Abstract
Background: Initial studies have shown that low-energy ultrasound stimulates living tissue cells to reduce regeneration or speed up their recovery. The purpose of this study was to examine the effects of various ultrasound parameters on the speed of recovery in injured sciatic nerves NMRI.Methods: NMRI mice (n = 200) with injured left paw, caused by crushing their sciatic nerves, were randomly selected. The animals were exposed to ultrasound radiation with various frequencies, intensities, and exposure time. They were allocated into 20 groups (19 treatment and 1 control groups). Sciatic functional index (SFI) test was used to evaluate the difference between the groups with respect to functional efficiency of the sciatic nerve and its recovery. SFI ، (P=0.000).Results: The results of SFI test obtained from the 14th day showed a significant difference among the groups (P<0.05). On the 14th day after treatment, one of the groups (US11) recovered up to 90%..Conclusion: Altered ultrasound exposure parameters had more favorable outcomes compared with our previous work.
Key Words: Sciatic nerve, Ultrasonic therapy, Regeneration
Introduction
Peripheral nerve injury is a widespread neuro-logical problem, which usually takes many months to regenerate [1, 2]. If the nerve is left to recover naturally during the regeneration period, the innervated muscles are inflected with atrophy leading to complete malformation. Different modalities have been used to address this issue, including operation, physiotherapy by using electric shock, and using magnetic fields or ultrasound [3, 4]. Moreover, the treatment may have positive effects on the therapeutic process of different tissues, such as the skin, muscle, tendons, and nerves [3-7].
Regarding the treatment with ultrasound, the first evaluations were done on conduction velocity of peripheral sensory nerves of Ulna and Radius [7], because the changes in conduction velocity of neurons due to different intensity and period of ultrasound implication are related to mechanical or thermal effects of the ultrasound [8-13].
Lowdon and colleagues [11] have studied the therapeutic effects of ultrasound on the recovery of stress-induced injury of rat tibial nerve. They applied continuous, 1-minute radiation (1 MHz, 0.5 and 1 W/cm2 intensity) for 2-3 weeks. They found that neural conduction speed was improved with 0.5 W/cm2 and more improvement was achieved with 1 W/cm2 compared with non-radiated nerve. They concluded that ultrasound radiations sped up the recovery of stress-induced injury of peripheral nerve, although intense radiation resulted in delayed recovery. In another study, Rat midportion W/cm2similar results were obtained by applying ultrasound radiation to crushed sciatic nerve of rat. The nerve was recovered using ultrasound radiation of 0.25 W/cm2 and 2.25 MHz repeated three times a week for one month [12].
Although previous studies showed the role of ultrasound in speeding up the recovery of injured sciatic nerve, only 1 or 2 parameters of ultrasound were examined in those studies to analyze the efficacy of such a modality [14-19] dutyTherefore, we aimed to examine the effects of various parameters of ultrasound (intensity, frequency, dutycycle SFI cycle, radiation time, and radiation mode [continuous/pulsed]) in various groups of rats to assess the performance of sciatic functional index (SFI) test, to obtain the optimal parameters of therapeutic ultrasound in healing sciatic nerve injury.
MATERIALS AND METHODS
Animals . NMRI.NMRI mice, weighing 27-34 g, were supplied by Pasteur Institute of Iran (Tehran) and were given adequate food and water. The animals were housed in a controlled colony room (temperature 21 ± 3°C), which was maintained under a 12:12 h light/dark cycle. The study was approved by the Ethics Committee of Pasteur Institute of Iran (Tehran).
Preparation . The mice were anesthetized with Xylazine mg/kg Ketamine mg/kg.xylazine (20 mg/kg) and ketamine (50 mg/kg). Their hair was cut and the skin was disinfected for the operations.
Sciatic nerve damage. .Firstly, the mice femur was cut 5 mm cross-sectionally and the sciatic muscles were cut with surgical scissors to expose the sciatic nerve. The nerve was pressed with special forceps for 20 seconds under a force of 50 Newton (N).
