Abstract
Background
Central renin angiotensin system has an important role on the cerebral microcirculation and metabolism. Our previous work showed that inhibition of angiotensin converting enzyme () activity prior to induction of ischemia protected the brain from severe ischemia/reperfusion (I/R) injuries. This study evaluated the impacts of post-ischemic inhibition of , enalapril, on brain infarction in normotensive rats.
Methods
Rats were anesthetized with chloral hydrate (400 mg/kg). Focal cerebral ischemia was induced by 60-min intraluminal occlusion of right middle cerebral artery (MCA). Intraperitoneal injection of enalapril (0.03 or 0.1 mg/kg) was done after MCA reopening (reperfusion). Neurological deficit score (NDS) was evaluated after 24 h and the animals randomly assigned for the assessments of infarction, absolute brain water content (ABWC) and index of brain edema
Results
Severe impaired motor functions (NDS = 2.78 ± 0.28), massive infarction (cortex = 214 ± 19 mm3, striatum = 86 ± 5 mm3) and edema (ABWC = 83.1 ± 0.46%) were observed in non-treated ischemic rats. Non-hypotensive dose of enalapril (0.03 mg/kg) significantly reduced NDS (1.5 ± 0.22), infarction (cortex = 102 ± 16 mm3, striatum = 38 ± 5 mm3) and edema (ABWC = 80.9 ± 0.81%). Enalapril at dose of 0.1 mg/kg significantly lowered arterial pressure could not improve NDS (2.0 ± 0.45) and reduce infarction (cortex = 166 ± 26 mm3, striatum = 71 ± 11 mm3).
Conclusion
Post-ischemic ACE inhibition in the normotensive rats without affecting arterial pressure protects the brain from reperfusion injuries; however, this beneficial action is masked by hypotension.
Key Words: Angiotensin converting enzyme (ACE) inhibitors, Enalapril, Brain edema, Cerebral infarction
Introduction
Ischemic brain injury is resulting from a complex sequence of pathophysiological events that is developed with time and space [1]. The extent of tissue damage occurring during stroke depends on both the duration and the intensity of focal cerebral ischemia [2]. Ischemic brain edema occurring during stroke, per se, is life threatening due to augmentation of intracranial pressure and herniation [1], and its conjunction with cerebral infarction intensifies the primary complications of ischemia injuries [3]. Pre-ischemic inhibition of angiotensin converting enzyme (ACE), using enalapril as ACE inhibitor, reduces brain edema and prevents the brain from severe ischemia/ reperfusion (I/R) injury in normotensive rats [4]. Now the question is whether these beneficial effects can be seen after stroke?
Some evidences exist about the role of ACE activity and angiotensin II (Ang II) in ischemic neuronal injury [5]. It is proposed that inhibition of the central renin-angiotensin system might be valuable not only in reducing the occurrence of stroke but also in alleviating neuronal injuries after stroke [6]. Recent clinical studies have revealed that some ACE inhibitors, which are used as anti-hypertensive drugs, diminish the risk and the severity of secondary attacks of the stroke [7, 8]. Previously, we showed that pre-ischemic treatment of normotensive rats with non-hypotensive dose of enalapril, an ACE inhibitor, improved neurological motor activity and concomitantly reduced cerebral edema and infarction [4]. As far as literature search is concerned, few studies have tackled the effectiveness of post-ischemic inhibition of ACE activity in reducing cerebral I/R injuries [7]. Therefore, this study was designed to evaluate the effects of ACE inactivation by administration of enalapril after termination of middle cerebral artery (MCA) occlusion on the brain injury in normotensive rats.
MATERIALS AND METHODS
Male normotensive Sprague Dawley rats (280-320 g) were obtained from Central Animal House Facility of Shiraz University of Medical Sciences (Shiraz, Iran). All protocols of the study were approved by the Institutional Animal Ethics Committee of the University, which follows the NIH guidelines for care and use of animals. Animals were housed at room temperature of 22-24°C, humidity of 40-60% and light period of 07.00-19.00 with controlled environments. Animals were food fasted overnight prior to surgery but had free access to water ad libitum. Anesthesia was made by intraperitoneal injection (i.p.) of chloral hydrate (400 mg/kg). Core body temperature was measured by a rectal probe connected to a thermistor and maintained at 37 ± 0.5ºC with a heating pad.
