Abstract
Background
The most important virulence factor which plays a central role in Candida albicans pathogenesis is the ability of this yeast to alternate between unicellular yeast and filamentous hyphal forms. Efg1 protein is thought to be the main positive regulating transcription factor, which is responsible for regulating hyphal-specific gene expression under most conditions. ALS3 is one of the Efg1-associated genes encoding a multi-functional adhesive polypeptide, which mediates adherence to diverse host substrates. In this study, the EFG1 gene was knocked down by using synthetic siRNA in C. albicans and the regulation in ALS3 as one of the Efg1-dependent genes was investigated.
Method
The 19-nucleotide siRNA was designed based on cDNA sequence of EFG1 gene in C. albicans. Transfection was performed using modified- plyethylen glycol/LiAc method. To quantify the level of EFG1 and the hyphal-specific ALS3 gene expression, the cognate EFG1 and ALS3 mRNA were measured in C. albicans by quantitative real-time RT-PCR.
Results
Fluorescent microscopy pictures indicated that transfection was performed successfully. Also, according to relative expression software tool, expression of EFG1 gene was decreased significantly with 500 nM siRNA as well as 1 µM siRNA (P<0.05). However, more significant down-regulations were observed in the expression of ALS3 in both concentrations of 500 nM and 1 µM siRNA (P<0.05).
Conclusion
conclusion, we demonstrated the down-regulation of ALS3 gene as a consequent of applying EFG1-specific siRNA in C. albicans. This may lead us to design anti-fungal-specific agents in order to face with C. albicans-associated infections.
Key Words: Candida albicans, ALS3, RNAi, EFG1
Introduction
Candida albicans is polymorph diploid yeast, which is widely recognized as the most pathogenic yeast species [1](Moran, 2012 #46). While the species is harmless to most individuals, it can opportunistically overgrow and elucidate a variety of infections under certain settings [2]. Identifying the virulence-associated factors in Candida species is rather elaborated due to the fact that they are opportunistic pathogens and are not usually able to cause infections unless the host deficiencies permit [1]. The most important virulence factor which plays a central role in C. albicans pathogenesis is the ability of the yeast to alternate between unicellular yeast and filamentous hyphal forms [3]. Although yeast cells are possibly essential for dissemination and initial colonization, the hyphal cells seems to have a key role in adhesion, invasion and biofilm formation [4]. Moreover, hyphal but not yeast cells are found to involve epithelial cells in infected locations [5]. In general, a combination of the serum and the temperature of 37°C are powerful and robust signals for germ tube formation from yeast cells [6]. However, elevated temperature is essential for hyphae formation, but in embedded matrixes [6]. Efg1 is thought to be the main positive regulating transcription factor, which is responsible for regulation of hyphal-specific gene expression under most conditions, including serum, CO2, neutral pH and GlcNAc in liquid media as well as on solid media [3, 7-9]. The activation region of ALS3 gene is one of the regions where Efg1 binds and as a consequent, this binding leads to ALS3 gene up-regulation [10]. Als3 protein acts as a multi-functional adhesive molecule which mediates adherence to diverse host substrates, such as endothelial cells, oral epithelial cells, gelatin, fibrinogen and laminin [11, 12]. The adhesive molecule Als3 is likely to help the yeast to be colonized on solid surfaces. This ability can facilitate biofilm formation, a specialized form of adherence [13]. In addition to adherence, it is well-known that Als3 can mediate C. albicans invasion to oral epithelial and vascular endothelial cells [14]. Therefore, the importance of expression of this hyphal-specific gene is clear in initiating C. albicans-associated infections.
RNAi-mediated gene silencing methods have been recently used in several cell systems [15, 16] and ARE NEWLY APPLIED IN fungi [17-20]. The procedure is initiated by the either production or introduction of small RNA (≈20-30 nucleotides). These RNA have a sequence complementary to a part of a target mRNA. siRNA generates from dsRNA by involving a member of the ribonulease Dicer family (RNase H). Afterward, siRNA associates with members of the Argonaute family of proteins to form RNA-induced silencing complexes. These complexes use the siRNA as a guide for the sequence-specific silencing of target messenger RNA through induction of the degradation of the mRNA or repressing their translation [21, 22]. In the present study, the gene EFG1, which encodes a major positive regulator of germ tube production, was knocked down by using synthetic siRNA in C. albicans and the regulation in ALS3 as one of the Efg1-dependent genes was investigated.