Groups . Mice (n = 200) with injured sciatic nerve were randomly assigned into 20 equal groups. The first group was treated with false ultrasound (control group, n = 10) and the other 19 groups were treated with ultrasound radiation 2 days after the injury. The groups are shown in Table 1 as US1~19 according to receiving different parameters of ultrasound (intensity, frequency, dutycycle SFI cycle, radiation time, radiation mode [continuous/pulsed]).
Table 1.
Different parameters of ultrasound used in various therapeutic groups.
| Groups | Intensity (W/cm 2 ) | Frequency (MHz) |
Pulse/
continuous |
Duty cycle (%) | Time (minute) |
|---|---|---|---|---|---|
| US1 | 0.2 | 1 | C | - | 2 |
| US2 | 0.5 | 1 | C | - | 2 |
| US3 | 1.0 | 1 | C | - | 2 |
| US4 | 2.0 | 1 | C | - | 2 |
| US5 | 1.0 | 1 | C | - | 5 |
| US6 | 0.2 | 3 | C | - | 2 |
| US7 | 0.5 | 3 | C | - | 2 |
| US8 | 1.0 | 3 | C | - | 2 |
| US9 | 0.2 | 1 | P | 20 | 5 |
| US10 | 0.2 | 1 | P | 20 | 2 |
| US11 | 0.5 | 1 | P | 20 | 2 |
| US12 | 1.0 | 1 | P | 20 | 2 |
| US13 | 0.5 | 1 | P | 5 | 2 |
| US14 | 0.5 | 1 | P | 5 | 5 |
| US15 | 0.5 | 3 | P | 5 | 2 |
| US16 | 0.5 | 3 | P | 20 | 2 |
| US17 | 0.2 | 3 | P | 5 | 2 |
| US18 | 0.2 | 3 | P | 20 | 2 |
| US19 | 1.0 | 3 | P | 5 | 2 |
Ultrasound radiation. We used the EMS 215A W/cm MHz duty cycle cm.sonotrophy device, which is a therapeutic device used in physiotherapy (EMS Co., UK). Two modes of 1 and 3 MHz frequencies with maximum 2 W/cm2 intensities, and two duty cycles (5% and 20%) were used. The daily duration of exposure was 2 and 5 minutes [20], and the area of the applied probe was 5 cm2. Sciatic nerves of the mice in 19 therapeutic groups were exposed to ultrasonic waves using different ultrasound parameters shown in Table 1cycle two days after the operation (Table 1). Radiation was continued for 14 intermittent days and coupling gel was used all over the injured area exposed to ultrasound.
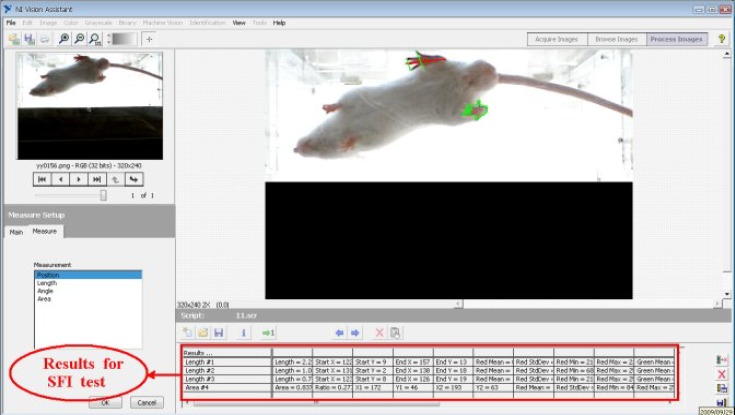
SFI Sciatic functional index test. (sciatic functional index) SFI.Sciatic nerve recovery rate of each mouse was assessed by SFI test. It was done by analyzing the back paw traces of the animals [13, 14]. A laboratory animal treadmill was used to process the images of mice paws. SFI was obtained by NI Vision Assistant 8.6 (National Instruments Co.) under LabVIEW 8.6 software (Fig. 1). SFI was calculated by the following formula 2, 4, 6, 8, 10, 12, 14 days after operation:
Fig. 1.
