800 000 emergency room visits per year in the United States.1,2 Reports indicate that 30% to 75% of patients with ankle sprains develop repeated sensations of giving way or “rolling”—known as functional ankle instability (FAI)—despite efforts to rehabilitate and mechanically stabilize the joint.3–5 Questions remain, however, because both injured and healthy patients may present with equivalent scores of mechanical laxity and sensory function, contrary to prevailing theories of joint stability that these deficits increase the likelihood of joint injury. Advances in technology now permit the simultaneous measurements of joint loading and laxity through arthrometry, as well as direct sensory recordings of individual mechanoreceptor populations through microneurography. The combination of these techniques may significantly advance our understanding of how these neuromechanical relationships related to joint stability differ among healthy individuals and those with repeated ankle sprains.
Some investigators4,6 originally suggested that FAI exists secondary to mechanical laxity of the lateral ligaments, because the excessive stress and strain directly damages these structures. Mechanical laxity has been investigated with a variety of tools including stress radiographs and ankle arthrometers; however, research has been inconclusive in establishing a relationship between mechanical laxity and FAI.6–8 Consequently, the joint stability paradigm includes sensory deficits that could lead to loss of neuromuscular control and, therefore, sensations of instability.9 According to this theory, laxity, whether congenital or secondary to injury, may cause sensory deficits because inadequate tension in loose capsuloligamentous tissues prevents embedded mechanoreceptors from being stimulated, such that not enough action potentials are generated to achieve conscious perception.10–12 Numerous authors13,14 have investigated this theory by testing proprioception, using joint angle-replication measures and observing patients' thresholds to detect passive motion (kinesthesia). However, across other joints, 10% to 40% of injured patients may exhibit sensory deficits yet excel functionally, whereas others are incapable of returning to their previous level of physical activity, even though mechanical laxity and sensation may be within normal limits.15,16 This evidence suggests that traditional beliefs regarding the relationship between joint laxity and proprioception may overlook essential neuromechanical factors influencing the perception of potentially injurious joint pathomechanics and the maintenance of joint stability.
Apart from the problem of joint laxity, discrete deficits in sensation and neuromuscular control have also been suggested as potential causes of FAI.4,14 This theory proposes that although the structural properties of the ligament may heal after a lateral ankle sprain, some mechanoreceptors located within the ankle may not undergo reinnervation.4 The diminished transmission of sensory signals originating from the articular mechanoreceptors would reduce proprioception and lead to alterations in neuromuscular control.11,17 However, current research has investigated these peripheral sensory signals using indirect methods. Joint angle replication, threshold to detect passive motion, and balance testing do not control for the spinal influences and cognitive abilities of the participant.12,13 Additionally, balance and muscle (peroneal) reaction time measures have been used to measure proprioception but may largely depend on visual and vestibular feedback along with neuromuscular coordination.18 The use of these indirect measures in assessing joint sensation complicates the interpretation of these results.14
Microneurography is an alternative technique that obtains real-time in vivo recordings directly from specific sensory receptors and, when combined with arthrometry, offers additional insight regarding the relationship among joint load, laxity, and mechanoreceptor function. The procedure involves insertion of a microelectrode directly into a peripheral nerve, where the summation of nerve action potentials is recorded, similar to electromyography (Figure 1).19,20 Therefore, researchers21 can observe and quantify real-time sensory events close to the peripheral source before conscious awareness arises in the brain. Although several types of afferent or efferent signals can be collected using microneurography, muscle spindle afferents (MSAs) are identifiable and contain highly relevant sensory information for understanding joint proprioception.22,23 Muscle spindles, through the fusimotor system, are responsive to changes in muscle length and the rate of change in length and, due to the muscle's arrangement across joints, may also provide accurate signals for joint position and loading.24 In addition, as reported by Johansson et al,24 articular mechanoreceptors have a potent influence on spindle discharge in cats via innervations of the muscle spindles by small gamma motor neurons.24–27 This suggests that changes in afferent feedback from capsuloligamentous mechanoreceptors may be reflected in the muscle spindle's signals, and for this reason, their sensory traffic is believed to serve as a “final common input” for sensory information to the central nervous system of joint position and movement.24 Muscle spindle activity has previously been studied using microneurography, but no authors have investigated the simultaneous response of the muscle spindle to a quantifiable joint load and position in patients complaining of ankle instability.
Figure 1. .
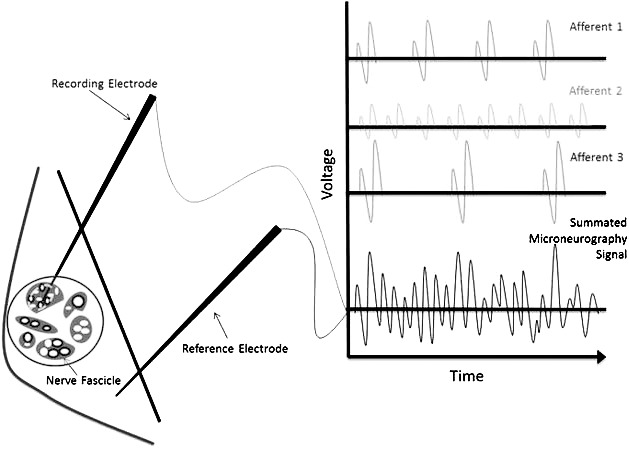
Microneurography. Schematic representation of recording electrode recording voltage differences of action potentials summated from various afferents within a recording sphere.