MATERIALS AND METHODS
Strains. C. albicans wild-type strain ATCC14053 was used in the present study. The strain was cultured on yeast extract/pepton/dextrose medium plates, incubated at 37°C for 24 h and maintained at 4°C until use.
siRNA. The 19-nucleotide siRNA was designed based on cDNA sequence of EFG1 gene of C. albicans (Accession number: XM_709104.1). The anti-sense and sense sequences are 5'-Fluorescein Amidite-ACAUUGAGCAAUUUGGUUC-3' and 5'-GAACCA AAUUGCUCAAUGU-3', respectively. Unrelated siRNA, which is a scramble sequence of anti-sense strand, having a sequence 5'-AUAUGCGCAACAUUG ACA-3' was synthesized as negative control (Metabion, Germany). Anti-sense strand of siRNA was labeled with a fluorescent dye, Fluorescein Amidite, so as to trace the siRNA localization in yeast cells. Sense/anti-sense annealing was performed in an annealing buffer (30 mM HEPES-KOH [pH 7.4], 100 mM KCL, 2 mM MgCl2 and 50 mM NH4Ac) according to the protocol for siRNA annealing (http:// www.metabion.com/downloads/ siRNA annealing.pdf) to generate siRNA duplex with symmetric 2-nt 3' overhangs.
C. albicans yeast culture and transfection. Yeast transfection was performed according to the basic protocol previously described for yeast transformation with some modifications [23]. C. albicans strain was grown on yeast nitrogen base medium at an initial absorbance of 0.2 at 623 nm (OD623 ≈ 0.2). The culture was incubated at 37°C with gentle shaking for 18-20 h. Afterward, when most C. albicans cells underwent exponential phase of their lifecycle, a final yeast concentration of 3 × 107 cells/ml (OD623 ≈ 0.6) was prepared and used for transfection. The cells were then harvested by centrifugation and washed twice with cold sterile PBS. The final solution was divided into microtubes in such a way that each 1.5 ml microtube contained 1 ml of the final solution (3 × 107 cells). Transfection was performed using modified- plyethylen glycol (PEG)/LiAc method. Briefly, to prepare competent yeasts, cells were re-suspended in 500 µl of 100 mM LiCl, mixed gently and centrifuged at 1,500 ×g for 2 min. Then, 240 µl 50% PEG 3350 and 36 µl 1.0 M LiCl were added to the pellet. siRNA was added in such a way that the final concentration of siRNA in each microtube reached to 1,000 nM (1 µM) and 500 nM. Therefore, a volume of 18 and 9 µl of annealed siRNA were added to the solution of each microtube. DEPC-treated water was lastly added to get a final volume of 360 µl. A positive (untransfected yeast cells) and a negative control (yeast cells treated with 1 µM of unrelated siRNA) were also run along with the experiment. Then, the cells suffered a heat shock in a water bath at 42°C for 40 min, followed by centrifugation at 3000 ×g for 30 s and by resuspension in 500 µl of yeast nitrogen base medium so as to allow the transfected cells to be recovered. Finally, the cultures were incubated without shaking at 37°C for 15 h. Transfected yeasts were then subjected to further studies.
siRNA entrance confirmation. To ensure that the entrance of siRNA was successful, samples of exponentially growing siRNA-treated C. albicans cultures were washed twice in sterile PBS. To remove any unused siRNA, samples were treated with RNase (Takara, Japan) and subjected to fluorescent microscopy. Samples of siRNA-treated cells (1 µM) were mounted on slides and examined. Fluorescence images were obtained using 100X objective lens on an Olympus fluorescent microscope (Olympus, UK) with the appropriate fluorescence filters. Photomicroscopy was performed using a Hitachi 12dp camera (Hitachi, USA). The locations of labeled siRNA were then defined.
