Abstract
Background
The aim of this study was to understand any association between differentiated thyroid carcinoma (DTC) and Ile3434Thr XRCC7 gene polymorphism (GenBank accession number: rs7830743). DTC is the most prevalent thyroid neoplasm, which includes papillary and follicular cell carcinoma. XRCC7 gene encodes a protein that functions in non-homologous end joining DNA repair pathway. Non-synonymous polymorphisms in this gene may alter DNA repair capacity of the cell and change the risk of developing cancers.
Methods
DTC patients (n = 173) and cancer free individuals (n = 204) were enrolled in a case-control study. The Ile3434Thr polymorphic alleles were discriminated by using amplification refractory mutation system-PCR method. The frequencies of this single nucleotide polymorphism in case and control groups were compared. Also, risk ratio for developing DTC in dichotomized genotypes was estimated by multivariate logistic regression analysis.
Results
Dichotomized genotypes into those with and without the 3434Thr allele showed that this allele was associated with DTC (OR [odd ratio]: 1.89, 95% confidence interval (CI) = 1.29-2.79, P<0.001). Also, TC genotype was significantly associated with increased risk of DTC (OR: 2.42, 95% CI = 1.55-3.81, P = 0.0001) in individuals carrying this genotype.
Conclusion
Allele 3434Thr in XRCC7 gene might be associated with differentiated thyroid cancer risk. Further studies with larger samples are needed to verify these initial findings.
Key Words: DNA repair enzymes, Thyroid neoplasms, Genetic polymorphism
Introduction
Thyroid cancer is the most prevalent endocrine cancer [1] and it is more prevalent in females. The worldwide estimated new case of thyroid cancer is 163,000 in females [2]. Differentiated thyroid carcinoma (DTC) mostly includes papillary thyroid carcinoma and follicular thyroid carcinoma. Exposure to radiation especially at young ages is a risk factor in thyroid cancer [3], though in most cases, specific risk factors for DTC cannot be identified [4]. As double-strand breaks (DSB) are produced by replication errors and exogenous agents such as ionizing radiation, it has been suggested that genetic variants in DNA repair pathways may be involved in DTC cancer risk [5]. There are two major DSB repair pathways in mammalian cells: homologous recombination and non-homologous end joining (NHEJ) [6]. NHEJ joins the ends of a broken DNA without need for homology sequence and it is an important repair mechanism for DSB in mammalian cells. This pathway requires DNA-dependent protein kinase (DNA-PK), so-called XRCC7 [7, 8]. This is a holoenzyme consisting of a Ku DNA-Binding domain and a catalytic subunit, which starts the NHEJ process. Ku binds a DSB and uses the catalytic subunit, DNA-PKc, to bind DNA termini. This process activates serine/threonine protein kinase enzymatic activity [9].
The association of DNA-PK variants with different kinds of cancer including thyroid [10] has been reported [11-13]. Up to now, more than 300 genetic variants have been reported in XRCC7 gene [14]. Among them, I3434T poly-morphism (GenBank accession number: rs7830743) is the most prevalent variant with mean allele frequency of 0.1439. The relationship between non-synonymous polymorphism of XRCC7 gene and occurrence of DTC was the subject of our case-control study, as the relevant amount of mean allele frequency provides smaller sample size for case-control study [15].
MATERIALS AND METHODS
Patients and controls. We recruited 173 patients with DTC (134 females and 39 males) and 204 controls (153 females and 51 males) from Research Institute for Nuclear Medicine of Shariati Hospital (Tehran, Iran) for GenBank (accession number: rs7830743) single nucleotide polymorphism (SNP) analysis during September 2008-2009. The individuals signed a written consent form for genetic tests. None of the following history was included in the selection criteria: cervical lymph node involvement, other cancer(s), being heavy smoker or alcoholism and a radiation exposure.
XRCC7 genotyping. Amplification refractory mutation system-PCR technique was used for discriminating the C and T alleles. A pair of common primers (CF: 5'-CAA GCC AAA AAG GGA AAG TG-3' and CR: 5'-GGC TCA AAG TCT CCT CTG GA-3’) was used based on previous work in the literature [16] to produce non-allele-specific amplicon and designated as common. Two allele-specific primers (SC: 5'-TGC AGT TCT GCA GAA TCA G-3' and ST: 5'-CTT TGG TGT CCT TGA TAG TTA T-3') were designed for production of allele-specific amplicons. A 241-bp DNA segment was amplified using CF and CR primers, while 116 bp and 165 bp allele-specific amplicons were amplified using CF-SC and CR-ST primer pairs, respectively. For each sample, two PCR reactions with three primers were performed: CF and CR were common in both reactions, while, SC and ST were specific primers for each one (designated as "C" and "T" reactions, respectively). The PCR mixture (25 μl) contained 2.5 μl PCR 10X buffer, 0.5 μl dNTP, 0.75 μl MgCl2, 10 pmol common primers, 24 pmol C or T primers, 0.1 unit Taq DNA polymerase, 19.25 μl double-distilled water, and 10-15 ng genomic DNA. The cycling conditions were at 95°C for 10 min, followed by 35 cycles (94°C, 30 s; 56°C, 30 s and 72°C, 60 s) and a final cycle at 72°C for 7 min. PCR products were separated by standard electrophoresis on 2.5% agarose gel containing ethidium bromide. For confidence, a few of samples were subjected to DNA sequencing.
