Abstract
Background
Ataxia with oculomotor apraxia type 1 (AOA1) shows early onset with autosomal recessive inheritance and is caused by a mutation in the aprataxin (APTX) gene encoding for the APTX protein.
Methods
In this study, a 7-year-old girl born of a first-cousin consanguineous marriage was described with early-onset progressive ataxia and AOA, with increased cholesterol concentration and decreased albumin concentration in serum. PCR and direct DNA sequencing was performed after DNA extraction.
Results
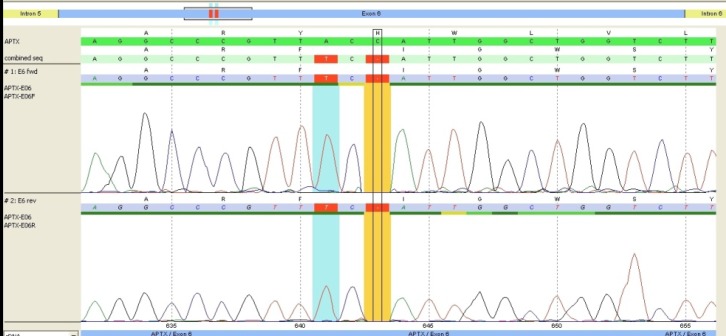
Sequencing analysis revealed a novel homozygous deletion in c.643 and A>T single nucleotide polymorphism in c.641 in exon 6 of the APTX gene [ENST00000379825].
Conclusion
It seems that this region of exon 6 is probably a hot spot; however, no deletions have been reported in exon 6 yet.
Key Words: Ataxia oculomotor apraxia 1 (AOA1), aprataxin (APTX), Iranian
Introduction
Ataxia with oculomotor apraxia (AOA, MIM # 208920) is a rare autosomal recessive progressive cerebellar ataxia associated with AOA and severe peripheral neuropathy. There are two types of ataxia with AOA, AOA1 (AOA type 1) and AOA2 (AOA type 2), which are very similar in several clinical features, such as AOA, chorea, myoclonus and neuropathy. The first manifestation of disease is cerebellar atrophy, followed by the inability to coordinate eye-head movements a few years after onset of disease. Besides, progressive cerebellar ataxia, AOA and peripheral neuropathy, hypoalbuminemia and hypercholesterolemia are the hallmark diagnostic features in AOA1 [1-3]. AOA1 is caused by a mutation in the aprataxin (APTX) gene at locus 9p13.3, which encodes for the APTX protein interacting with poly adenosine diphosphate-ribose polymerase-1[1, 4]. AOA1 shows early onset (mean age of onset: 4.3 years; range: 2-10 years), where the first symptom is progressive gait imbalance followed by dysarthria, mild intention tremor, AOA and peripheral axonal neuropathy [2]. In this study, a 7-year-old girl with ataxia and AOA1 was described.
Patient and molecular analysis.
Clinical features of patient. A 7-year-old girl who was born in Iran from a first-cousin consanguineous marriage with no family history of neurodegenerative disease was described. Her mother had no problem during pregnancy and labor. She was born at term with normal birth weight, normal height and normal head circumstance. She underwent normal childhood development, including walking and speaking, up to 4 years of age. At age 4, a progressive gait imbalance was appeared, followed by intention tremor and dysarthria. When she was referred to the Genetics Department of Special Medical Center at the age of 7, ataxia with intention tremor, dysarthria and AOA were observed. The parents of the patient were informed of the aims of the study and the patient gave her informed consent for the genetics analysis.
Neurological and paraclinical examination. Neurological reflexes were exaggerated and electro-myography revealed severe axonal sensory-motor neuropathy. MRI of the brain showed cerebellar atrophy. A routine blood examination revealed an increase in cholesterol concentration and a decrease in albumin concentration in serum. Her serum creatine kinase and alpha-fetoprotein levels were normal. The clinical features of the patient have been summarized in Table 1. With regards to the patient’s symptoms, the diagnosis of ataxia with AOA1 was probable, which was later confirmed by molecular diagnosis.
Table 1.
The clinical characteristics of the patient.
| Clinical feature | Patient |
|---|---|
| Gender | female |
| Age of onset | 7 |
| Ataxia | + |
| Dysarthria | + |
| Gaze-evoked nystagmus | + |
| Mental retardation | - |
| Deep tendon reflexes | Areflexic |
| Spasticity in the lower limbs | + |
| Scoliosis | + |
| Muscle weakness in upper limbs | Distal |
| Muscle weakness in lower limbs | Prox* and Distal |
| Muscle atrophy in upper and lower limbs | Distal |
| Cardiomyopathy | - |
| Alpha-fetoprotein level | Normal |
| Serum creatine kinase | Normal |
| Hypercholesterolemia concentration | + |
| Hypoalbuminemia concentration in serum | + |
| MRI signs of cerebellar atrophy | + |
| Presence (+); Absence (-). |
*Prox, proximal
PCR of the APTX gene and direct sequencing. Genomic DNA of the patient and her parents was isolated from peripheral EDTA-treated blood cells by Qiagen DNA Mini kit (cat No: 51304). Genomic DNA (200 ng) of the patient and her parents was subjected to the PCR amplification of coding exons and flanking intronic sequences of the APTX gene. PCR reactions were carried out according to the method and primers described by Habeck and colleagues [5]. The PCR products were analyzed by direct sequencing of DNA fragments amplified by the ABI 3700 capillary sequencer (Macrogen Korea). Sequence results were compared with the published sequence (ENST00000379825). To confirm the results, all procedures of the molecular analysis were double checked.
