Abstract
Background: A growing body of preclinical data indicates that statins may possess antineoplastic properties; however, some studies have raised the possibility that statins may also have carcinogenic potential. Methods: An air pouch model was used for angiogenesis. Single or multiple applications of croton oil on the back of Swiss albino mice with or without initiation by dimethylbenz(a)antheracene (DMBA) were used to evaluate the skin tumorgenesis, ultrastructural and histological alterations. Results: Atorvastatin (orally, 10 mg/kg/day) produced a significant (P<0.05) reduction in angiogenesis. Concurrent administration of mevalonate reversed the anti-angiogenic effect of atorvastatin. However, local injection of atorvastatin (200 µg) into the pouches induced a significant (P<0.5) increase in angiogenesis that was not reversed by co-administration of mevalonate. The disturbance of cell polarity, inflammatory response, thickness of epidermal layer, and mitotic index induced by croton oil were inhibited markedly and dose-dependently (P<0.001) by pre-treatment with atorvastatin. In spite of the strong anti-inflammatory and anti-proliferative effects of atorvastatin on epidermal cell proliferation, it was identified that the same doses of atorvastatin in DMBA-initiated and croton oil-promoted skin tumorgenesis in mice increased the incidence of tumors and their conversion into malignant carcinoma. Conclusion: The reasons for these discrepancies remain unclear, and could be related to ambivalent effects of atorvastatin on angiogenesis or to specific differences in the experimental conditions. It is suggested that the pro-angiogenic effect of the drug, which could be responsible for promotion of skin tumors, is independent of the 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibition that can be mediated directly by atorvastatin.
Key Words: Atorvastatin, Angiogenesis, Cell Proliferation, Cancer
Introduction
Statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, are used routinely in the treatment of patients with cardiovascular disease. Statins have been shown to exert ‘pleiotropic effects’, that are independent of their cholesterol-lowering action, such as anti-inflammatory properties [1], reduction of plaque thrombogenicity [2], inhibition of cellular proliferation and migration [3], and improvement of endothelial function [4]. Furthermore, these drugs increase the expression of endothelial progenitor cells that are involved in vascular repair [5, 6] as well as in the expression and activity of endothelial nitric oxide synthase [7, 8]. Statins have been reported to have contradictory effects on angiogenesis, a major pathological component of diseases such as cancer and coronary heart disease. The stimulation of angiogenesis considered a potential approach in the treatment of coronary artery disease, and its blocking is considered an effective tactic for the treatment of malignancies. A report has indicated that simvastatin promote collateral vessel formation in the hearts subjected to ischemia [9]. On the contrary, another study has demonstrated that atorvastatin impairs the myocardial angiogenic response to chronic ischemia [10] or interrupt angiogenesis [11]. Besides, several studies have indicated a biphasic effect of statins on angiogenesis in cultured endothelial cells in a dose-dependent manner [12, 13]. However, the reasons for these ambivalent behaviors have not been fully understood as yet [12, 14]. It has been suggested that the diverse effects of statins on angiogenesis are related to the drug potency and dose, cell type used, and the microenvironment [15]. The potential effect of statins on cancer is controversial. The aggressiveness of cancers can be significantly increased when angiogenesis is stimulated, it can be speculated that the pro-angiogenic effects of statins could promote cancer growth and metastasis.
Statins inhibit the rate-limiting step in the mevalonate pathway [16], which is essential for the synthesis of various compounds that include cholesterol and a number of non-sterol products. The findings show that non-sterol products of the mevalonate pathway, such as farnesyl diphosphate and other phosphorylated products are essential for various cellular processes suggests and the statins may influence tumor cell growth and differentiation [16, 17]. Based on experimental data and several clinical observations, it has been claimed that extrahepatic and cholesterol-lowering independent effects of statins may play a potentially beneficial role in the treatment of cancer [18, 19]. However, the majority of clinical trials that aimed to demonstrate the anticancerous effects of statins did not obtain conclusive results [20].
Moreover, the most recent reports published on these effects do not support the hypothesis that statins strongly reduce the risk of cancer [21-23]. Among different biological effects of statins, some effects could inhibit tumor growth (e.g. by inhibiting Ras and Rho oncoproteins), whereas other actions may stimulate cancer aggressiveness (e.g. through angiogenic effects). For instance, fluvastatin was found to reduce proliferation and increase apoptosis in women with high-grade breast cancer [24]. Similarly, it has been reported that lovastatin inhibits growth and induces apoptosis in a number of cultured cancer cell lines, possibly due to its ability to block farnesylation and geranylgeranylation of small proto-oncogenic guanosine triphosphate-ases, such as Ras and Rho [18].