Recordings of arterial blood pressure and regional cerebral blood flow (rCBF). After induction of anesthesia, four rats of each group were randomly selected and cannulation of posterior tail artery was performed for continuous recording of arterial blood pressure and taking blood samples. In the same rats, laser Doppler flowmetry (AD Instrument, model: ML191, Australia) was performed as described previously [9], to continuously record the rCBF of the areas of the right hemisphere that were nourished from the right MCA.
Induction of transient focal cerebral ischemia. Right MCA occlusion was carried out by intraluminal filament method described by Longa et al. [10] and modified by Panahpour et al. [4]. In short, the right common carotid artery was exposed through a midline neck incision. Through the external carotid artery, a surgical nylon thread (3-0, coated with poly-L-lysine) was placed in the internal carotid artery and gently advanced up until feeling a resistance and seeing a sharp decline in the blood flow trace. MCA occlusion terminated after 60-min ischemia by gently pooling out the thread. After re-establishment of the blood flow of the ischemic region, all the incisions were sutured, the animals were allowed to recover from anesthesia, and returned to a warm cage for recuperation during 24 h reperfusion period.
Experimental protocol and groups. Sham (n = 16) rats underwent the surgery at the neck region and received a single i.p. injection of the vehicle (1 ml/kg distilled water) without being exposed to MCA occlusion.
Control ischemia (CI). After neck surgery, similar group, rats (n = 12) underwent 60 MCA occlusion. A single i.p. injection of the vehicle (1 ml/kg distilled water) was done after the start of MCA reopening (reperfusion).
Post-ischemic enalapril treated -1 (PIEnT-1) . Rats of this group (n = 12) were treated similar to CI, but received a single i.p. injection of 0.03 mg/kg enalapril (Sigma Chemicals, UK).
Post-ischemic enalapril treated -2 (PIEnT-2). Rats of this group (n = 11) were treated similar to PIEnT-1, but the dose of enalapril was increased to 0.1 mg/kg. The number of animals presented for each group was the number of rats that survived during 24 h reperfusion period. The obtained data of the animals that died during 24 h reperfusion period were excluded from the study. The number of deceased animals with respect to the total number of rats in each group was regarded as the percent of mortality rate, which was 0%, 25%, 25% and 31.3% for sham, CI, PIEnT-1 and PIEnT-2 groups, respectively.
Evaluation of neurological outcome. Behavioral tests were performed in blinded fashion 24 h after the start of the reperfusion (MCA reopening) in rats survived from ischemic trauma and at comparable periods in the sham group. A five-point grading scale of neurological deficit score (NDS) test was carried out to evaluate the neurological outcome [11]. After evaluation of motor neuron activity, rats of each group were randomly divided into two subgroups to quantify the extent of cerebral infarction volumes and brain edema.
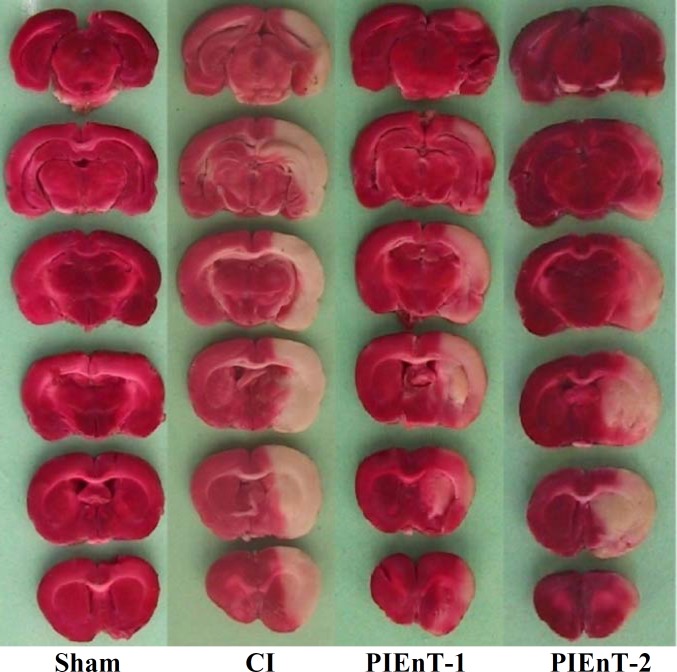
Quantification of cerebral lesions . Animals were deeply anesthetized and guillotined. The brain was immediately removed, cleaned and for solidification, immersed in 4C cold normal saline and kept in a refrigerator for 5 min. Frontal sections of 2-mm thick slices were prepared using a brain matrix and stained with triphenyl-tetrazolium chloride as described previously [11, 12]. In this staining technique, non-ischemic brain regions were colored red by converting triphenyl-tetrazolium chloride to a deep red formazan compound and ischemic areas remained white due to failure of this conversion (Fig. 1). After staining, the slices were photographed and their infarction areas were determined with computer based NIH image analyzer software [11, 12].