Contemporary scientific paradigms regarding joint stability do not fully explain existing data. More research is necessary to reconcile how joint load and laxity manifest within the nervous system and whether other receptors, such as muscle spindles, have the capacity to provide timely feedback on joint position and loading. The purpose of our study was to use microneurography to evaluate whether the progressive onset of ligamentous stress causes changes in MSA activity and to investigate potential sensory deficits that may exist in unstable ankles.
METHODS
Experimental Design
A within-subject, posttest-only control group design was used. The independent variables were group (healthy or unstable ankles), condition of the recording (test or sham), and magnitude of load on the ankle joint. The dependent variables included sensory traffic amplitude as measured via microneurography and ankle laxity as measured via arthrometry. Variability of our pilot data yielded the most conservative estimate of the sample size for this experimental study.
Participants
A total of 29 participants between the ages of 18 and 30 years (mean = 20.9 ± 2.2 years) from the university community volunteered for this study. We recruited 50 participants for this study; however, recordings were only obtained on 29 participants due to the success rate of microneurography. Participants were stratified as having a healthy ankle (HA) or an unstable ankle (UA) using the Cumberland Ankle Instability Tool28 (CAIT), as well as an Ankle Injury History Questionnaire. The HA group had a score above 27 and no complaints of the ankle giving way, and the UA group had a score below 25, in addition to complaints of recurrent ankle sprains and giving way. Volunteers with scores between 25 and 27 were excluded from this study. Group demographics are presented in Table 1. All participants were free of lower extremity injury for the past 6 months and had no history of neurologic conditions. Institutional review board approval was obtained, and informed consent was provided by all participants. If a patient had complaints of bilateral ankle instability, the ankle with the lower score on the CAIT was selected for testing.
Table 1. .
Group Demographics
| Characteristic |
Healthy Ankles |
Unstable Ankles |
t Score |
P Value |
| Number, n | 19 | 10 | ||
| Age, y | 21 ± 2.3 | 20.6 ± 2.1 | 0.48 | .65 |
| Height, cm | 172.8 ± 9.4 | 173.7 ± 8.1 | −0.28 | .78 |
| Mass, kg | 75.4 ± 13.1 | 72.8 ± 12.3 | 0.52 | .61 |
| Cumberland Ankle Instability Tool score | 29.4 ± 0.8 | 17.4 ± 5.5 | 6.85a | <.001a |
| Previous ankle sprains, n | 0.3 ± 0.6 | 3.3 ± 2.5 | −3.55a | .006a |
Significant difference (P < .05).
Procedures
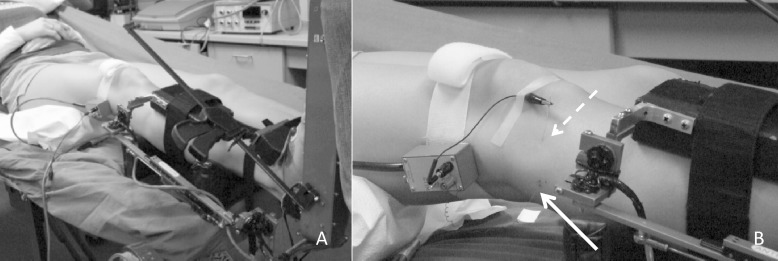
Each participant was tested once. Participants were positioned on a padded table with the trunk flexed comfortably at approximately 30° from parallel and the knee flexed to 15°. Padding was used to support the lower leg, and a strap around the distal ankle secured the lower leg while allowing free motion of the ankle joint. External electrical stimulation was applied using a wand stimulator (model S48 Stimulator; Grass Technologies, West Warwick, RI) near the fibular head to identify the location of the common peroneal nerve, which was identified as the point where a motor response of foot eversion could be observed using the lowest intensity possible. Once the location of the nerve was identified and marked with ink, a modified instrumented ankle arthrometer (Blue Bay Research Inc, Milton, FL) was affixed to the ankle to monitor position, force, and torque on the ankle. The ankle arthrometer consists of a heel clamp securing the calcaneus, a dorsal clamp securing the anterior ankle, and a tibial plate fastened to the tibial shaft (Figure 2A). The arthrometer was suspended to support the weight of the ankle and limit extraneous motion in the joint.
Figure 2. .
Setup. A, Attachment of the ankle arthrometer. B, Insertion of the microelectrodes (solid arrow, recording electrode; dashed arrow, reference electrode).
Microneurography was performed using a nerve-traffic analyzer (model 662c-3, Nerve Traffic Analyzer, University of Iowa Bioengineering, Iowa City, IA) and executed as described by Hagbarth et al.21 A recording electrode was placed in the common peroneal nerve, and a reference microelectrode was placed in the nearby skin (Figure 2B). The recording electrode was adjusted until muscle afferent activity was identified by observing the response to short and slow muscle stretches, silence during shortening of the muscle, and silence during brushing of the skin.22,29 Once sensory activity was identified, ligamentous stress was applied using the ankle arthrometer. An anterior translation stress (AP) was then applied to the ankle 3 times at a force of 125 N, followed by 3 separate inversion stresses (IE) applied at a torque of 4 Nm.30 Participants were instructed to remain relaxed throughout testing, a process that was confirmed by the investigators through real-time force, motion, and microneurography signals. A trial was discarded if there was indication of efferent activity or displacement of the microelectrode. The same examiner (A.R.N.) performed all ankle arthrometer measurements, and a separate examiner (W.B.F.) performed all microneurography recordings; all measurements were performed at a slow, controlled rate. After test recordings, the recording electrode was adjusted so that it was no longer within the nerve but still within the skin and no response to stretch or shortening was observed. Three sham AP translations and IE rotations were then applied to the ankle. Sham trials were used to confirm that observed nerve traffic was actually recordings from the muscle spindle, rather than signal noise from motion of the ankle.