Image processing software on animal treadmill for imaging and image analysis of animal foot parameter (sciatic function index).
Where PL is print length or maximum distance between the tip of the longest paw to heel, TS is the toe space or spaces of the 1st and 5th toes and IT is middle toes or distance between the 2nd and 4th toes. N is the normal value and E is the experimental value. All the mice were tested for running for a few minutes before operation.
By using SFI values, we can calculate the following values to evaluate the improvement of each group; how every group is recovered, how much is the improvement and how much is the median percentile of each treatment days.
The percent of recovery at the end of treatment days was calculated using the following formula:
The percent of recovery at the end of all treatment days was calculated using the following formula:
Analysis of data. Data were analyzed using SPSS software, version 16.0. The results were shown as mean ± SD. One way analysis of variance (ANOVA) and Post-hoc Tukey test were used as appropriated and P<0.05 was considered as statistically significant.
Results
The toe of the left paw of all the in the 20 groups was strained and twitched after the operation. They were almost unable to stand on the left paw in the early days, and it took many days to be recovered. On the 14th day, they were almost returned to the normal condition, and especially the mice in the 11th group (US11) achieved 90%.
Sciatic functional index . .All back paw images of 200 mice were recorded by treadmill system. They were processed before operation and analyzed in 2, 4, 6, 8, 10, 12, 14 days after operation. Mean and standard deviations of SFI are shown in Table 2. Parameters of ultrasound used in US11 group resulted in a better improvement both for the primary and final days of the treatment compared with the other groups (Table 2). We observed that ultrasound of lower intensities had more desirable results in the final days of the treatment, but increased intensities (i.e. I = 0.5 W/cm2) had more desirable results in the primary days of the treatment.
Table 2.
Mean and standard deviation of sciatic functional index before and after the injury for different ultrasound therapeutic groups.
| Groups |
Days
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |
| control | -3.4 ± 6.19 | -101.5 ± 4.86 | -97.7 ± 3.77 | -88.7 ± 3.79 | -90.2 ± 4.06 | -78.1 ± 3.23 | -62.0 ± 6.18 | -43.6 ± 4.85 |
| US1 | -4.3 ± 3.51 | -100.2 ± 4.02 | -100.1 ± 4.01 | -96.3 ± 2.23 | -83.1 ± 3.81 | -68.3 ± 5.20 | -49.6 ± 4.90 | -40.2 ± 2.02 |