Statistical analysis: Hardy-Weinberg equilibrium (p2 + 2pq + q2 = 1, p = frequency of the variant allele, q = 1-p) was tested by χ2 test to compare the observed genotype frequencies with the estimated ones within the control group. Risk ratio for developing DTC in dichotomized genotypes was estimated by multivariate logistic regression analysis, and A P value of less than 0.05 indicated statistical significance. In this analysis, data for genotype frequencies, without sex or age adjustment, were used for calculation of the of odd ratio (OR). The statistical analyses were performed by SPSS version 13.
RESULTS AND DISCUSSION
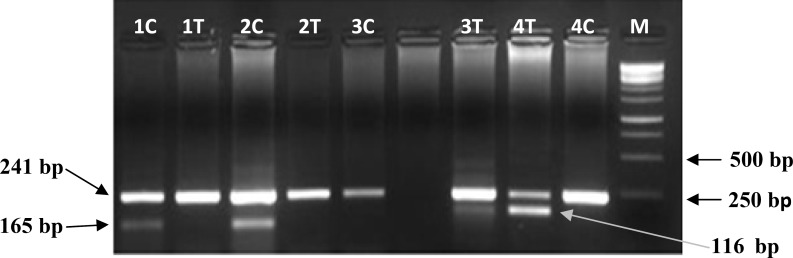
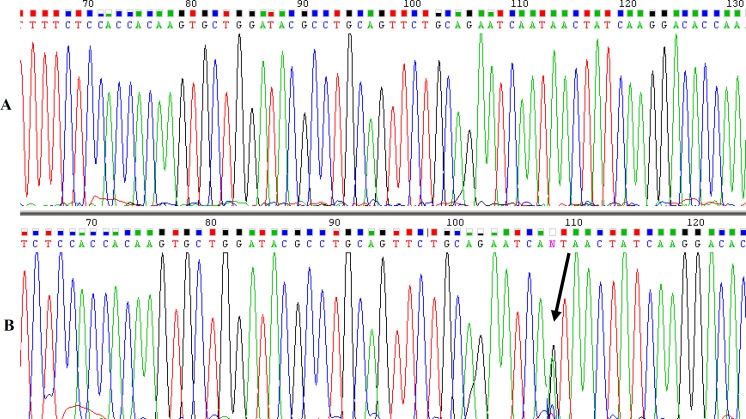
Allele discrimination was performed by amplification refractory mutation system PCR method (Fig. 1). Presence of bands with expected size in relevant PCR product reactions showed presence of one or two allele(s). Common amplicon was used as internal PCR control. The results of DNA sequencing of a wild type and a variant carrier have been shown in Figure 2.
Fig. 1.
Amplification refractory mutation system PCR for allele discrimination of Ile3434Thr polymorphism in XRCC7 gene on 2.5% gel electrophoresis. Two PCR reactions were performed for each sample: C and T; C allele and T allele reactions, respectively. Samples numbers 1 and 2, CC genotype; samples 3 and 4, TT genotype; M, 1 kb DNA markers. The 241, 165 and 116 bp bands correspond to common, C allele and T allele PCR products, respectively.
Fig. 2.
Electropherograms of XRCC7 gene PCR amplicons using CF and CR primers (see text). (A) Homozygous wild allele and (B) heterozygous variant allele. Solid arrow shows nucleotide position change.
The demographic characteristics of the study subjects have been summarized in Table 1. There was no significant difference in gender and age between DTC cancer patients and controls.
Table 1.
Demographic information of case and control subjects.
| Variable |
DTC (n = 173
)
|
P value |
Controls (n = 204)
|
||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | 0.577 | ||||
| Male | 39 | 22.54 | 51 | 25.00 | |
| Female | 134 | 77.45 | 153 | 75.00 | |
| Age | 0.301 | ||||
| <=50 | 142 | 82.08 | 159 | 77.94 | |
| >50 | 31 | 17.91 | 45 | 22.05 | |
P<0.05 is significant.
The observed genotype distributions of XRCC7 gene among cases and controls have been shown in Table 2. Based on Hardy-Weinberg equation, the observed genotypes showed no deviation from that expected. The frequency of C allele observed in DTC patients was about 75%, which was significantly higher than that in controls (52%, P = 0.001). Also, the calculated OR (1.89, 95% CI = 1.29-2.79) demonstrated a higher risk in DTC patients for having T allele. On the other hand, a significant difference in genotype distribution was found among DTC cancer and control groups. Furthermore, different OR were calculated to evaluate the risk of DTC in different genotypes (Table 3).