Data Acquisition and Reduction
Raw data were amplified (80 000-fold), passed through a bandpass filter (700 to 2000 Hz), rectified (full), and integrated (time constant = 0.1 second) within the nerve-traffic analyzer. The signal was then synchronized with applied ankle force, torque, and laxity data (anterior displacement or inversion rotation) at 1000 Hz using the data-acquisition card within the ankle arthrometer. Custom LabVIEW software (National Instruments, Austin, TX) was used to smooth data with a 30-millisecond moving average, correct for DC offset, and identify peak nerve activity. The peak amplitudes for the trials were averaged to determine a mean peak amplitude value for that participant. This value was used for normalization of all sensory traffic data. Representative data from a single AP trial are presented in Figure 3, demonstrating a marked rise in both displacement and afferent traffic as a force is applied to the ankle. Displacement or rotation and sensory traffic amplitude at 0, 30, 60, 90, and 125 N of AP force and at 0, 1, 2, 3, and 4 Nm of IE torque were determined and used for statistical analysis.
Figure 3. .
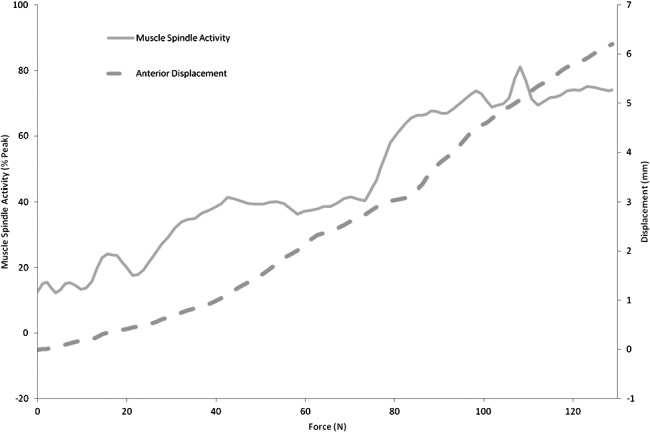
Sample nerve traffic and displacement with force application. Data from a single anterior translation trial.
Statistical Analysis
A 2-factor repeated-measures analysis of variance (ANOVA) was first used to detect differences in sensory traffic amplitude between test and sham trials across all levels of force and torque for the purpose of validating the technique. Separate 2-factor repeated-measures ANOVAs were used to detect differences in displacement, rotation, and sensory traffic amplitude between HA and UA across all levels of force and torque. When appropriate, pairwise comparisons were used to determine where differences occurred in the data. Intraclass correlation coefficients (2,1) were calculated to determine the reliability of peak displacement, rotation, and afferent traffic across an individual's trials. An α level of P = .05 was set a priori to determine statistical significance. The software program SPSS (version 16.0; SPSS Inc, Chicago, IL) was used for all data analysis.
RESULTS
The AP recordings were obtained from all 29 participants (UA = 10, HA = 19), whereas IE recordings were obtained from 22 participants (UA = 8, HA = 14). Test trials revealed an increase in sensory traffic amplitude with each increase in AP force and IE torque (P < .001; Table 2), whereas sensory traffic amplitude did not increase in sham trials until 90 to 125 N of AP force were applied. Sensory traffic during test trials was greater than during sham trials across all levels of force and torque (P < .001). Results of intraclass correlation coefficients for peak measures are presented in Table 3, with strong reliability observed for all measures.
Table 2. .
Afferent Activity as Load Increased in Test and Sham Trials
| Condition |
Sensory Traffic Amplitude, % |
|||||||||
| Force, N |
Torque, Nm |
|||||||||
| 0 |
30 |
60 |
90 |
125 |
0 |
1 |
2 |
3 |
4 |
|
| Test | 10.55 ± 7.87a | 25.70 ± 13.71a,b | 48.45 ± 13.66a,b | 66.10 ± 19.76a,b | 74.00 ± 9.04a,b | 0.80 ± 12.60 | 31.56 ± 22.42a,b | 50.53 ± 17.61a,b | 66.81 ± 19.23a,b | 71.11 ± 18.86a |
| Sham | 2.70 ± 8.11 | 2.41 ± 7.70 | 0.32 ± 6.03 | 1.90 ± 8.36 | 9.04 ± 13.26b | −0.72 ± 2.37 | 1.06 ± 5.80 | 2.97 ± 8.33 | 7.45 ± 11.69 | 5.20 ± 6.05 |
Significant difference from sham trial (P < .01).
Significant increase from previous level (P < .01).
Table 3. .