RNA extraction and quantitative real-time RT-PCR assay. In order to quantify the level of EFG1 and the hyphal-specific ALS3 gene expression, the cognate EFG1 and ALS3 mRNA were measured in C. albicans by quantitative real-time RT-PCR. Total RNA was extracted from both siRNA-treated (EFG1-specific and unrelated siRNA) as well as untreated cells using FastPureTM RNA kit (Takara, Japan) according to manufacturer's protocol. RNA concentrations and RNA purity were determined spectrophotometrically using an Eppendorf BioPhotometer (Germany). An equal amount of RNA (1 µg in 20 µl) was subjected to cDNA synthesis by using PrimeScript RT reagent kit (Takara, Japan). EFG1 and ALS3 primers were designed on the bases of published sequences of the EFG1 (NCBI, Accession no.: XM_709104.1), and ALS3 (NCBI, Accession no.: XM_705343.1 ) genes in C. albicans. The β-actin gene (ACT1) was used as endogenous reference gene. The sequences of forward and reverse primers have been shown in Table 1. Standard curves for each gene were established with four serially diluted cDNA, which was obtained from cells grown to mid-logarithmic phase at 37°C using specific primers under the appropriate PCR conditions. Real-time RT-PCR was performed with a StepOnePlusTM real-time PCR system (Applied Biosystems, USA) and SYBR® Premix Ex Taq™ II was used as a reagent specifically designed for intercalator-based real-time PCR using SYBR Green I. All PCR reaction mixtures contained: 10 µl SYBR® Premix Ex TaqTM II (2×), 2 µl first strand cDNA, 0.4 µM each primer, 0.4 µl ROX Reference Dye (50×) and dH2O up to the final volume of 20 µl. The program for amplification was 95°C for 30 s as initial denaturation step, followed by 40-cycle PCR consisting of 95°C for 5 s and 60°C for 30 s. Negative control (water as template) was included in each run. Expression of each investigated gene was normalized to the housekeeping ACT1 gene and analyzed by applying REST© (2008 V2.0.7) software, which analyzes data by use of comparative Ct method (ΔΔCt). Expression of EFG1 and ALS3 genes from cells grown under siRNA-treatment condition was indicated as relative expression to that of gene from untreated yeast cells. Each experimental condition was performed in duplicate and each experiment was repeated twice on two different days for reproducibility.
Table 1.
PCR primers for real-time RT-PCR analysis.
| Gene | Primer name | Sequence (5'-3') | PCR product size (bp) | GenBank |
|---|---|---|---|---|
| EFG1 | Fefg140 Refg140 |
TgCCAATAATgTgTCggTTg CCCATCTCTTCTACCACgTgTC |
140 | XM_709104.1 |
| ACT1 | Fact110 Ract110 |
ACggTATTgTTTCCAACTgggACg TggAgCTTCggTCAACAAAACTgg |
110 | XM_717232.1 |
| ALS3 | Fals141 Rals141 |
gCTggTggTTATTggCAACgTgC TggTAAggTggTCACggCgg |
141 | XM_705343.1 |
Results
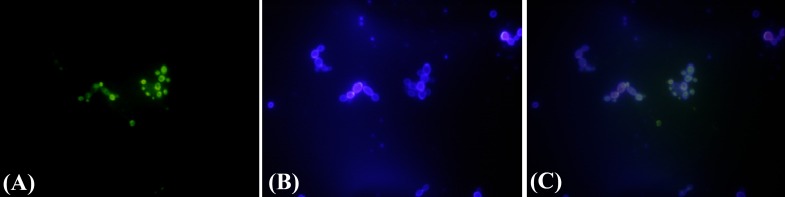
Transfection and siRNA entrance confirmation. To assess the potential of RNAi as a means of gene silencing in C. albicans, we benefited from the modified PEG/LiAc method for double-stranded RNA to be introduced into the cells. Florescent microscopy method was used at the first step of evaluating the efficiency of C. albicans transfection and more important, to trace the siRNA localization in yeasts. Yeast cells were harvested 15 h post transfection, washed, mounted and visualized. Apparently, only yeast cells with labeled-siRNA inside were luminous enough to be traced. Figure 1 indicates that the transfection was performed successfully.