Table 2.
Genotype frequencies of case and control subjects.
| Variable |
DTC (n = 173
)
|
P value |
Controls (n = 204)
|
||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Genotypes | 0.0005 | ||||
| TT | 101 | 58.38 | 156 | 76.47 | |
| TC | 69 | 39.88 | 44 | 21.56 | |
| CC | 3 | 1.73 | 4 | 1.96 | |
| Alleles | 0.001 | ||||
| C | 75 | 52 | |||
P<0.05 is significant.
Table 3.
Odd ratio (OR) and confidence interval (CI) of case and control subjects.
| Variable | Crude OR | 95% CI | P value |
|---|---|---|---|
| CC vs TT | 1.16 | 0.25- 5.28 | 0.8490 |
| TC vs TT | 2.42 | 1.55- 3.81 | 0.0001 |
| (CC or TC) vs TT** | 2.32 | 1.49-3.61 | 0.0002 |
| CC vs (TC or TT)*** | 0.88 | 0.19-4.00 | 0.8710 |
P<0.05 is significant; **dominant C allele; ***recessive C allele
There are many factors in human carcinogenesis; one of which is genetic susceptibility that is involved at different stages of the cancer process [17]. In the present study, we examined the relationship between XRCC7 gene polymorphism and DTC patients in a subpopulation in Iran. The product of this gene participates in NHEJ pathway, which is one of the DSB repair pathways [18]. It has been shown that cells lacking XRCC7 are sensitive to ionizing radiation and other DNA damaging agents and display impaired DNA repair [19]. It can be assumed that variants of XRCC7 gene may alter the function of the so-called product [20], which per se may contribute to change the risk of cancer development.
There are only limited studies evaluating the association of different SNPs in NHEJ genes with the risk of different types of cancer. One report from China revealed a negative association of XRCC7 6721G allele (GenBank accession number: rs7003908, OR = 0.70, 95% CI = 0.47-1.03) with risk of bladder cancer (OR = 1.53, 95% CI = 1.04-2.25) [13]. However, the association of the same SNP with urothelial bladder cancer (OR = 4.45, P = 0.001) and prostate cancer (OR = 1.529, P = 0.002) showed an opposite manner in northern population of India [12, 21]. Also, a significant relationship between XRCC7 6721GG genotype with hepatocellular cell carcinoma has been shown [11]. However, no significant difference was noted in genotypes of several XRCC7 SNPs between glioma patients and controls [22].
Here, we performed a case-control study to analyze the association of Ile3434Thr change (rs: 7830743, T>C) in XRCC7 gene with DTC in an Iranian subpopulation. Our study shows a significant association of Ile3434Thr polymorphism in XRCC7 gene with DTC in Iranian population. The risk of DTC was increased in a dose-response manner as the number of C alleles (Thr) was increased. Also, when we used TT as the reference, the dominant model (CC or TC) was associated with 2.32 increased risk of DTC (P<0.0002). However, the relationship between this SNP with papillary thyroid carcinoma has not been shown in a similar study in Saudi Arabia with 223 cases and 229 controls [16]. This discrepancy between the results may probably be due to different populations and different factors, which influence developing thyroid carcinomas, small sample size or other unknown phenomena.
This non-synonymous polymorphism results in substitution of isoleucine with threonine in position 3434 in the FRAP-ATM-TRRAP domain of DNA-PK protein. This domain interacts with kinase domain to stabilize the C-terminus region of enzyme. It can be assumed that substitution of a non-polar isoleucine with a polar threonine may alter the function of this domain and eventually the DSB repair capacity of the genome.
Although the potential involvement of defects in the XRCC7 gene in inducing chromosomal instability is conceivable, to date, no direct clinical evidence has been obtained in patients with thyroid cancer. Therefore, to our knowledge, this is the first report that shows the relationship between XRCC7 gene and thyroid cancer.
In summary, this study provides the evidences that the polymorphism of XRCC7 rs: 7830743 may be a risk factor for development of DTC in an Iranian population. Because this is the first study which reports the significant association between XRCC7 polymorphism and DTC cancer susceptibility, to confirm our findings, additional studies with larger sample size are recommended in different ethnic populations.