Intraclass Correlation Coefficients (ICC [2,1]) Across Trials for Peak Variables
| Dependent Variable |
ICC (2,1) |
Mean |
SEM |
| Maximum anterior displacement | 0.922 | 8.069 | 0.301 |
| Maximum inversion rotation | 0.955 | 26.913 | 0.444 |
| Peak anterior-displacement nerve signala | 0.917 | −0.767 | 0.050 |
| Peak inversion-rotation nerve signal | 0.963 | −0.699 | 0.025 |
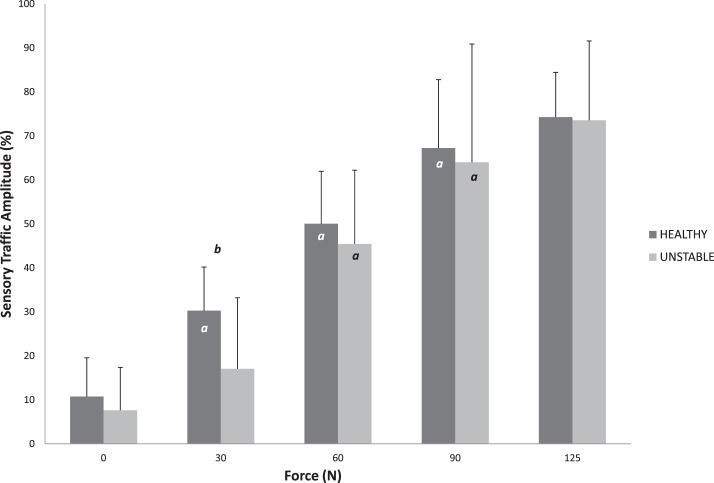
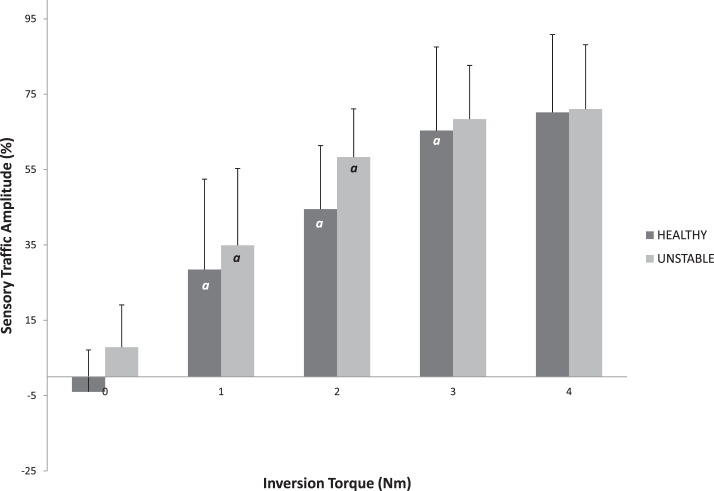
Anterior displacement (Table 4) increased with each increase in force in both the HA and UA groups (P < .001). However, no differences in displacement were noted between groups at any level of force (P > .05). Inversion rotation (Table 4) increased with each increase in torque in both the HA and UA groups (P < .001); however, no differences were observed between groups (P > .05). In HA, sensory traffic amplitude increased with increasing AP force up to 90 N (P < .01), whereas UA demonstrated increases only between 30 and 60 N of force and between 60 and 90 N of force (P < .01; Figure 4). Neither group displayed a difference in sensory traffic amplitude between 90 and 125 N of AP force (P > .05). In addition, the UA group had decreased sensory traffic at 30 N of AP force (P = .01, effect size = 0.985). For IE trials, increases in sensory traffic amplitude were observed at each level of torque up to 3 Nm in HA, whereas UA increased from 0 to 1 Nm and 1 to 2 Nm of torque (P < .05). Neither group demonstrated an increase in sensory traffic amplitude between 3 and 4 Nm of torque (P > .05; Figure 5). No sensory traffic amplitude differences in IE trials were observed between groups (P > .05).
Table 4. .
Mechanical Laxity Changes as Load Increased in Healthy and Unstable Ankles (Mean ± SD)a
| Group |
Anterior Displacement, mm |
Inversion Rotation, ° |
||||||||
| Force, N |
Torque, Nm |
|||||||||
| 0 |
30 |
60 |
90 |
125 |
0 |
1 |
2 |
3 |
4 |
|
| Healthy | 0.44 ± 0.30 | 2.09 ± 0.91 | 4.09 ± 1.45 | 6.21 ± 2.15 | 8.31 ± 2.41 | 0.36 ± 0.27 | 6.99 ± 3.30 | 14.81 ± 4.96 | 20.85 ± 6.19 | 25.46 ± 6.69 |
| Unstable | 0.30 ± 0.26 | 1.51 ± 0.77 | 3.36 ± 5.15 | 5.15 ± 1.96 | 7.24 ± 1.98 | 0.48 ± 0.41 | 6.83 ± 4.48 | 15.70 ± 6.12 | 22.06 ± 7.23 | 26.74 ± 7.99 |
Significant increase at each level of force for both groups (P < .001).
Figure 4. .
Sensory traffic as force increases in healthy and unstable ankles. a Indicates difference from previous force level (P < .05).b Indicates difference between groups (P < .05).
Figure 5. .
Sensory traffic as torque increases in healthy and unstable ankles. a Indicates difference from previous torque level (P < .05). No differences between groups were observed (P > .05).
DISCUSSION
Laboratory and clinical outcomes research suggests that in many patients, mechanical laxity may not correlate with functional joint stability or traditional sensory test scores; despite several hypothetical neural pathways, limited real-time data are available on potential compensatory mechanisms or alternative theories to explain these inconsistencies.8,15,16,31 In this study, we explored the neuromechanical relationships of joint stability using an ankle arthrometer and microneurography to simultaneously measure joint load, mechanical laxity, and sensory traffic from muscle spindles. Healthy participants and those with ankle instability demonstrated similar mechanical laxity during anterior translation and inversion rotation; however, differences were evident in the muscle spindle behavior during joint loading. Patients with ankle instability demonstrated decreased sensory traffic amplitude, compared with patients with healthy ankles, during the low-level initial application of AP force. In addition, sensory traffic from the UA group appears to plateau earlier than that from the HA group during inversion rotation. These data provide evidence that potential alterations in the fusimotor system, rather than mechanical laxity alone, are implicated in patients complaining of FAI.