| US2 | -4.0 ± 2.32 | -99.3 ± 5.61 | -99.5 ± 5.08 | -93.1 ± 6.20 | -84.2 ± 4.19 | -71.2 ± 3.87 | -52.3 ± 3.91 | -43.9 ± 4.01 |
| US3 | -4.1 ± 7.72 | -97.5 ± 6.13 | -100.6 ± 4.79 | -94.9 ± 5.02 | -87.4 ± 4.46 | -74.3 ± 4.87 | -56.4 ± 5.06 | -45.1 ± 5.24 |
| US4 | -4.6 ± 5.06 | -81.1 ± 5.93 | -99.2 ± 4.43 | -101.0 ± 0.00 | -101.0 ± 0.00 | -101.0 ± 0.00 | -101.0 ± 0.00 | -101.0 ± 0.00 |
| US5 | -5.0 ± 2.78 | -96.3 ± 3.01 | -100.2 ± 2.71 | -93.1 ± 5.63 | -89.2 ± 3.71 | -99.3 ± 5.01 | -102.1 ± 0.00 | -102.1 ± 0.00 |
| US6 | -4.2 ± 3.42 | -98.4 ± 3.46 | -99.3 ± 4.52 | -98.2 ± 5.91 | -85.3 ± 2.95 | -70.2 ± 3.08 | -53.2 ± 5.12 | -45.3 ± 5.61 |
| US7 | -5.1 ± 2.91 | -101.1 ± 4.51 | -99.0 ± 3.91 | -95.2 ± 4.88 | -87.6 ± 3.65 | -75.8 ± 2.99 | -56.2 ± 3.18 | -47.9 ± 2.43 |
| US8 | -4.2 ± 2.56 | -99.3 ± 2.23 | -98.5 ± 5.21 | -96.3 ± 3.43 | -90.7 ± 4.36 | -77.3 ± 4.83 | -60.3 ± 4.31 | -50.7 ± 2.53 |
| US9 | -4.7 ± 5.70 | -103.0 ± 8.88 | -99.5 ± 4.36 | -76.6 ± 5.89 | -80.3 ± 5.60 | -50.1 ± 4.18 | -41.4 ± 5.80 | -30.7 ± 6.69 |
| US10 | -4.3 ± 2.49 | -99.6 ± 3.09 | -95.8 ± 3.96 | -86.5 ± 2.59 | -81.6 ± 4.38 | -51.5 ± 3.61 | -39.0 ± 4.97 | -27.2 ± 4.51 |
| US11 | -6.6 ± 2.94 | -98.8 ± 2.50 | -97.0 ± 3.69 | -78.8 ± 2.79 | -60.6 ± 5.42 | -43.9 ± 4.61 | -25.2 ± 4.03 | -15.7 ± 3.55 |
| US12 | -5.5 ± 2.69 | -95.9 ± 3.59 | -92.3 ± 3.27 | -86.9 ± 2.93 | -69.7 ± 4.47 | -46.9 ± 5.01 | -37.9 ± 4.10 | -28.0 ± 4.38 |
| US13 | -4.5 ± 2.84 | -93.4 ± 4.16 | -89.4 ± 3.04 | -76.6 ± 3.38 | -74.6 ± 5.30 | -53.7 ± 4.93 | -39.0 ± 3.31 | -32.7 ± 3.26 |
| US14 | -3.5 ± 2.44 | -89.8 ± 3.11 | -90.0 ± 2.51 | -81.6 ± 2.73 | -75.1 ± 4.02 | -64.8 ± 5.09 | -50.7 ± 4.76 | -34.9 ± 5.30 |
| US15 | -4.9 ± 2.85 | -95.0 ± 3.56 | -102.0 ± 2.55 | -93.5 ± 4.58 | -84.4 ± 4.01 | -79.3 ± 6.80 | -59.5 ± 7.36 | -40.6 ± 6.48 |
| US16 | -4.1 ± 3.01 | -96.1 ± 4.56 | -99.7 ± 5.12 | -94.3 ± 3.47 | -82.1 ± 3.49 | -78.1 ± 5.12 | -57.3 ± 6.13 | -39.7 ± 3.14 |
| US17 | -3.8 ± 2.86 | -101.0 ± 3.03 | -100.5 ± 2.95 | -96.4 ± 2.90 | -91.2 ± 3.30 | -77.9 ± 6.52 | -57.9 ± 6.27 | -37.9 ± 6.34 |
| US18 | -3.2 ± 2.16 | -101.1 ± 2.34 | -98.6 ± 2.73 | -96.6 ± 2.06 | -83.7 ± 4.08 | -67.5 ± 3.24 | -57.3 ± 3.47 | -41.7 ± 2.96 |
| US19 | -5.2 ± 3.22 | -99.3 ± 4.03 | -98.4 ± 3.69 | -95.2 ± 4.58 | -87.3 ± 5.01 | -84.2 ± 3.19 | -63.2 ± 6.12 | -45.7 ± 2.18 |
Statistically, parameters of ultrasound used in US11 group resulted in a better improvement both for the primary and final days of the treatment. Moreover, only US11 group in the final days (from 8 to 14 days) showed a significant difference compared with the other groups (P<0.05).