In a study, Klement and Rak [25] have demonstrated that fluvastatin blocks the action of angiogenic factors on cultured endothelial cells, but is ineffective against highly angiogenic and aggressive tumors in mice. They also concluded that the antitumor and anti-angiogenic activities of fluvastatin in vitro are not recapitulated in vivo. This study aimed to investigate the effects of atorvastatin on angiogenesis in an air pouch model. Also, a single topical application of croton oil on the back of Swiss albino mice was used to evaluate the effect of the drug on the epidermal cell proliferation. To identify whether these effects act in synergistically with the effect of atorvastatin on skin tumors, tumorgenesis induced by multiple applications of croton oil on the back of mice with initiation by DMBA was used as an in vivo model.
MATERIALS AND METHODS
Reagents. Atorvastatin was kindly provided by Sobhan Pharmaceutical Inc. (Tehran, Iran). Carrageenan, carmine red dye, DMSO, dimethylbenz [a]antheracene (DMBA), and croton oil were purchased from Sigma chemical Co. (USA). All reagents were of analytical grade.
Experimental animals. Male Wistar rats (200-250 g, n = 6-8) and Swiss albino female mice (20-22 g, n = 6-8) were used as experimental animals in this study. The animals were given food and water ad libitum, and they were housed in the Animal House of Tabriz University of Medical Sciences at a controlled ambient temperature of 25 ± 2°C with 50 ± 10% relative humidity in a 12-h light/12-h dark cycle (lights on at 7:00 a.m.). This study was performed in accordance with the guidelines specified in the Guide for the Care and Use of Laboratory Animals of Research affairs of Tabriz University of Medical Sciences, Tabriz, Iran.
Determination of angiogenesis in granulation tissue. Rats were lightly anesthetized with diethyl ether; then, 8 ml of air was injected subcutaneously in the back to make an air pouch that was oval in shape. Twenty-four hours later, 4 ml of 1% (w/v) solution of carrageenan in saline was injected into the air pouch under light anesthesia with diethyl ether anesthesia. The carrageenan solution had been sterilized by autoclaving at 121ºC for 15 min and supplemented with antibiotics (0.1 mg penicillin G potassium and 0.1 mg dihydrostreptomycin sulfate per milliliter), after cooling to 40-45°C. The level of angiogenesis was evaluated 6 days after the carrageenan injection. Animals were anesthetized by intraperitoneal injection of a mixture of ketamine (60 mg/kg), xylazine (10 mg/kg), and acepromazin (10 mg/kg). In addition, 3 ml of 5% (w/v) carmine dye in 5% (w/v) gelatin in a saline vehicle at 37°C was injected into the jugular vein of each rat and the carcasses were chilled by placing them on ice for 3 h. After this time, the entire granulation tissue was dissected, weighed, and washed with PBS (pH 7.4). The content of carmine dye in the granulation tissue was used as an indicator of angiogenesis and measured according to the methods described by Ghosh et al. [3], albeit with slight modifications. Briefly, the entire granulation tissue was homogenized in two volumes of 0.5 mM sodium hydroxide by processing in a basic homogenizer (IKA Labortechnik, Italy) at 9500 rpm for 4 min while placed on an ice bed. The tissue homogenate was centrifuged at 10000 ×g and 4°C for 30 min. The supernatant (500 ml) was diluted 2-fold with 0.5 mM sodium hydroxide and the mixture was centrifuged again at 14,000 ×g and 4°C for 30 min. The concentration of the dye in 200 µl of the supernatant was determined spectrophotometrically by measuring absorbance at a wavelength of 490 nm. For standard curve, known amounts of carmine dye were added to the final supernatant of granulation tissue obtained from control rats, which were injected with 3 ml of a 10% (w/v) gelatin solution in a saline vehicle without carmine dye, and the absorbance determined. The amount of carmine dye in the entire granulation tissue was then calculated.
Drug treatment. Atorvastatin was dissolved in DMSO. The final concentration of DMSO in the saline solution was adjusted to 1% (v/v). Stock solutions were diluted with saline, and 0.5 ml of the diluted solution containing either 100 or 200 µg of the drug was injected locally into the pouch immediately before and every 24 h after the carrageenan injection. Control rats received the same amount of saline solution containing DMSO at 1% (v/v). To assess the systemic effect of atorvastatin on angiogenesis, a preparation with 10 mg/kg atorvastatin in 0.5% carboxymethyl cellulose was given by gavages to pouch-bearing rats. In addition, the present study tested whether intraperitoneal injection of 10 mg/kg mevalonate in saline or intra-pouch injection of 50 g mevalonate in DMSO (%1) could reverse atorvastatin effect.