ACKNOWLEDGEMENTS
This project was supported by the Pasteur Institute of Iran.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009 Aug;115(16):3801–7. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, Brill AB, et al. A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006 Jul;98(13):897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 4.Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, et al. The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. 2011 Dec;32(6):798–826. doi: 10.1210/er.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adjadj E, Schlumberger M, de VathaireF. Germ-line DNA polymorphisms and susceptibility to differentiated thyroid cancer. Lancet Oncol. 2009 Feb;10(2):181–90. doi: 10.1016/S1470-2045(09)70020-8. [DOI] [PubMed] [Google Scholar]
- 6.West SC. Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol. 2003 Jun;4(6):435–45. doi: 10.1038/nrm1127. [DOI] [PubMed] [Google Scholar]
- 7.Meek K, Gupta S, Ramsden DA, Lees-Miller SP. The DNA-dependent protein kinase: the director at the end. Immunol Rev. 2004 Aug;200:132–41. doi: 10.1111/j.0105-2896.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 8.Lees-Miller SP, Meek K. Repair of DNA double strand breaks by non-homologous end joining. Biochimie. 2003 Nov;85(11):1161–73. doi: 10.1016/j.biochi.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Pawelczak KS, Turchi JJ. A mechanism for DNA-PK activation requiring unique contributions from each strand of a DNA terminus and implications for microhomology-mediated nonhomologous DNA end joining. Nucleic Acids Res. 2008 Jul;36(12):4022–31. doi: 10.1093/nar/gkn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long XD, Yao JG, Huang YZ, Huang XY, Ban FZ, Yao LM, et al. DNA repair gene XRCC7 polymorphisms (rs#7003908 and rs#10109984) and hepatocellular carcinoma related to AFB1 exposure among Guangxi population, China. Hepatol Res. 2011 Nov;41(11):1085–93. doi: 10.1111/j.1872-034X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 11.Mandal RK, Kapoor R, Mittal RD. Polymorphic variants of DNA repair gene XRCC3 and XRCC7 and risk of prostate cancer: a study from North Indian population. DNA Cell Biol. 2010 Nov;29(11):669–74. doi: 10.1089/dna.2010.1047. [DOI] [PubMed] [Google Scholar]
- 12.Wang SY, Peng L, Li CP, Li AP, Zhou JW, Zhang ZD, et al. Genetic variants of the XRCC7 gene involved in DNA repair and risk of human bladder cancer. Int J Urol. 2008jun;15(6):534–9. doi: 10.1111/j.1442-2042.2008.02049.x. [DOI] [PubMed] [Google Scholar]
- 13.Sturgis EM, Zhao C, Zheng R, Wei Q. Radiation response genotype and risk of differentiated thyroid cancer: a case-control analysis. Laryngoscope. 2005 Jun;115(6):938–45. doi: 10.1097/01.MLG.0000163765.88158.86. [DOI] [PubMed] [Google Scholar]
- 14. Available from: http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?geneId=5591.
- 15.Kasiulevičius V, Šapoka V, Filipavičiūtė R. Sample size calculation in epidemiological studies. Gerantologija. 2006;7(4):225–31. [Google Scholar]
- 16.Siraj AK, Al-Rasheed M, Ibrahim M, Siddiqui K, Al-Dayel F, Al-Sanea O, et al. RAD52 polymorphisms contribute to the development of papillary thyroid cancer susceptibility in Middle Eastern population. J Endocrinol Invest. 2008 Oct;31(10):893–9. doi: 10.1007/BF03346438. [DOI] [PubMed] [Google Scholar]
- 17.Wood RD, Mitchell M, Lindahl T. Human DNA repair genes. Mutat Res. 2005 Sep;577(1-2):275–83. doi: 10.1016/j.mrfmmm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Chen BP, Chan DW, Kobayashi J, Burma S, Asaithamby A, Morotomi-Yano K, et al. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J Biol Chem. 2005 Apr;280(15):14709–15. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- 19.Olsen BB, Fischer U, Rasmussen TL, Montenarh M, Meese E, Fritz G, et al. Lack of the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) is accompanied by increased CK2alpha' levels. Mol Cell Biochem. 2011 Oct;356(1-2):139–47. doi: 10.1007/s11010-011-0954-7. [DOI] [PubMed] [Google Scholar]
- 20.Convery E, Shin EK, Ding Q, Wang W, Douglas P, Davis LSetal. Inhibition of homologous recombination by variants of the catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs) Proc Natl Acad Sci USA. 2005 Feb;102(5):1345–50. doi: 10.1073/pnas.0406466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangwar R, Ahirwar D, Mandhani A, Mittal RD. Do DNA repair genes OGG1, XRCC3 and XRCC7 have an impact on susceptibility to bladder cancer in the North Indian population. Mutat Res. 2009 Nov-Dec;680(1-2):56–63. doi: 10.1016/j.mrgentox.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Zhang H, Zhou K, Chen L, Xu Z, Zhong Y, et al. Tagging SNPs in non-homologous end-joining pathway genes and risk of glioma. Carcinogenesis. 2007 Sep;28(9):1906–1. doi: 10.1093/carcin/bgm073. [DOI] [PubMed] [Google Scholar]