Generally, ultrasound frequency of 3 MHz was less effective than 1 MHz low duty cycle does not result in very effective recovery and by using 20% duty cycle, the recovery was improved.
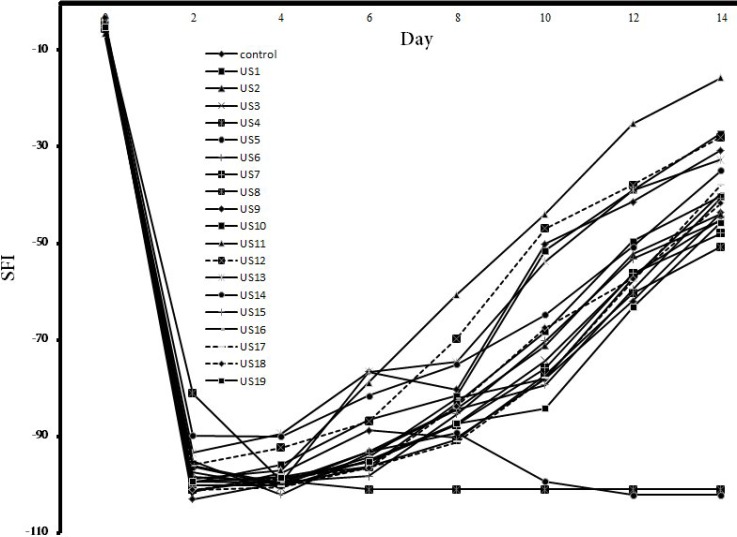
.It seems that 2-minute radiation had more desirable results, but continuous mode of radiation produced higher heat leading to less effective results compared with the pulse mode. Figure 2 shows the graph drawn according to the data of Table 1, which can be used to analyze the trend of recovery among the mice in various groups.
Fig. 2.
Sciatic function index of the 20 groups compared with continuous treatment days and pre operation day.
Table 3 shows the total recovery in the last day of treatment and mean total recovery days in various groups of mice. These values are very important to compare the improvement among groups, because we can compute the amount of the recovery of each group with regard to changes in ultrasound exposure parameters. Hence, the best ultrasound exposure parameters among groups can be obtained.
Table 3.
Total recovery in the last day of treatment and mean total recovery days in various groups of mice.
| Groups |
average total
recovery days (%) |
last recovery day (%) |
|---|---|---|
| control | 19.67 | 59.03 |
| US1 | 25.74 | 62.56 |
| US2 | 21.60 | 58.13 |
| US3 | 19.67 | 56.11 |
| US4 | 1.94 | -26.01 |
| US5 | 5.34 | -15.43 |
| US6 | 22.01 | 56.36 |
| US7 | 20.22 | 55.41 |
| US8 | 21.89 | 51.10 |
| US9 | 32.89 | 73.56 |
| US10 | 30.09 | 75.98 |
| US11 | 38.81 | 90.14 |
| US12 | 31.24 | 75.12 |
| US13 | 29.28 | 68.28 |
| US14 | 21.21 | 63.62 |
| US15 | 15.61 | 60.38 |
| US16 | 19.02 | 61.30 |
| US17 | 18.59 | 64.92 |
| US18 | 21.68 | 60.68 |
| US19 | 20.48 | 56.96 |
The most improved sciatic nerves are related to the US11 group with 90% recovery (P<0.05) .As shown in Table 4, the best procedures for treatment belonged to US13 group on the 4th day after operation, US9 on the 6th day, and US11 on the final day.
Table 4.