Epidermal cell proliferation experiments. Female Swiss mice (6 mice in each group) were pretreated with atorvastatin (3, 6, and 9 mg/kg/day) for 2 weeks. The dorsal skin of the mice were shaved with electric clippers and followed by the application of hair-removing cream at least 2 days prior to the induction of proliferation. Only mice that did not show signs of hair re-growth were used in the experiments. A 0.2-ml quantity of acetone solution containing croton oil (0.5%) was applied to the shaved areas of individual mice. Control mice were treated with the same volume of acetone. The mice were sacrificed by neck fracture 24 h after the topical application of croton oil; thereafter, the skin over the back was excised and processed for light microscopic examination in accordance with standard procedures and the sections were stained with hematoxylin and eosin. Histopathological changes were analyzed using a microscope image analyzer (Model: Olympus AX8, Japan) to investigate: (1) disturbances of cell polarity in 2500 µm2; (2) inflammatory response (number of poly-morphonuclear neutrophils (PMN) in 2500 µm2); (3) nucleated cell layer (number of cells layers in 2500 µm2); (4) thickness of the epidermal layer, and (5) mitotic index (the number of cells in mitosis per 100 basal cells) in 2500 µm2 using a modification of the procedure described by Raick [26]. All parameters were measured in the same area and at the same magnification (×40).
Skin tumorgenesis. Female Swiss mice (n = 20) were orally administered atorvastatin (3, 6, and 9 mg/kg per day) for 2 weeks before induction of skin tumor or, in another set of experiments, for the whole period following induction of skin tumor. The dorsal skin of the mice were shaved and prepared by the same method described for the proliferation experiments. Tumors were initiated by topical application of a single dose of DMBA (40 μg in 100 μl acetone per mouse) and promoted with twice-weekly applications of croton oil (200 µl of 0.5% in acetone per mouse) for 32 weeks. The mice were observed weekly, and the appearance of first tumor (latency period) and percentage of mice with papillomas (percentage of tumor incidence) were recorded.
Statistics . Data are presented as mean ± SD. Comparisons between groups were made using the analysis of variance (ANOVA). If the ANOVA analysis indicated significant differences, a Student-Newman-Keuls post-test was performed to compare mean values between treatment and control groups. Differences between groups were considered significant at P<0.05.
Results
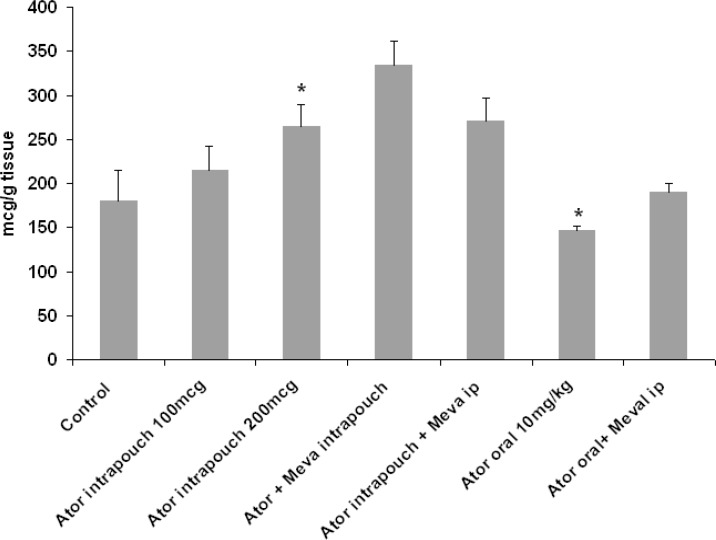
Effect of atorvastatin on angiogenesis in granulation tissue. Six days after the injection of carrageenan into the air pouch, a dissectible granulation tissue was observed in the subcutaneous tissue. Following a single intravenous injection of carmine red dye in anesthetized animals, the dye was accumulated in the granulation tissue, and the amount of the dye was assessed as an index of angiogenesis. As shown in Figure 1, local injection of 100 and 200 µg atorvastatin into the pouch caused a dose-dependent increase (19% and 47%, respectively) in the content of the dye, and the concentration attained a significant level (P<0.05) at 200 µg. Neither intra-pouch nor intraperitoneal injection of mevalonate inhibited the angiogenic action of locally administrated atorvastatin. On the contrary, oral administration of atorvastatin (10 mg/kg) produced a significant (P<0.05) reduction in angiogenesis that could be reversed by intraperitoneal injection of mevalonate (Fig. 1).