Fig. 1.
Fluorescent microscopy images of the cells transfected by siRNA specific to EFG1 gene. (A) The fluorescent images of the yeasts carrying labeled-siRNA, (B) calcoflour white staining of yeasts cell wall, and (C) merged figures of A and B indicating the location of siRNA in the yeast cells.
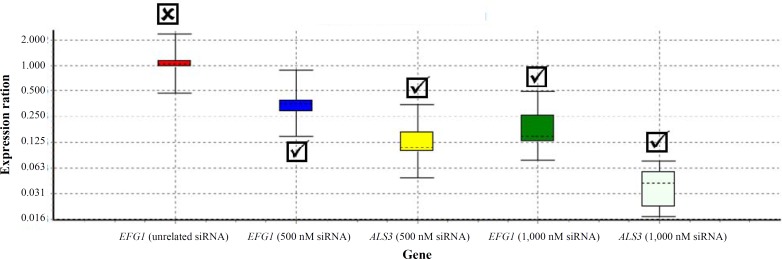
Quality control and effect of siRNA on EFG1 gene expression. EFG1, ALS3 and ACT1 mRNA levels were monitored over a 15-h period of yeast incubation with both unrelated and EFG1-specific siRNA. Positive control (untreated C. albicans cells) was also included in each run of experiment. EFG1, ALS3 and ACT1 primers demonstrated similar efficiency in titration experiment using C. albicans cDNA in serial dilutions (data not shown). Expression of each gene was indicated as expression ratio relative to that of untreated logarithmic-phase grown yeasts. REST© (2008 V2.0.7) software was applied to analyze the obtained data of quantitative real-time RT-PCR. On the basis of REST© output, expression of EFG1 gene was decreased about 2 folds using 500 nM siRNA. Meaningfully, a 5.5-fold decrease in EFG1 gene expression was observed when applying 1 µM of siRNA (P<0.05). The level of EFG1 mRNA in cells affected with unrelated siRNA was the same as positive control and unchanged (P>0.05). Figure 2 shows the relative expression ratio of EFG1 and ALS3 genes under different conditions. Furthermore, the expression of ALS3, the hyphal-specific genes regulated by EFG1, was considerably down-regulated and there were significant differences between ALS3 gene expression in positive control (untreated cells) and test sample yeasts (P<0.05). An 8-fold reduction in the expression ALS3 gene was observed when 500 nM siRNA was added to EFG1 mRNA (Table 2).
Fig. 2.
Effect of siRNA on EFG1 and ALS3 gene expression in C. albicans exposed to unrelated siRNA (500 and 1,000 nM). Relative gene expression indicates expression ratio relative to that of untreated logarithmic-phase grown yeasts (The expression ratio in yeast is considered 1 for positive control). The expression of EFG1 gene was not significantly reduced in the presence of 1 µM unrelated siRNA. However, significant reductions were indicated for EFG1 and ALS3 genes in the presence of 500 nM as well as 1 µM specific siRNA. Boxes represent the interquartile range, or the middle 50% of observations. The dotted line represents the median gene expression. Error bars represent the minimum and maximum observations. Check marks ( ) represent the effectiveness of siRNA on EFG1 and ALS3 genes down regulation. ( ) represents that siRNA was not effective on EFG1 and ALS3 gene regulation.
Table 2.