Simultaneous Measurement of Load, Laxity, and Sensory Traffic
We examined a novel technique for simultaneously measuring joint load, mechanical laxity, and sensory traffic from the ankle joint. Although microneurography is a common tool in neurophysiology and cardiovascular research, the integration of these data with measures of joint load and motion has not been investigated. Combining these measurements could be a valuable tool in understanding the relationships between static restraint and sensory aberrations leading to instability. As a means of validating our technique, we compared the recordings of trials in which the recording electrode collected confirmed afferent traffic with sham trials in which the recording electrode was within the skin but not collecting data from any axons. As expected, we observed a marked rise in sensory traffic amplitude during test trials and virtually no increase in sham trials, demonstrating an increase in muscle spindle afferent activity with loading of the ankle joint and subsequent stress on the capsuloligamentous structures.
Muscle spindles are typically recognized for their role in detecting stretch within a muscle; however, they are considerably more complex, having a highly modifiable sensitivity to distinguish immediate muscle length, changes in length, and the velocity at which the muscle changes length.25 Through these functions, they have a crucial role in maintaining muscle tone and regulating reflexive contractions.26 As joint loading was applied by the arthrometer, stimulation of the muscle spindle occurred, leading to action potentials traveling along afferent axons.21,22 Higher loads increased the stimulus, causing the generation of more action potentials. The microneurography electrode detected these electrical signals as the spatial and temporal summation of sensory signals (Figure 1). With the electrode situated in the peroneal nerve, we believed that action potentials in a recording sphere of approximately 6 axons could be detected.22 Previous authors32,33 have observed that similar mechanoreceptors are organized in clusters within individual nerve fascicles. This technique is similar to electromyography, in which motor action potentials are recorded through fine wire or surface electrodes.21
Mechanical Laxity in HA and UA Groups
Previous investigators34 attempting to quantify differences in mechanical laxity have used the total ranges of anterior-posterior displacement or inversion-eversion rotation6 or have reported only maximal values. In this study, we examined group differences at several levels of force and torque. Although displacement and rotation increased at each level of force and torque, we did not observe differences between HAs and UAs, reinforcing previous evidence that suggests no differences exist in mechanical laxity between patients with FAI and healthy participants.7,35 Collectively, these findings provide growing evidence that contradicts conventional theories, which have predicted that participants with complaints of multiple ankle sprains or repeated “rollover” events have increased mechanical laxity. Several conclusions may be drawn from these results.
One potential explanation for the absence of a difference in laxity between HAs and UAs is that the loading forces imparted during arthrometer tests do not replicate the physiologic stresses and strains experienced during physical activity, which would invariably alter the tissue's loading response curve. Testing this hypothesis would necessitate exposing participants to dangerously high loads and invasive procedures, which may be unnecessary, because anecdotal evidence suggests that many rollover episodes do not result from abnormally high loads. Alternatively, the simplest explanation for the absence of a difference in laxity is that the normal healing process of the injured tissue has restored the joint's capsuloligamentous mechanical properties.36 This suggests that FAI may predominantly be a problem within the sensorimotor system, causing a persistent sense of joint instability, even when mechanical laxity is within normal limits.
Muscle Spindle Function in HA and UA Groups
Previous researchers14 investigating somatosensory deficits and ankle instability have shown mixed results, primarily because a variety of indirect measurements are used, including joint position sense, passive movement detection, reflexes, and balance. More noteworthy is the fact that these dependent variables do not control for such factors as cognitive abilities, visual and vestibular system involvement, and motor responses.13,18 Therefore, these measures may lack sensitivity for quantifying peripheral deficits in joint sensation. Even the apparently simple tests of proprioception and kinesthesia measure conscious perception, which occurs at the cortical level, far removed from the signal's origin at the ankle.12
Prior microneurography studies23,25 have focused largely on the role of muscle spindles to examine their regulation of activity and sensitivity to dynamic stretching or to differentiate between location and type of afferent fibers. However, this body of research has not explored the role of muscle spindles simultaneously with joint loading patterns common to clinically relevant diagnostic tests or in patients with ankle-joint instability. We observed that during ankle anterior translation, nerve activity from muscle afferents increased at each level of force up to 90 N in HAs; yet in UAs, it did not increase until 60 N of anterior force. In addition, the amplitude of sensory traffic was less in the UA group at 30 N of anterior force. These data suggest that in patients with ankle instability, muscle spindles have a diminished response at lower levels of joint force when compared with HAs. This diminished response could potentially explain a mechanism by which patients with ankle instability are unable to properly detect changes in forces in the early stages of an impending rollover event.