Fig. 1.
The effect of oral or local administration of atorvastatin on carmine dye content (as an index of angiogenesis) in granulation tissue in an air pouch model of angiogenesis in rat. Data represented as mean SD. *P<0.05 compared with control group using one-way ANOVA. ator, atorvastatin.
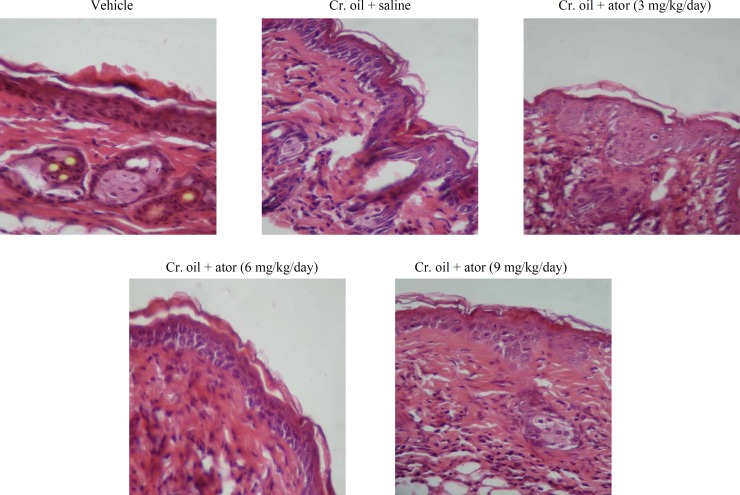
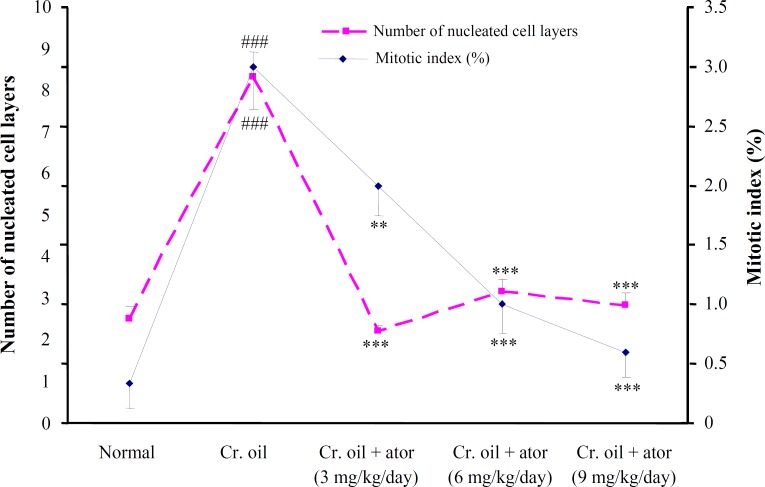
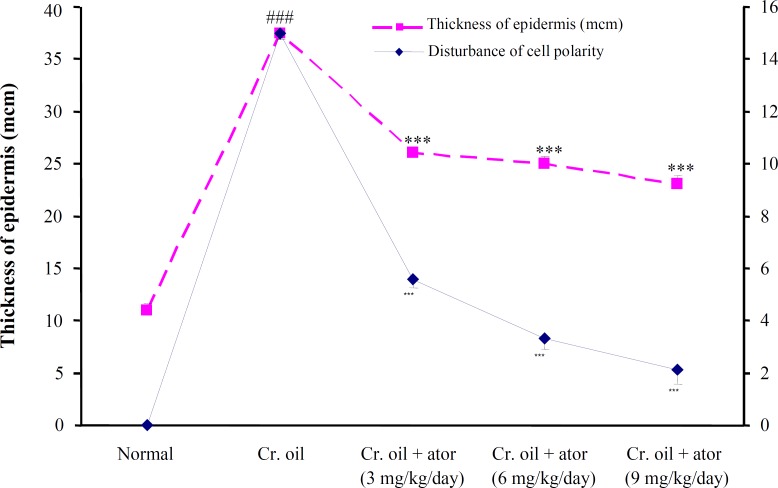
Effect of atorvastatin against proliferative alter-ations produced by croton oil on mouse epidermis. A single application of croton oil on mouse skin was induced, in addition to inflammation and hyperplasia, an early and rapid increase in the thickness, number of nucleated cell layers, and mitotic index of the interfollicular epidermis that were evident at 24 h (Fig. 2). All three doses of atorvastatin obviously prevented epidermal hyperplasia, inflammation, and cell disorientation in a dose-dependent manner (Fig. 2). As shown in Figure 3, the number of cells undergoing mitosis in 2500 µm2 of interfollicular epidermis was reduced from 3 ± 0.3 in the untreated group to 2 ± 0.2, 1 ± 0.2 (P<0.01), and 0.6 ± 0.2 (P<0.001) by 3, 6, and 9 mg/kg of atorvastatin, respectively. Similarly, the disturbance of cell polarity was also significantly (P<0.001) and dose-dependently reduced by atorvastatin pre-treatment (Fig. 4). Atorvastatin markedly (P<0.001) prevented croton oil-induced increase in the thickness and number of nucleated cell layers; however, this prevention was not dose dependent (Figs. 3 and 4).