Fig. 1.
Representative of brain slices stained with triphenyl-tetrazoliumchloride in sham and ischemic rats receiving vehicle (CI) and enalapril at doses 0.03 mg/kg (PIEnT-1) or 0.1 mg/kg (PIEnT-2) during reperfusion. Ischemic regions are colored white and non-ischemic regions are red. For more details see the text.
Evaluation of brain edema of ischemic hemispheres. To evaluate brain edema, the wet/dry technique was used to directly measure the absolute brain water content (ABWC) of ipsilateral ischemic and contralateral non-ischemic hemispheres [13]. The percentage of ABWC for each hemisphere was calculated as described previously [14].
Statistical analyses. Values are expressed as mean ± S.E.M. One-way ANOVA with post-hoc of Duncan’s test was used to compare the mean values of mean arterial blood pressures (MAP). Holm-Sidack method was also used for pair wise comparisons of other parameters. Statistical significance was accepted at P<0.05.
Results
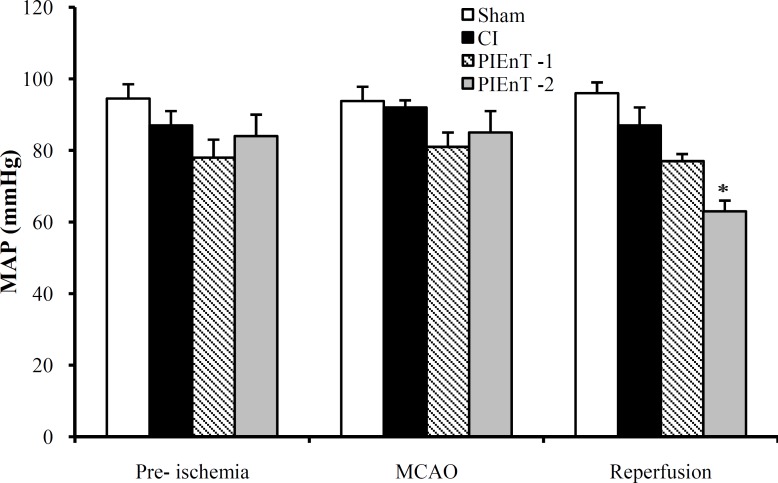
Mean arterial blood pressure. The average MAP of the selected rats of four groups has been presented in Figure 2. The similarities of MAP of CI group at times of pre- and during MCA occlusion and after reopening with each other and with sham are indicating that the maneuver of 60-min MCA occlusion and reopening did not significantly alter arterial blood pressure. These data also indicated that in normotensive rats post-ischemic administration of enalapril at dose 0.03 mg/kg (PIEnT-1, Fig. 2) did not influence arterial blood pressure, whereas at dose of 0.1 mg/kg significantly reduced MAP, during the reperfusion time (PIEnT-2, Fig. 2).
Fig. 2.