The potential reasons for this decreased sensory traffic must first be qualified with the knowledge that the spindle afferent fibers transmit a “final common input” to the nervous system, because spindle sensitivity is partially dependent on the descending gamma motor drive from the brain and spinal pathways synapsing with other joint receptors' afferents. In other words, the signal from the MSA is influenced by sensory information from the mechanoreceptors in the joint, muscle, and tendon. Additional studies would be needed to determine the temporal and spatial influences that may be contributing to the group differences in amplitude of muscle spindle sensory traffic we observed.24 We will speculate that the decreased response in UAs at lower levels of force could result for a variety of reasons. The first potential reason is that the muscle spindles may not be sensitive to lower loads of force due to a decrease in gamma motor neuron drive.25 Direct damage to articular mechanoreceptors could lead to a decrease in reflexive gamma motor drive and, therefore, the muscle spindle would be less sensitive to changes in muscle length and tension, especially at low levels of joint loading. Also, mechanoreceptors may not repopulate the capsuloligamentous tissue in similar kind and quantity as before the injury.10,37 Golgi tendon organs (GTOs) in the ankle evertor musculotendinous tissue may also have a diminished response to low loads of tension as a result of repetitive injury.37 The GTOs detect loads as low as 5 N and are typically able to provide excitatory feedback to the muscle spindles at these low force levels.11 Additionally, the potential for plastic changes in the central nervous system at the spinal or supraspinal level after ligamentous injury38,39 could result in decreased gamma motor drive to the muscle spindles, lowering the sensitivity to joint loading.24
A secondary theme to explain the diminished muscle spindle sensory traffic is that in UAs, the relative length change in the muscle at lower levels of force is simply insufficient to evoke action potentials. Although no differences were seen in AP laxity between HAs and UAs at low levels of force, it is possible that lower loads are absorbed to a greater degree by the static restraints in UAs. After injury, scar tissue forms to replace damaged capsuloligamentous structures. This scar tissue has greater stiffness than native connective tissue structures, and in participants with a history of multiple ankle sprains, an excessive amount of relatively inelastic scar tissue may be unable to absorb loading.40 Therefore, at lower levels of force, more tension may be transmitted into scar tissue, and hence, relatively less change in length is transmitted to the muscle spindle to elicit a neural response.
No differences in sensory traffic amplitude were seen between HAs and UAs for IE trials. This is unlike the sensory traffic amplitude deficit seen in UAs at low levels of force in AP trials. One reason for this result may be that the IE trials predominantly stressed the calcaneofibular ligament, with the anterior and posterior talofibular ligaments and the peroneal muscles providing support, whereas the anterior translations predominantly stressed the anterior talofibular ligament.41 These UAs may only have damage to the anterior talofibular ligament and its mechanoreceptors, because this structure is typically the first to accept load and sprain after an inversion stress. Therefore, stressing these additional structures may provide enough feedback to compensate for any sensory deficits due to neuromechanical damage of the anterior talofibular ligament.
Another observation noted in both AP and IE trials was a plateau effect. Neither HAs nor UAs showed an increase in nerve activity between 90 and 125 N of AP force. This effect was also observed in IE trials, with no differences seen in HAs between 3 and 4 Nm of IE torque and no differences in UAs between 2 and 4 Nm of IE torque. These results lend support to the hypothesis that a saturation phenomenon may be occurring, whereby mechanoreceptors become maximally stimulated before maximal force and torque are achieved. This could indicate that neither HAs nor UAs may be able to differentiate between higher levels of force on the ankle.42 This effect may be amplified in UAs during inversion rotation.
Clinical Relevance
Because these recordings were believed to be obtained directly from muscle spindle afferents, proximal to the site of potentially damaged tissue and the stresses imparted on static capsuloligamentous stabilizers, clinicians and researchers may be encouraged to explore promising conditioning and therapeutic techniques in promoting beneficial changes in spindle function. These adaptations may occur in other types of mechanoreceptors, from reflexive pathways, or in the brain, because all are capable of manipulating gamma motor drive and thus changing the muscle spindles' level of sensitivity and responsiveness to joint loading. We anticipate that this fosters greater somatosensory awareness and functional joint stability. The observed increase in activity of the MSAs during joint loading for all participants lends support to the complex and potentially important compensatory role they serve for joint proprioception and kinesthesia. Their response to joint motion and loads, as well as their capacity to change levels of sensitivity, may partially explain why mechanical joint laxity does not always correlate with decreases in joint sensation8,31 and why many individuals who suffer a joint sprain may not develop deficits in joint sensation or functional instability.16 In those patients who subjectively complain of instability and experience episodes of giving way or rolling, this is the first study to simultaneously record deficits in muscle spindle sensory traffic during mechanical loading of the ankle joint. The results suggest that patients complaining of joint instability may predominantly suffer from dysfunction of the fusimotor system rather than mechanical laxity.
Limitations
It is not always possible to obtain usable microneurography recordings. Additional participants were recruited for this study; however, recordings were only obtained on the 29 participants presented. Furthermore, IE recordings were not obtained on approximately 30% of included participants because motion and stretch of the skin and muscle caused the electrode to dislodge. This problem is not uncommon for this method,43 and success rates were similar for both HAs and UAs.
Moreover, although all recordings included afferent activity confirmed from the muscle spindle, as well as an absence of afferent activity from skin and efferent activity, we cannot definitively rule out the possible contribution of other joint mechanoreceptors, such as free nerve endings and capsuloligamentous receptors, to our recordings.22,32,33 Slow-adapting type II skin receptors may exhibit activity in response to the arthrometer used in this study. In the hand, these receptors responded to deformation and lateral stretching of the skin and would produce an increased discharge in response to the types of load applied.44 However, these receptors may also continue to spontaneously discharge in the absence of skin deformation, and it would be difficult to postulate why UAs would have diminished sensitivity to load in these receptors when compared with HAs.