Fig. 2.
Representative photomicrograph of dorsal skin tissue sections 24 h after a single application of vehicle (acetone) or croton oil from mice pretreated with saline or atorvastatin. Sections were stained with hematoxylin and eosin (original magnification ×40). Oral administration of atorvastatin markedly inhibited croton oil-induced epidermal hyperplasia and inflammation. Cr., croton; ator, atorvastatin.
Fig. 3.
The effects of a single application of croton oil on the number of nucleated cell layers and mitotic index of interfollicular epidermis in control and atorvastatin-treated mice. Data are mean SD (n = 6) and were analyzed with ANOVA and the Student-Newman-Keuls post test. ###P<0.001 vs. control and **P<0.01, and ***P<0.001 vs. croton oil group. Cr., croton; ator, atorvastatin.
Fig. 4.
The effects of a single application of croton oil on the thickness of epidermis, and disturbance of cell polarity in control and atorvastatin-treated mice. Data are mean SD (n = 6) and were analyzed with ordinary ANOVA and Student-Newman-Keuls post test. ###P<0.001 vs. normal control and ***P<0.001 vs. croton oil group. Cr., croton; ator, atorvastatin.
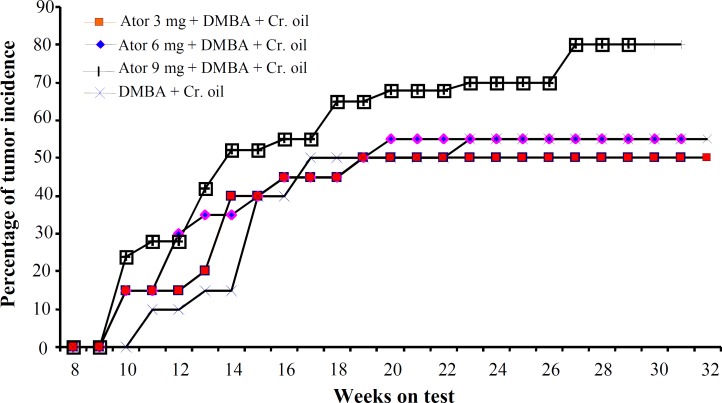
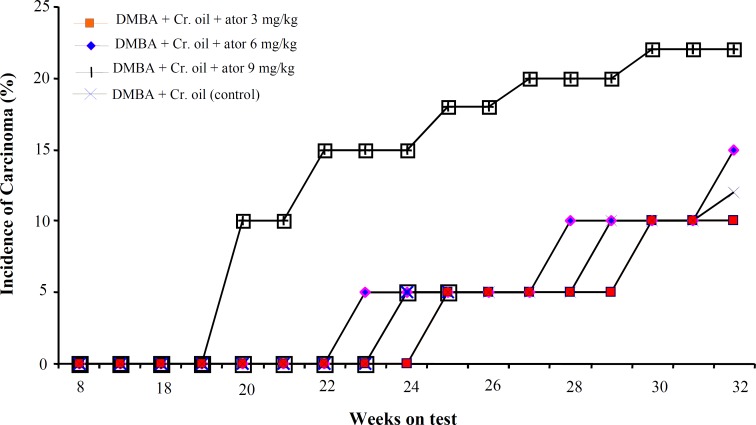
E ffect of atorvastatin on d imethylbenz(a) antheracene - i nitiated and c roton oil-promoted s kin t umorgenesis . The effect of atorvastatin, administered orally for 2 weeks prior to tumor induction, on the incidence of skin tumor has been shown in Figure 5. In this set of experiments, most tumors appeared as papillomas. The percentage of mice with papillomas in the control group (DMBA + croton oil) and in groups treated with 3 and 6 mg/kg per day of atorvastatin was nearly similar. However, in atorvastatin-treated animals, the tumors appeared earlier. Compared with the control group, mice pre-treated with high dose of atorvastatin (9 mg/kg) showed an increased incidence of tumors. At week 13, 42% and at week 27, 80% of the animals treated with high doses of atorvastatin showed development of papillomas, whereas these values were 15% and 55% in the control group. In the experimental group, that wherein atorvastatin was administered orally for the entire duration of the experiment after tumor induction, the incidence of skin tumors did not differ between groups. Whereas the conversion of tumor into malignant carcinoma was significantly higher in animals treated with 9 mg/kg of atorvastatin (Fig. 6).