The best treatment procedure in different days of experiment.
| Rank |
Days
|
|||||
|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 14 | |
| 1 | US13 | US9 | US11 | US11 | US11 | US11 |
| 2 | US10 | US11 | US12 | US9 | US9 | US10 |
| 3 | control | US13 | US9 | US12 | US10 | US9 |
| 4 | US12 | US10 | US13 | US10 | US12 | US12 |
| 5 | US9 | control | US10 | US13 | US13 | US17 |
| 6 | US18 | US12 | US18 | US18 | US1 | US13 |
| 7 | US7 | US14 | US1 | US1 | US2 | US1 |
| 8 | US11 | US2 | US2 | US6 | US6 | US18 |
| 9 | US19 | US7 | US14 | US2 | US7 | control |
| 10 | US8 | US17 | US16 | US7 | US18 | US16 |
| 11 | US17 | US18 | US7 | US14 | US17 | US2 |
| 12 | US1 | US19 | US6 | control | US3 | US14 |
| 13 | US14 | US1 | US19 | US3 | control | US15 |
| 14 | US2 | US5 | control | US17 | US14 | US19 |
| 15 | US6 | US8 | US15 | US8 | US8 | US7 |
| 16 | US3 | US3 | US3 | US16 | US16 | US6 |
| 17 | US16 | US16 | US17 | US15 | US19 | US3 |
| 18 | US5 | US15 | US8 | US19 | US15 | US8 |
| 19 | US15 | US6 | US5 | US5 | US5 | US5 |
| 20 | US4 | US4 | US4 | US4 | US4 | US4 |
Statistically, only US11 group in the final days (from 8 to 14 days) showed significant difference compared with the other groups (P<0.05). But the other groups did not have significant difference with the other groups (P>0.05).
Discussion
We found that the groups were not different before the operation and 2, 4, and 6 days after the operation (P>0.05), but for US11 group and 8, 10, 12 and 14 days after operation, a significant difference was found (P<0.05). The trend had fully been effective and there was a significant difference among the groups, which was remarkable in the final days. According to Figures 1 and 2 and Table 3, we conclude that changes in ultrasound parameters and their increase or decrease may help us to interpret and study the improvement trend. Therefore, it is anticipated that by using more groups and selecting precise parameters of ultrasound radiation, the improvement process may be interpreted more precisely [21-25].
As shown in Table 4, the US11 group had Table 4 showsAthe best improvement percentile compared with the other groups. For the US11 group, the parameters of ultrasound were as follows: US5: Intensity= 0.5 W/cm², Frequency= 1 MHz, pulse mode,intensity = 0.5 W/cm², frequency = 1 MHz, pulse mode, dDuty cycle = 20%, time = 2 min, 14 treatmentddduty cycle = 20%, duration = 2 min, and 14 treatment days. The recovery in the last day of treatment was 90% and total recovery was 39% (Table 3). Therefore, it is concluded that the experimented mice had more improvement which is remarkable compared with the previous studies [1-5].
According to Table 4, the best procedure for treatment was that used for the US13 group on the 4th day after operation, US9 on the 6th day, and US11 at the final day. It may be a general instruction for physiotherapists and researchers to recover nerve and speed up the improvement process. As our results were achieved by experimenting on mice, the future instruction of our findings for human beings should be performed with caution. Selecting the continuous mode could produce more heat for NMRI mice, which can cause reverse effects on the recovery. Thus, it can be considered that non-thermal effects of ultrasound are superior to its thermal effects [15-17, 26-30].
In this study, we examined the effects of ultrasound on the recovery of peripheral nerve injury by altering parameters of ultrasound with 20 groups. Based on the tests, we obtained some optimal combination of values in ultrasound parameters compared with previous studies [5-8]. Those desirable parameters could be obtained just for one or two groups. It is considered that these tests are not enough to precisely interpret the improvement of nerve recovery. Therefore, this is the first study to systematically examine and compare the effects of ultrasound parameters on the recovery of injured peripheral nerve.
ACKNOWLEDGEMENTS
This study was supported by a grant (no. 470) from Pasteur Institute of Iran (Tehran).