Output for relative expression of EFG1 and ALS3 genes by use of ΔΔCt method (REST©, 2008 V2.0.7). Results indicate that the differences between EFG1 expression in the yeast of control and sample groups are significant as well as the expression of ALS3 gene.
| Gene | Type | Reaction Efficiency | Expression | Standard Error | 95% C.I. | P(H1) * | Result |
|---|---|---|---|---|---|---|---|
| ACT1 | REF | 1.0 | 1.000 | ||||
| EFG1 (unrelated siRNA) | TRG | 1.2 | 1.046 | 0.636 - 1.820 | 0.482 - 2.277 | 0.638 | |
| EFG1 (500 nM siRNA) | TRG | 1.2 | 0.357 | 0.209 - 0.674 | 0.153 - 0.858 | 0.000 | DOWN● |
| ALS3 (500 nM siRNA) | TRG | 0.8 | 0.122 | 0.066 - 0.288 | 0.049 - 0.347 | 0.000 | DOWN |
| EFG1 (1,000 nM siRNA) | TRG | 1.0 | 0.179 | 0.101 - 0.394 | 0.080 - 0.487 | 0.000 | DOWN |
| ALS3 (1,000 nM siRNA) | TRG | 0.8 | 0.037 | 0.021 - 0.068 | 0.017 - 0.075 | 0.000 | DOWN |
*P(H1), probability of alternate hypothesis that difference between sample and control groups is due only to chance. TRG, target; REF, reference; ●down-regulation of ALS3 and EFG1 genes after using siRNA; C.I., confidence interval.
Discussion
During C. albicans infection, epithelial cells are invaded by two distinct routes: in the first route, hyphal cells induce endocytosis by the host cells in which the adhesive molecule ALS3 is necessary [24]. In the second rout, hyphal cells penetrate actively in the plasma membrane [8]. Invasion of oral epithelial cells occurs through both routes, while active penetration is the only route by which C. albicans invades intestinal epithelial cells [8]. Despite the roles of hyphae during infections due to C. albicans, the issue that the hyphae are required for virulence is still difficult to prove [6]. One of the crucial functions of Efg1 is up-regulation of ALS3 gene expression [10]. ALS3 is one of the members of agglutinin-like sequence gene family in C. albicans [25, 26]. The family of this gene comprises eight members (ALS1-7 and ALS9), which encode cell surface proteins with the same overall structure [27]. ALS3 gene encodes a protein called Als3, which is now considered as a multi-functional adhesion and invasion. This adhesive molecule mediates adherence to various kinds of substrates, such as laminin, gelatin, salivary pellicles etc. This function can be leaded to produce biofilm, which is a kind of specialized form of adherence [11, 12]. Moreover, endocytosis is one of the strategies by which C. albicans can invade to both epithelial and endothelial cells. However, hyphae of C. albicans are more likely to be endocytosed rather than yeast-phase organisms. This suggests that hyphae express specific molecule such as Als3, which can bind to one or more receptors in host cells and induce endocytosis [24]. This adhesive molecule is able to form a mixed-species biofilm with Streptococcus gordonii through adherence to this bacterial flora besides to host cells. In addition, it has been demonstrated that S. gordonii cells stimulate hyphal growth of C. albicans and bind to them in an Als3-dependent manner [28]. Moreover, Als3 molecule in C. albicans is a receptor for ferritin and mediates iron acquisition from host cells [29]. According to the above reasons, EFG1 gene was targeted to be silenced by RNAi technology in this study. Furthermore, the expression of EFG1 as well as ALS3 genes, have been investigated and significant reductions in both gene regulations were indicated. Outputs for real-time RT-PCR demonstrated the outstanding influence of EFG1 gene expression on pathogenicity of C. albicans again so that even a 2-fold decrease in EFG1 gene expression can cause ALS3 to be down-regulated. Based on our obtained results, the RNAi pathway does exist functionally in the yeast C. albicans.
Suppression of gene expression by a dsRNA-expressing plasmid or other related-systems has been shown in many fungal species [30]. Rappleye et al. [17] reported the role of α-(1,3)-glucan in virulence of the fungus H. capsulatum through a plasmid-based RNAi system. In 2007, Khatri et al. [19] explored the utility of RNA interference as a tool for specific silencing of gene expression in A. nidulans [19]. Disney et al. [31] reported that oligonucleotides can be introduced into C. albicans in an energy-dependent manner. In addition, they reported anti-fungal activity for those oligonucleotides [31]. Janbon et al. [32] characterized the role of the RNAi pathway in regulation of many cellular processes in C. neoformans as a model system. In all mentioned studies, vector-based approaches were applied for fungal cells transfection, except for Khatri et al. [19] who has reported that siRNA can be directly uptaken by the germinated spores from media. However, the method for yeast transfection described here had not been previously applied before.