Mean arterial blood pressure (MAP) in sham rats (n = 4), control ischemic (CI) rats (n = 4) and ischemic rats receiving enalapril at dose of 0.03 mg/kg (PIEnT-1, n = 4) or 0.1 mg/kg (PIEnT-2, n = 4) 10 minutes before middle cerebral artery occlusion (MCAO), 30 minutes after MCAO and 10 minutes after the beginning of reperfusion. Values are mean ± S.E.M. *significant difference from respective vehicle-treated control rats
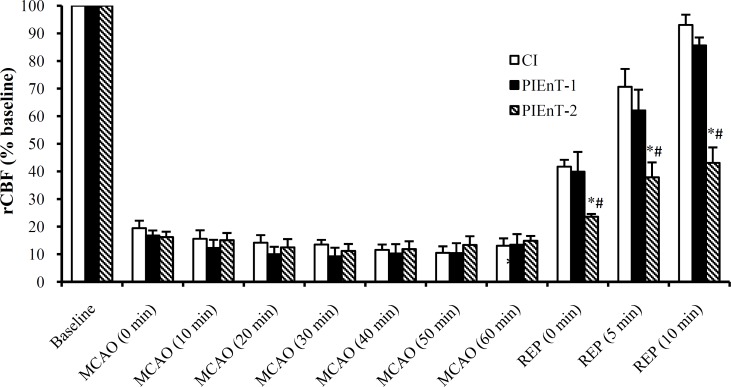
Regional Cerebral blood flow ( rCBF). Alterations of rCBF (% baseline) have been presented in Figure 3. The differences among rCBF of all groups prior to induction of ischemia were not significant. There was about 80% reduction in rCBF of ischemic rats during 60-min MCA occlusion. After the start of reperfusion, the reduced levels of rCBF were increased significantly, and with the exception of PIEnT-2 group, reached to their pre-occlusion levels. Meanwhile, the level of rCBF in group PIEnT-2 steadily increased during reperfusion period but never reached to its pre-ischemic values and still was significantly lower than sham group (Fig. 3).
Fig. 3.
Regional cerebral blood flow (rCBF) in control ischemic (CI) rats received the vehicle (n = 5) and ischemic rats received enalapril at doses of 0.03 mg/kg (PIEnT-1, n = 4) and 0.1 mg/kg (PIEnT-2, n = 5) before and during MCAO as well as during reperfusion period. Animals received enalapril at the beginning of reperfusion. Values are mean ± S.E.M. *significantly different (P<0.05) from control ischemia, #significantly different (P<0.05) from PIEnT-1.
.
Physiological parameters. All physiological parameters measured during the experiment, such as arterial blood gas, blood pH, blood glucose and body temperature were at normal physiological range and inter- and intra-group comparisons indicated no significant differences among their proper values.
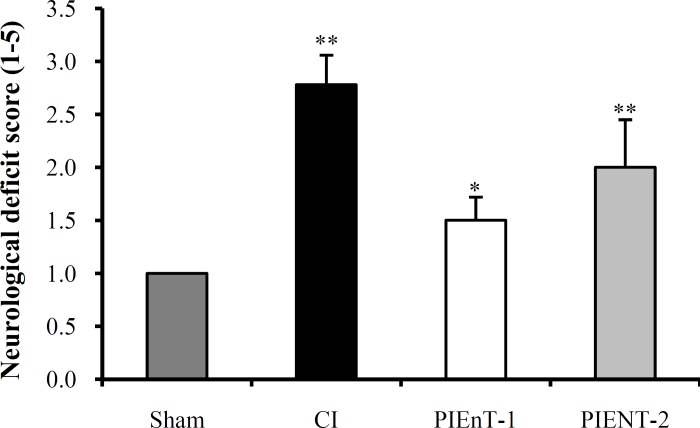
Evaluation of neurological deficit score. The NDS of ischemic rats have been presented in Figure 4. NDS of CI group (2.78 ± 0.28) was significantly higher than both sham and PIEnT-1 groups (1.5 ± 0.22). However, NDS of PIEnT-2 group (2.0 ± 0.45) was not significantly different from CI group, but was significantly higher than sham group (Fig. 4).
Fig. 4.
Neurological deficit score in sham rats (n = 16), control ischemic (CI) rats (n = 12) and ischemic rats received enalapril at doses of 0.03 mg/kg (PIEnT-1, n = 12) and 0.1 mg/kg (PIEnT-2, n = 11) during reperfusion period. Values are mean ± S.E.M. *significant difference (P<0.05) from ischemic control rats, **significant difference (P<0.01) from sham operated rats.