Finally, we demonstrated a deficit in afferent activity observed in UAs, compared with a group of HAs. Yet we obtained these values with patients in a relaxed, supine position. Further research should be conducted to observe whether these deficits may be detected in a standing or functional position for the ankle, because this may accordingly alter muscle spindle sensitivity.
CONCLUSIONS
In this study we used direct nerve recordings in conjunction with an ankle arthrometer to simultaneously quantify load, laxity, and sensory traffic in HAs and UAs. No differences were seen in mechanical laxity between HAs and UAs, suggesting that mechanical laxity may not be a factor in FAI. Our results also suggest that unstable ankles may have difficulty sensing initial, low-level loads on the ankle. This diminished sensation may impair the ability of a patient to sense an impending episode of rolling until it is too late for the musculotendinous structures to stiffen and stress-shield the capsuloligamentous structures from excessive loads. Additionally, a plateau effect was noted in the AP and IE conditions, with the plateau occurring earlier for inversion stress of UAs. These data lend support to the hypothesis that sensations of joint instability depend on more than just static restraints and that muscle spindle function may be crucial in the maintenance of functional joint stability.
ACKNOWLEDGMENTS
We thank the National Athletic Trainers' Association Foundation (Dallas, TX) for financial support of this study through the Osternig Master's Grant. Additionally, we thank Dr. J. Marcus Hollis and Blue Bay Research for assistance with customization of the ankle arthrometer.
REFERENCES
- 1.Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ., Jr The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279–2284. doi: 10.2106/JBJS.I.01537. [DOI] [PubMed] [Google Scholar]
- 2.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 3.Yeung MS, Chan KM, So CH, Yuan WY. An epidemiological survey on ankle sprain. Br J Sports Med. 1994;28(2):112–116. doi: 10.1136/bjsm.28.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47(4):678–685. [PubMed] [Google Scholar]
- 5.Anandacoomarasamy A, Barnsley L. Long-term outcomes of inversion ankle injuries. Br J Sports Med. 2005;39(3) doi: 10.1136/bjsm.2004.011676. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubbard TJ, Kaminski TW, Vander Griend RA, Kovaleski JE. Quantitative assessment of mechanical laxity in the functionally unstable ankle. Med Sci Sports Exerc. 2004;36(5):760–766. doi: 10.1249/01.mss.0000126604.85429.29. [DOI] [PubMed] [Google Scholar]
- 7.Hertel J, Denegar CR, Monroe MM, Stokes WL. Talocrural and subtalar joint instability after lateral ankle sprain. Med Sci Sports Exerc. 1999;31(11):1501–1508. doi: 10.1097/00005768-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard TJ, Kramer LC, Denegar CR, Hertel J. Correlations among multiple measures of functional and mechanical instability in subjects with chronic ankle instability. J Athl Train. 2007;42(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- 9.Lephart SM, Henry TJ. The physiological basis for open and closed kinetic chain rehabilitation for the upper extremity. J Sport Rehabil. 1995;5(1):71–87. [Google Scholar]
- 10.Michelson JD, Hutchins C. Mechanoreceptors in human ankle ligaments. J Bone Joint Surg Br. 1995;77(2):219–224. [PubMed] [Google Scholar]
- 11.Grigg P. Peripheral neural mechanisms in proprioception. J Sport Rehabil. 1994;3(1):2–17. [Google Scholar]
- 12.Salles JI, Costa F, Cunha-Cruz V et al. Electrophysiological analysis of the perception of passive movement. Neurosci Lett. 2011;501(2):61–66. doi: 10.1016/j.neulet.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Riemann BL, Myers JB, Lephart SM. Sensorimotor system measurement techniques. J Athl Train. 2002;37(1):85–98. [PMC free article] [PubMed] [Google Scholar]
- 14.Munn J, Sullivan SJ, Schneiders AG. Evidence of sensorimotor deficits in functional ankle instability: a systematic review with meta-analysis. J Sci Med Sport. 2010;13(1):2–12. doi: 10.1016/j.jsams.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Brown C, Padua D, Marshall SW, Guskiewicz K. Individuals with mechanical ankle instability exhibit different motion patterns than those with functional ankle instability and ankle sprain copers. Clin Biomech (Bristol, Avon) 2008;23(6):822–831. doi: 10.1016/j.clinbiomech.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Eastlack ME, Axe MJ, Snyder-Mackler L. Laxity, instability, and functional outcome after ACL injury: copers versus noncopers. Med Sci Sports Exerc. 1999;31(2):210–215. doi: 10.1097/00005768-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez GM, Kaminski TW, Douex AT. Neuromuscular control and ankle instability. PM R. 2009;1(4):359–365. doi: 10.1016/j.pmrj.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Hirabayashi S, Iwasaki Y. Developmental perspective of sensory organization on postural control. Brain Dev. 1995;17(2):111–113. doi: 10.1016/0387-7604(95)00009-z. [DOI] [PubMed] [Google Scholar]
- 19.Hagbarth KE, Vallbo AB. Afferent response to mechanical stimulation of muscle receptors in man. Acta Soc Med Ups. 1967;72(1):102–104. [PubMed] [Google Scholar]