Fig. 5.
Percentage of mice with tumors (benign papillomas) in dimethylbenz(a)antheracene (DMBA) + croton oil-applied mice with or without atorvastatin pretreatment. The data were recorded every week and plotted as a function of weeks on test. Each value represents percentage incidence data calculated for 20 animals. Cr., croton; ator, atorvastatin.
Fig. 6.
The effect of atorvastatin, when administered orally for the entire duration of experiment after induction of tumor, on the conversion of papillomas into malignant carcinoma. These data were recorded every week and plotted as a function of weeks on the test. Each value represents the percentage incidence data calculated for 20 animals. Cr., croton; ator, atorvastatin.
Discussion
The present study demonstrated that local injection of atorvastatin into the pouch, in an air pouch model of angiogenesis in rats, caused a significant pro-angiogenic effect which was not reversed by either systemic or local administration of mevalonate. However, oral administration of atorvastatin produced mevalonate-dependent anti-angiogenic action. Angio-genesis plays a crucial role in tumor growth and metastasis, and has been considered as a target for intervention in cancer therapy. Statins are competitive inhibitors of HMG-CoA reductase, the enzyme that catalyzes the synthesis of mevalonate and its isoprenoid intermediates such as farnesylpyro-phosphate and geranylgeranyl pyrophosphate. These intermediates have been implicated in several post-translational protein isoprenylation processes that are essential for various cellular processes. Therefore, statins may influence cell growth, differentiation, proliferation, and angiogenesis [4, 27, 28] through inhibition of mevalonate formation. Regardless of the widespread benefits of statins, the effect of statins on angiogenesis and, therefore on cancer is the subject of contradictory debate [29]. Several preclinical reports have indicated that statins may possess antineoplastic properties [16, 19]; however, some other studies have raised the possibility that statins may possess a carcinogenic potential [18, 20]. Recent epidemiologic data and commentaries do not demonstrate a consistent reduction in cancer risk among statin users; however, the chemopreventive effect or any potential increase in the risk effect of statin in humans remains to be confirmed [30-32]. It has been reported that simvastatin accelerates revascularization in vitro through the activation of protein kinase Akt [9]. On the contrary, a diminished endogenous angiogenic response was reported to have been observed with atorvastatin treatment in chronic myocardial ischemia in normocholesterolemic swine [10]. Several studies have also shown that HMG-CoA reductase inhibition has a biphasic dose-dependent effect on angiogenesis that is associated with alterations in endothelial apoptosis and vascular endothelial growth factor signaling. Statins have pro-angiogenic effects at low therapeutic concentrations, but at high concentrations, they have anti-angiogenic effects that can be reversed by geranylgeranyl pyrophosphate [12, 13].
We have previously demonstrated that high doses of atorvastatin induced pro-inflammatory effect in air-pouch inflammation model when injected directly into the pouch [1]. Concurrent administration of mevalonate along with atorvastatin failed to inhibit the pro-inflammatory action of the drug. Under steady-state conditions, small amounts of the parent drug can be found in the systemic circulation. The high dose of drug might produce a high level of free drug and cause a mevalonate-independent pro-inflammatory and pro-angiogenic effects. On the other hand, the accumulation of metabolites of high doses can create different effects from that of the parent drug. Serum level of atorvastatin, prescribed at doses of 10-80 mg/day, ranged between 2 and 200 µg/l. However, such small concentration of statins, especially potent statins like atorvastatin with a long plasma half life of 20 hours, may demonstrate direct significant biological effects beyond the mevalonic acid pathway.
Based on the results of the present study, it can be determined that oral administration of atorvastatin (3, 6, and 9 mg/kg per day) for 2 weeks prior to single application of croton oil on dorsal skin of mice showed a very strong and dose-dependent anti-proliferation effect. All doses of atorvastatin effectively (P<0.001) decreased the number of nucleated cell layers, mitotic index of interfollicular epidermis, thickness of epidermis, and disturbance of cell polarity.