Due to the considerably differences in RNA silencing proteins among fungal species, RNA silencing pathways appears to have been diversified in this kingdom [30]. In the case of C. albicans, it was reported that the RNA silencing machinery is probably absent because the Dicer involved in the silencing pathway lacks both a helicase and PAZ (Piwi Argonaut and Zwille) domain [33]. According to Candida Genome Database, the genome of C. albicans holds a typical Argonaute homologue (orf19.2903; www. candidagenome.org), and a non-canonical Dicer (orf19.3796; www.candidagenome.org). Interestingly, the nucleotide sequence of the Dicer (orf19.3796) is only conserved among Candida species. However, the deduced amino acid sequence has quite high homology with ribonuclease of other fungal species. Moreover, the yeast C. albicans harbors a probable ribonuclease III protein. Remarkably, the homology of the amino acid sequences of ribonuclease III protein and the so called Dicer protein (orf19.3796) is 100% after performing alignment (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The probable ribonuclease III protein contains DSRM (double-stranded RNA binding motif) and ribonuclease III C terminal domain. Despite that Candida Dicer lacks a PAZ and helicase domain, it contains a DSRM and ribonuclease III C terminal domain. DSRM domain binding is not sequence specific, but is highly specific for double stranded RNA. Therefore, based on the above mentioned arguments and the results obtained in this study, it supposed that the Dicer of C. albicans is functional and effectively participate in RNAi silencing pathway. The Argonaute protein of the pathogenic yeast C. albicans holds both PiWi and PAZ domains, which are essential motifs for Argonaut function and dsRNA binding. Therefore, it can be suggested that the RNAi gene silencing pathway is functional in this microorganism.
In conclusion, we established that RNAi is an applicable tool for functional silencing of EFG1 gene expression in C. albicans in a dose-dependent manner. We have also demonstrated the consequence effect of this gene silencing on the down-regulation of ALS3 gene expression in C. albicans. Post transcriptional gene silencing is likely to be considered as a promising approach to discover new gene targets. This may lead us to design anti-fungal-specific agents in order to face with C. albicans-associated infections via inhibiting the production of true hyphae and subsequently hyphal-wall specific proteins of the yeast C. albicans.
Acknowledgment
The authors thank the Central Research Laboratory of School of Public Health, Tehran University of Medical Sciences (Iran), for providing laboratory facility. This research has been financially supported by Tehran University of Medical sciences (TUMS), grant No. 11424-87-03-89.