Results
The patient’s features were in accordance with symptoms that had been reported in the diagnosis of AOA1, which is caused by mutation in the APTX gene. Direct sequencing of all exons of the APTX gene amplified from the patient and her parents revealed a novel homozygous single nucleotide polymorphism (SNP) and a single homozygous nucleotide deletion in exon 6 of the patient’s APTX gene. The parents were heterozygous for the same mutation. The SNP is a single nucleotide change of A to T in c.641, which changes the amino acid tyrosine to phenylalanine at position 214 (Y>F 214) [ENST00000379825]. The nonsense mutation was a single nucleotide (cytosine) deletion located at c.643delC [ENST00000379825] (Fig. 1), which caused a frame shift resulting in a premature stop codon at amino acid 227. Sequence analysis of APTX gene in parents showed that they were also carrier of SNP polymorphism and deletion in exon 6 of APTX gene.
Fig. 1.
Comparison of a single nucleotide change in exon 6 of APTX gene at location c.641 A> T [214 (Y>F 214)] and a single nucleotide deletion located at c.643delC [AA227> STOP] [ENST00000379825] in the patient with normal sequence of cDNA and protein.
Discussion
In this study, the patient showed two main diagnostic features of early onset ataxia with AOA1 including sever primary motor peripheral neuropathy and AOA. In addition, the total cholesterol and serum albumin levels were higher (>5.6 mmol/L) and lower (< 3.5 g/L) than normal level, respectively. However, her serum creatine kinase and alpha-fetoprotein levels were normal [3]. Regarding to the clinical and par clinical features, AOA1 was a probable diagnosis for the patient.
Clinical diagnosis was confirmed by double check of the molecular test in the affected patient. This is the first report of a single nucleotide change in c.641 A> T [214 (Y>F 214)] [ENST00000379825] and a single nucleotide deletion located at c.643delC [AA227> STOP] [ENST00000379825] in exon 6 of the APTX gene, which caused a frame shift resulting in a premature stop codon at amino acid 227.
APTX consists of seven coding exons. According to The Human Gene Mutation Database, to date, 28 different mutations, deletions and insertions have been found in the APTX gene [human gene mutation database]. According to Ensemble Database, about 8 other SNP and 5 other mutations have been reported in exon 6, where 3 of them are located in codons 213, 215 and 220, which are very near to our patient’s mutation region in codon 214 [ENST00000379825]. Therefore, it seems that this region of exon 6 is probably a hot spot; however, no deletions have been reported in exon 6 yet [ENST00000379825].
It seems that there is a genotype-phenotype correlation for this disease. For instance, missense mutations of APTX may be associated with a later onset of approximately 9 years. But nonsense or frame shift mutations have an onset age ranging between 2-12 years of age (mean: 4.6 years), that is in accordance with our results [1, 2, 5, 6]. Also, there is a relationship between the type of mutation and severity of cognitive impairments, chorea and dystonia [1, 2, 4, 6], but cerebellar ataxia, cerebellar atrophy and peripheral neuropathy are common symptoms in all patients.
Acknowledgment
This work was supported by a grant from the Genetics Department, Special Medical Center.
References
- 1.Moreira MC, Barbot C, Tachi N, Kozuka N, Uchida E, Gibson T, et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new hit/zn-finger protein aprataxin. Nat Genet. 2001 Oct;29(2):189–93. doi: 10.1038/ng1001-189. [DOI] [PubMed] [Google Scholar]
- 2.Shimazaki H, Takiyama Y, Sakoe K, Ikeguchi K, Niijima K, Kaneko J, et al. Tsuji S: Early-onset ataxia with ocular motor apraxia and hypoalbuminemia. Neurology. 2002;59:590–5. doi: 10.1212/wnl.59.4.590. [DOI] [PubMed] [Google Scholar]
- 3.Date H, Onodera O, Tanaka H, Iwabuchi K, Uekawa K, Igarashi S, et al. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new hit superfamily gene. Nat. Genet. 2001 Oct;29(2):184–8. doi: 10.1038/ng1001-184. [DOI] [PubMed] [Google Scholar]
- 4.Le BerI, Moreira MC, Rivaud‐Péchoux S, Chamayou C, Ochsner F, Kuntzer T, et al. Cerebellar ataxia with oculomotor apraxia type 1: Clinical and genetic studies. Brain. 2003 Dec;126(Pt 12):2761–72. doi: 10.1093/brain/awg283. [DOI] [PubMed] [Google Scholar]
- 5.Habeck M, Zühlke C, Bentele KHP, Unkelbach S, Kreß W, Bürk K, et al. Aprataxin mutations are a rare cause of early onset ataxia in germany. J Neurol. 2004 May;251(5):591–4. doi: 10.1007/s00415-004-0374-7. [DOI] [PubMed] [Google Scholar]
- 6.Barbot C, Coutinho P, Chorao R, Ferreira C, Barros J, Fineza I, et al. Recessive ataxia with ocular apraxia: Review of 22 portuguese patients. Arch Neurol. 2001 Feb;58(2):201–5. doi: 10.1001/archneur.58.2.201. [DOI] [PubMed] [Google Scholar]