From the discussion above, it can be inferred that statins, by inhibition of HMG-CoA reductase, efficiently suppress the production of prenylated proteins. However, the same doses and duration of oral administration of atorvastatin in DMBA-initiated and croton oil-promoted skin tumorgenesis in mice increased the incidence of tumors and their conversion into malignant carcinoma. The reasons for this discrepant effect remain unclear, and could be attributed to the ambivalent action of statins on angiogenesis or on immune system or to specific differences in the experimental conditions of the study. It is suggested that the pro-angiogenic effect of the drug, which can be a reason for the promotion of skin tumors, is independent of HMG-CoA reductase inhibition and can be mediated directly by atorvastatin. In fact, among the diversity of biological effects of statins some could, at least in theory, inhibit tumor growth (e.g. by inhibiting Ras oncoproteins), while other actions may stimulate cancer aggressiveness (e.g. through angiogenic or immunosuppressive effects).
Acknowledgment
The present study was supported by grants from the Research Vice Chancellors of Tabriz University of Medical Sciences; Tabriz, Iran and from the Pharmaceutical Research Network, Ministry of Health, Tehran, Iran.
References
- 1.Garjani A, Andalib S, Ziaee M, Maleki-Dizaji N. Biphasic effects of atorvastatin on inflammation. Pak J Pharm Sci. 2008 Apr;21(2):125–30. [PubMed] [Google Scholar]
- 2.Son JW, Koh KK, Ahn JY, Jin DK, Park GS, Kim DS, et al. Effects of statin on plaque stability and thrombogenicity in hypercholesterolemic patients with coronary artery disease. Int J Cardiol. 2003;88(1):77–82. doi: 10.1016/s0167-5273(02)00368-6. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh A, Hirasawa N, Niki H, Ohuchi K. Cyclo-oxygenase-2-mediated angiogenesis in carrageenan-induced granulation tissue in rats. J Pharmacol Exp Ther. 2000;295(20):802–9. [PubMed] [Google Scholar]
- 4.Liu M, Wang F, Wang Y, Jin R. Atorvastatin improves endothelial function and cardiac performance in patients with dilated ardiomyopathy: the role of inflammation. Cardiovasc Drugs Ther. 2009 Oct;23(5):369–76. doi: 10.1007/s10557-009-6186-3. [DOI] [PubMed] [Google Scholar]
- 5.Minami Y, Satoh M, Maesawa C, Takahashi Y, Tabuchi T, Itoh T, et al. Effect of atorvastatin on microRNA 221/222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. Eur J Clin Invest. 2009 May;39(5):359–67. doi: 10.1111/j.1365-2362.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Liu X, Chen J, Zacharek A, Cui X, Savant-Bhonsale S, et al. Simvastatin enhances bone marrow stromal cell differentiation into endothelial cells via notch signaling pathway. Am J Physiol Cell Physiol. 2009 Mar;296(3):C535–43. doi: 10.1152/ajpcell.00310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heeba G, Moselhy ME, Hassan M, Khalifa M, Gryglewski R, Malinski T. Anti-atherogenic effect of statins: role of nitric oxide, peroxynitrite and haem oxygenase-1. Br J Pharmacol. 2009 Apr;156(8):1256–66. doi: 10.1111/j.1476-5381.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Wang P, Xu X, Wang Y, Xia Y, Wang D. Simvastatin increases the activity of endothelial nitric oxide synthase via enhancing phosphorylation. J Huazhong Univ Sci Technolog Med Sci. 2009 Jun;29(3):286–90. doi: 10.1007/s11596-009-0304-0. [DOI] [PubMed] [Google Scholar]
- 9.Llevadot J, Asahara T. Effects of Statins on angiogenesis and vasculogenesis. Rev Esp Cardiol. 2002 Aug;55(8):838–44. doi: 10.1016/s0300-8932(02)76713-4. [DOI] [PubMed] [Google Scholar]
- 10.Boodhwan , M , Mieno S, Feng J, Sodha NR, Clements RT, Xu SH, et al. Atorvastatin impairs the myocardial angiogenic response to chronic ischemia in normocholesterolemic swine. J Thorac Cardiovasc Surg. 2008 Jan;135(1):117–22. doi: 10.1016/j.jtcvs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Muck AO, Seeger H, Wallwiener D. Class-specific pro-apoptotic effect of statins on human vascular endothelial cells. Z Kardiol. 2004 May;93(5):398–402. doi: 10.1007/s00392-004-0081-5. [DOI] [PubMed] [Google Scholar]