References
- 1.Moran GP, Coleman DC, Sullivan DJ. Candida albicans versus Candida dubliniensis: Why is C. albicans more pathogenic? Int J Microbiol. 2012 doi: 10.1155/2012/205921. doi:10.1155/2012/ 205921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruhnke M. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr Drug Targets. 2006 Apr;7(4):495–504. doi: 10.2174/138945006776359421. [DOI] [PubMed] [Google Scholar]
- 3.Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004 Jul;12(7):317–24. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011 Feb;9(2):109–18. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherwitz C. Ultrastructure of human cutaneous candidosis. J Invest Dermatol. 1982 Mar;78(3):200–5. doi: 10.1111/1523-1747.ep12506451. [DOI] [PubMed] [Google Scholar]
- 6.Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011 Oct;9(10):737–48. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 7.Odds FC. Candida and Candidosis: A Review and Bibliography. London: Baillière Tindall; 1988. [Google Scholar]
- 8.Dalle F, Wächtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010 Feb;12(2):248–71. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 9.Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006 Dec;2(12):e129. doi: 10.1371/journal.ppat.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng P, Lee PR, Wu H, Brown AJP. Efg1, a morphogenetic regulator in Candida albicans, is a sequence-specific DNA binding protein. J Bacteriol. 2001 Jul;183(13):4090–93. doi: 10.1128/JB.183.13.4090-4093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobbs AH, Vickerman MM, Jenkinson HF. Heterologous expression of Candida albicans cell wall-associated adhesins in Saccharomyces cerevisiae reveals differential specificities in adherence and biofilm formation and in binding oral Streptococcus gordonii. Eukaryot Cell. 2010 Oct;9(10):1622–34. doi: 10.1128/EC.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, et al. Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem. 2004 Jul;279(29):30480–9. doi: 10.1074/jbc.M401929200. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Daniels KJ, Oh SH, Green CB, Yeater KM, Soll DRetal. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology. 2006 Aug;152(Pt 8):2287–99. doi: 10.1099/mic.0.28959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One. 2011 Feb;6(2):e17046. doi: 10.1371/journal.pone.0017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cottrell TR, Doering TL. Silence of the strands: RNA interference in eukaryotic pathogens. Trends Microbiol. 2003 Jan;11(1):37–43. doi: 10.1016/s0966-842x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 16.Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005 Nov;23(11):1399–405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- 17.Rappleye CA, Engle JT, Goldman WE. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol Microbiol. 2004 Jul;53(1):153–65. doi: 10.1111/j.1365-2958.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Cottrell TR, Pierini LM, Goldman WE, Doering TL. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics. 2002 Feb;160(2):463–70. doi: 10.1093/genetics/160.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatri M, Rajam MV. Targeting polyamines of Aspergillus nidulans by siRNA specific to fungal ornithine decarboxylase gene. Med Mycol. 2007 May;45(3):211–20. doi: 10.1080/13693780601158779. [DOI] [PubMed] [Google Scholar]
- 20.Janbon G, Maeng S, Yang DH, Ko YJ, Jung KW, Moyrand F, et al. Characterizing the role of RNA silencing components in Cryptococcus neoformans. Fungal Genet Biol. 2010 Dec;47(12):1070–80. doi: 10.1016/j.fgb.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev. 2009 Jul;61(9):746–59. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009 Jan;457(7228):405–12. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 23.Gietz RD, Woods RA. Genetic transformation of yeast. Biotechniques. 2001 Apr;30(4):816–20. doi: 10.2144/01304rv02. [DOI] [PubMed] [Google Scholar]
- 24.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007 Mar;5(3):e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998 Jun;33(6):451–9. doi: 10.1007/s002940050359. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer LL, Scherer S, Shatzman A, Livi GP. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol Microbiol. 1995;15(1):39–54. doi: 10.1111/j.1365-2958.1995.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell. 2011 Feb;10(2):168–73. doi: 10.1128/EC.00279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bamford CV, d'Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun. 2009 Sep;77(9):3696–704. doi: 10.1128/IAI.00438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida RS, Brunke S, Albrecht A, Thewes S, Laue M, Edwards JE, et al. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008 Nov;4(11):e1000217. doi: 10.1371/journal.ppat.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayashiki H. RNA silencing in fungi: mechanisms and applications. FEBS Lett. 2005 Oct;579(26):5950–7. doi: 10.1016/j.febslet.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Disney MD, Haidaris CG, Turner DH. Uptake and antifungal activity of oligonucleotides in Candida albicans. Proc Natl Acad Sci USA. 2003 Feb;100(4):1530–4. doi: 10.1073/pnas.0337462100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janbon G, Maeng S, Yang DH, Ko YJ, Jung KW, Moyrand F, et al. Characterizing the role of RNA silencing components in Cryptococcus neoformans. Fungal Genet Biol. 2010 Dec;47(12):1070–80. doi: 10.1016/j.fgb.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staab JF, White TC, Marr KA. Hairpin dsRNA does not trigger RNA interference in Candida albicans cells. Yeast. 2011 Jan;28(1):1–8. doi: 10.1002/yea.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]