- 20.Basmajian JV. Electromyography: its structural and neural basis. Int Rev Cytol. 1967;21:129–140. doi: 10.1016/s0074-7696(08)60813-x. [DOI] [PubMed] [Google Scholar]
- 21.Hagbarth KE, Vallbo AB. Mechanoreceptor activity recorded percutaneously with semi-microelectrodes in human peripheral nerves. Acta Physiol Scand. 1967;69(1):121–122. doi: 10.1111/j.1748-1716.1967.tb03498.x. [DOI] [PubMed] [Google Scholar]
- 22.Burke D. Unit identification, sampling bias and technical issues in microneurographic recordings from muscle spindle afferents. J Neurosci Methods. 1997;74(2):137–144. doi: 10.1016/s0165-0270(97)02244-9. [DOI] [PubMed] [Google Scholar]
- 23.Edin BB, Vallbo AB. Stretch sensitization of human muscle spindles. J Physiol. 1988;400:101–111. doi: 10.1113/jphysiol.1988.sp017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson H, Sjolander P, Sojka P. A sensory role for the cruciate ligaments. Clin Orthop Relat Res. 1991;(268):161–178. [PubMed] [Google Scholar]
- 25.Bergenheim M, Johansson H, Pedersen J. The role of the gamma-system for improving information transmission in populations of Ia afferents. Neurosci Res. 1995;23(2):207–215. doi: 10.1016/0168-0102(95)00941-l. [DOI] [PubMed] [Google Scholar]
- 26.Johansson H. Role of knee ligaments in proprioception and regulation of muscle-stiffness. J Electromyogr Kinesiol. 1991;1(3):158–179. doi: 10.1016/1050-6411(91)90032-Z. [DOI] [PubMed] [Google Scholar]
- 27.Johansson H, Sjolander P, Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of joint afferent fibres in the hind limb of the cat. J Physiol. 1986;375:137–152. doi: 10.1113/jphysiol.1986.sp016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiller CE, Refshauge KM, Bundy AC, Herbert RD, Kilbreath SL. The Cumberland Ankle Instability Tool: a report of validity and reliability testing. Arch Phys Med Rehabil. 2006;87(9):1235–1241. doi: 10.1016/j.apmr.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Edin BB, Vallbo AB. Classification of human muscle stretch receptor afferents: a Bayesian approach. J Neurophysiol. 1990;63(6):1314–1322. doi: 10.1152/jn.1990.63.6.1314. [DOI] [PubMed] [Google Scholar]
- 30.Kovaleski JE, Hollis J, Heitman RJ, Gurchiek LR, Pearsall AW., 4th Assessment of ankle-subtalar-joint-complex laxity using an instrumented ankle arthrometer: an experimental cadaveric investigation. J Athl Train. 2002;37(4):467–474. [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett DS, Cobb AG, Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br. 1991;73(1):53–56. doi: 10.1302/0301-620X.73B1.1991775. [DOI] [PubMed] [Google Scholar]
- 32.Wu G, Ekedahl R, Stark B, Carlstedt T, Nilsson B, Hallin RG. Clustering of pacinian corpuscle afferent fibres in the human median nerve. Exp Brain Res. 1999;126(3):399–409. doi: 10.1007/s002210050746. [DOI] [PubMed] [Google Scholar]
- 33.Wu G, Ekedahl R, Hallin RG. Clustering of slowly adapting type II mechanoreceptors in human peripheral nerve and skin. Brain. 1998;121:265–279. doi: 10.1093/brain/121.2.265. [DOI] [PubMed] [Google Scholar]
- 34.Chandnani VP, Harper MT, Ficke JR et al. Chronic ankle instability: evaluation with MR arthrography, MR imaging, and stress radiography. Radiology. 1994;192(1):189–194. doi: 10.1148/radiology.192.1.8208935. [DOI] [PubMed] [Google Scholar]
- 35.Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47(4):669–677. [PubMed] [Google Scholar]
- 36.Hubbard TJ, Hicks-Little CA. Ankle ligament healing after an acute ankle sprain: an evidence-based approach. J Athl Train. 2008;43(5):523–529. doi: 10.4085/1062-6050-43.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takebayashi T, Yamashita T, Minaki Y, Ishii S. Mechanosensitive afferent units in the lateral ligament of the ankle. J Bone Joint Surg Br. 1997;79(3):490–493. [Google Scholar]
- 38.Courtney C, Rine RM, Kroll P. Central somatosensory changes and altered muscle synergies in subjects with anterior cruciate ligament deficiency. Gait Posture. 2005;22(1):69–74. doi: 10.1016/j.gaitpost.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic ankle instability alters central organization of movement. Am J Sports Med. 2010;38(4):829–834. doi: 10.1177/0363546509351562. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson KE, Liu SH. Anterolateral impingement of the ankle. J Med Assoc Ga. 1992;81(6):297–299. [PubMed] [Google Scholar]
- 41.Tohyama H, Yasuda K, Ohkoshi Y, Beynnon BD, Renstrom PA. Anterior drawer test for acute anterior talofibular ligament injuries of the ankle. How much load should be applied during the test? Am J Sports Med. 2003;31(2):226–232. doi: 10.1177/03635465030310021201. [DOI] [PubMed] [Google Scholar]
- 42.Bahrick HP. An analysis of stimulus variables influencing the proprioceptive control of movements. Psychol Rev. 1957;64(5):324–328. doi: 10.1037/h0043391. [DOI] [PubMed] [Google Scholar]
- 43.Eckberg DL, Wallin BG, Fagius J, Lundberg L, Torebjork HE. Prospective study of symptoms after human microneurography. Acta Physiol Scand. 1989;137(4):567–569. doi: 10.1111/j.1748-1716.1989.tb08804.x. [DOI] [PubMed] [Google Scholar]
- 44.Johansson RS, Vallbo AB. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983;6(0):27–32. [Google Scholar]