- 12.Katsumoto M, Shingu T, Kuwashima R, Nakata A, Nomura S, Chayama K. Biphasic effect of HMG-CoA reductase inhibitor, pitavastatin, on vascular endothelial cells and angiogenesis. Circ J. 2005 Dec;69(12):1547–55. doi: 10.1253/circj.69.1547. [DOI] [PubMed] [Google Scholar]
- 13.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002 Feb;105(6):739–45. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 14.Skaletz-Rorowski A, Walsh K. Statin therapy and angiogenesis. Curr Opin Lipidol. 2003 Dec;14(6):599–603. doi: 10.1097/00041433-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Khaidakov M, Wang W, Khan JA, Kang BY, Hermonat PL, Mehta JL. Statins and angiogenesis: Is it about connection? Biochem Biophysic Res Commun. 2009 Sep;387(3):543–7. doi: 10.1016/j.bbrc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Graaf MR, Richel DJ, van NoordenCJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004 Nov;30:609–41. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Elson CE, Peffley DM, Hentosh P, Mo H. Isoprenoid-mediated inhibition of mevalonate synthesis: potential application to cancer. Proc Soc Exp Biol Med. 1999 Sep;221(4):294–311. doi: 10.1046/j.1525-1373.1999.d01-87.x. [DOI] [PubMed] [Google Scholar]
- 18.Jakobisiak M, Golab J. Potential antitumor effects of statins (Review) Int J Oncol. 2003 Oct;23(4):1055–69. [PubMed] [Google Scholar]
- 19.Wong WWL, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002 Apr;16(4):508–19. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 20.Dulak J, Józkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-Cancer therapy. Curr Cancer Drug Targets. 2005 Dec;5(8):579–94. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonovas S, Filioussi K, Flordellis CS, Sitaras NM. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol. 2007 Aug;25(23):3462–8. doi: 10.1200/JCO.2007.10.8936. [DOI] [PubMed] [Google Scholar]
- 22.Frits S, Olsen JH. Statin use and cancer risk: an epidemiologic (review) Cancer Invest. 2006 Jun-Jul;24(4):413–24. doi: 10.1080/07357900600705532. [DOI] [PubMed] [Google Scholar]
- 23.Moorman PG, Hamilton RJ. Statins and cancer risk: what do we know and where do we go from here? Epidemiology. 2007 Mar;18(2):194–6. doi: 10.1097/01.ede.0000254699.31405.e2. [DOI] [PubMed] [Google Scholar]
- 24.Garwood ER, Kumar AS, Behner FL, Moore DH, Au A, Hylton N, et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010 Jan;119(1):137–44. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klement H, Rak J. A disconnect between antitumor and antiangiogenic effects of fluvastatin in vitro and in vivo. Neoplasma. 2006;53(2):111–8. [PubMed] [Google Scholar]
- 26.Raick AN. Ultrastructural, histological, and biochemical alteration produced by 12-O-tetradecanoyl-phorbol-13-acetate on mouse epidermis and their relevance to skin tumor promotion. Cancer Res. 1973 Feb;33(2):269–86. [PubMed] [Google Scholar]
- 27.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996 Jan;275(1):55–60. [PubMed] [Google Scholar]
- 28.Glynn SA, O'Sullivan D, Eustace AJ, Clynes M, O'Donovan N. The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, simvastatin, lovastatin and mevastatin inhibit proliferation and invasion of melanoma cells. BMC Cancer. 2008 Jan;16:8–9. doi: 10.1186/1471-2407-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elewa HF, El-Remessy AB, Somanath PR, Fagan SC. Diverse effects of statins on angiogenesis: new therapeutic avenues. Pharmacotherapy. 2010 Feb;30(2):169–76. doi: 10.1592/phco.30.2.169. [DOI] [PubMed] [Google Scholar]
- 30.Azoulay L, Suissa S. Statins in the prevention of prostate cancer recurrence: are certain biases exaggerating the results. J Clin Oncol. 2010 Nov;28(31):e644. doi: 10.1200/JCO.2010.31.1720. [DOI] [PubMed] [Google Scholar]
- 31.Castelo-Branco C. Statins and cancer: harmful or helpful? Maturitas. 2011 Feb;68(2) [Google Scholar]
- 32.Suissa S, Dell'aniello S, Vahey S, Renoux C. Time-window bias in case-control studies: Statins and Lung cancer. Epidemiology. 2011 Mar;22(2):228–31. doi: 10.1097/EDE.0b013e3182093a0f. [DOI] [PubMed] [Google Scholar]