References
- 1.Jiménez-Díaz F, Jimena I, Luque E, Mendizábal S, Bouffard A, Jiménez-Reina L, et al. Experimental muscle injury: Correlation between ultrasound and histological findings. Muscle Nerve. 2012 May;45(5):705–12. doi: 10.1002/mus.23243. [DOI] [PubMed] [Google Scholar]
- 2.Wu YH, Liang HW, Chen WS, Lai JS, Luh JJ, Chong FC. Electrophysiological and functional effects of shock waves on the sciatic nerve of rats. Ultrasound Med Biol. 2005 Oct;34(10):1688–96. doi: 10.1016/j.ultrasmedbio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Stratmeyer ME, Greenleaf JF, Dalecki D, Salvesen KA. Fetal ultrasound: mechanical effects. J Ultrasound Med. 2008 Apr;27(4):597–605. doi: 10.7863/jum.2008.27.4.597. [DOI] [PubMed] [Google Scholar]
- 4.Hasuike A, Sato S, Udagawa A, Ando K, Arai Y, Ito K. In vivo bone regenerative effect of low-intensity pulsed ultrasound in rat calvarial defects. Oral Surg Radiol Endod. 2011 Jan;111(1):112–20. doi: 10.1016/j.tripleo.2010.09.061. [DOI] [PubMed] [Google Scholar]
- 5.Madduri S, Gander B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J Control Release. 2011 Dec; doi: 10.1016/j.jconrel.2011.11.036. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Chen WZ, Qiao H, Zhou W, Wu J, Wang ZB. Upgraded nerve growth factor expression induced by low intensity continuous-wave ultrasound accelerates regeneration of neurotometicly injured sciatic nerve in rats. Ultrasound Med Biol. 2010 Jul;36(7):1109–17. doi: 10.1016/j.ultrasmedbio.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Farmer WC. Effect of intensity of ultrasound on conduction of motor axons. Phys Ther. 1986;48:1233–7. doi: 10.1093/ptj/48.11.1233. [DOI] [PubMed] [Google Scholar]
- 8.Currier DP, Greathouse D, Swift T. Sensory nerve conduction: effect of ultrasound: effect of ultrasound. Arch Phys Med Rehab. 1987 Apr;59(4):181–5. [PubMed] [Google Scholar]
- 9.Halle JS, Scoville CR, Greathouse DG. Ultrasound's effect on conduction latency ofsuperficial radial nerve in man. Phys Ther;61:345–50. doi: 10.1093/ptj/61.3.345. [DOI] [PubMed] [Google Scholar]
- 10.Moore JH, Gieck JH, Saliba EN, Perrin DH, Ball DW, Mccue FC. The biophysical effects of ultrasound on median nerve distal latencies. Electromyogr Clin Neurophysiol. 2000 Apr;40(3):169–80. [PubMed] [Google Scholar]
- 11.Lowdon IM, Seaber AV, Urbaniak JR. An improved method of recording rat tracks for measurement of the sciatic functional index of de Medinaceli. J Neurosci Methods. 1988 Jul;24(3):279–81. doi: 10.1016/0165-0270(88)90173-2. [DOI] [PubMed] [Google Scholar]
- 12.Mourad PD, Lazar DA, Curra FP, Mohr BC, Andrus KC, Avellino AM, et al. Ultrasound accelerates functional recovery after peripheral nerve damage. Neurosurgery. 2001 May;48(5):136–40. doi: 10.1097/00006123-200105000-00035. [DOI] [PubMed] [Google Scholar]
- 13.DeMedinaceliL , Freed WJ, Wyatt RJ. An index of the functional condution of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982 Sep;77:634–43. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 14.DeMedinaceli L, Derenzo E, Wyatt RJ. Rat sciatic funcional index data management system with digited input. Comp Biom Res. 1984 Apr;17:185–92. doi: 10.1016/0010-4809(84)90031-4. [DOI] [PubMed] [Google Scholar]
- 15.Raso VV, Barbieri CH, Mazzer N, Fasan VS. Can therapeutic ultrasound influence the regeneration of peripheral nerves. J Neurosci Methods. 2005 Mar;142(2):185–92. doi: 10.1016/j.jneumeth.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Chang CJ, Hsu SH. The effects of low-intensity ultrasound on peripheral nerve regeneration in poly (DL-lactic acid-CO-glycolic acid) conduits seeded with Schwann cells. Ultrasound Med Biol. 2004 Aug;30(8):1079–84. doi: 10.1016/j.ultrasmedbio.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W, Chen W, Zhou K, Zhibiao W. Low-intensity ultrasound for regeneration of injured peripheral nerve. Neural Regeneration Res. 2006 Jul;1(7):605–8. [Google Scholar]
- 18.Crisci AR, Ferreira AL. Low-Intensity pulsed ultrasound accelerates the regeneration of the sciatic nerve after neurotomy in Rats. Ultrasound Med Biol. 2002 Oct;28(10):1335–41. doi: 10.1016/s0301-5629(02)00576-8. [DOI] [PubMed] [Google Scholar]
- 19.Lazar DA, Curra FP, Mohr B, McNutt LD, Kliot M, Mourad PD. Acceleration of recovery after injury to the peripheral nervous system using ultrasound and other therapeutic modalities. Neurosurg Clin N Am. 2001 Apr;12(2):353–7. [PubMed] [Google Scholar]
- 20.Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol. 2009 Oct;35(10):1737–47. doi: 10.1016/j.ultrasmedbio.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paik NJ, Cho SH, Han TR. Ultrasound therapy facilitates the recovery of acute pressure-induced conduction block of the median nerve in rabbits. Muscle Nerve. 2002 Sep;26(3):356–61. doi: 10.1002/mus.10209. [DOI] [PubMed] [Google Scholar]
- 22.Michlovitz SL. Is there a role for ultrasound and electrical stimulation following injury to tendon and nerve. J Hand Ther. 2005 Apr;18(2):292–6. doi: 10.1197/j.jht.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Gebauer D, Mayer E, Northner E, Ryaby JP. Low-intensity pulsed ultrasound: effects on nounions. Ultrasound Med Biol. 2005 Oct;31(10):1391–1402. doi: 10.1016/j.ultrasmedbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007 Jan;93:212–55. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De KoolBS, Blok JH, Walbeehm ET, Van NeckJW, Hovius SE, Visser GH. Ultrasound-guided near-nerve neurography for early evaluation of nerve regeneration. J Neurosci Methods. 2008 Sep;174(2):265–71. doi: 10.1016/j.jneumeth.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Lin X, Wan H, Li JH, Li JM. Effect of low-intensity pulsed ultrasound on the expression of neurotrophin-3 and brain-derived neurotrophic factor in cultured Schwann cells. Microsurgery. 2009 Mar;29(6):479–85. doi: 10.1002/micr.20644. [DOI] [PubMed] [Google Scholar]
- 27.Van NeckJW, De KoolBS, Hekking-Weijma JI, Walbeehm ET, Visser GH, Blok JH. Histological validation of ultrasound-guided neurography in early nerve regeneration. Muscle Nerve. 2009 Dec;40(6):967–75. doi: 10.1002/mus.21405. [DOI] [PubMed] [Google Scholar]
- 28.Park SC, Oh SH, Seo TB, Namgung U, Kim JM, Lee JH. Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. J Biomed Mater Res B Appl Biomater. 2010 Aug;94(2):359–66. doi: 10.1002/jbm.b.31659. [DOI] [PubMed] [Google Scholar]
- 29.Kuffler DP. Ultrasound imaging of regenerating rat sciatic nerves in situ. J Neurosci Methods. 2010 May;188(2):276–9. doi: 10.1016/j.jneumeth.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Fernanda GJ, Nilton M, Vanessa VM, Anita SLL, Cláudio HB. Therapeutic ultrasound on the spinal cord accelerates regeneration of the sciatic nerve in rats. Acta Ortop Bras. 2011 Jun;19(4):213–8. [Google Scholar]