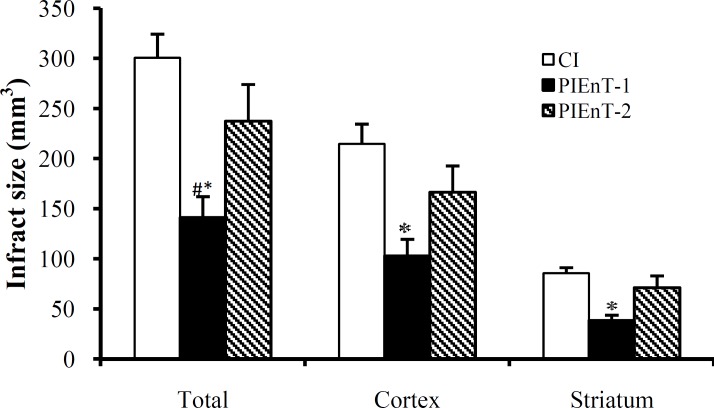
Assessment of cerebral infarct volumes. Figure 1 is a photograph of the coronal sections of rat brains stained with triphenyl-tetrazolium chloride. The uniform dark red color of the slices of the right and the left hemispheres of sham is an induction that anesthesia, neck surgery or other surgical maneuvers per se did not provoke brain injury. This was also true in the left hemispheres (non-lesioned hemispheres) of ischemic rats, meaning that 60-min right MCA occlusion did not affect the left side. The appearance of white color combined with dark red color areas in the right hemispheres of ischemic rats indicated that 60-min right MCA occlusion induced different magnitudes of infarctions without affecting the left hemispheres. MCA occlusion of the right hemispheres in the ischemic rats induced various intensities of infarctions. In addition, quantitative comparisons of these intensities indicated that 60-min MCA occlusion provoked severe infarctions in the cortical and subcortical regions of CI group. Enalapril could reduce the size of infarction about 53% at dose of 0.03 mg/kg but not at dose of 0.1 mg/kg (Fig. 1). Quantitative comparisons of cerebral infarction volumes have been shown in Figure 5. The sham-operated rats had no cerebral infarctions and the total cerebral infarct volume of PIEnT-1 (141 ± 21 mm3) was significantly lower than either CI (301 ± 23 mm3) or PIEn-T2 (237 ± 36 mm3). More specifically, comparison of cortical infarction of ischemic rats indicated that there was a substantial reduction in their infarct volumes when the rats were treated with enalapril at 0.03 mg/kg but not at 0.1 mg/kg (Fig. 1). Comparisons of subcortical infarction volumes of PIEnT-1 group (38 ± 5 mm3) with CI group (86 ± 5 mm3) and PIEnT-2 group (71 ± 11 mm3) indicated that enalapril at dose of 0.03 mg/kg could significantly reduce infarction volumes of this region but its higher dose (0.1 mg/kg) failed to do so (Fig. 5).
Fig. 5.
Cerebral infarct volumes in control ischemic (CI, n = 6) and ischemic rats treated with enalapril during reperfusion at doses of 0.03 mg/kg (PIEnT-1, n = 6) or 0.1 mg/kg (PIEnT-2, n = 6). Values are mean ± SEM. *significantly different (P<0.05) from CI, # significantly different (P<0.05) from PIEnT-2.
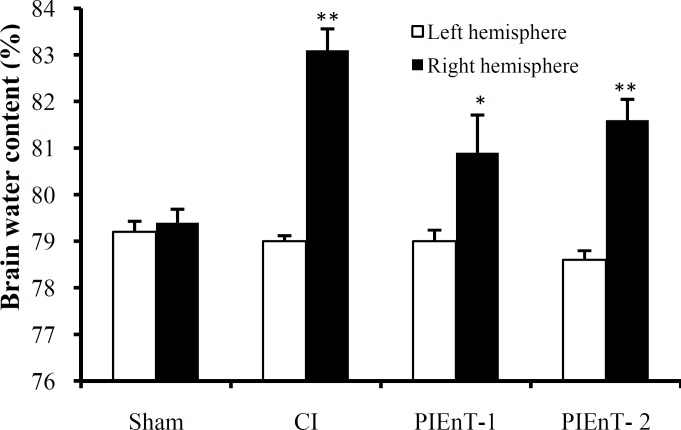
Absolute brain water content. ABWC of the left (non-ischemic) hemispheres of all experimental groups were similar to each other and to the right hemispheres of sham group (Fig. 6). However, the ABWC of the right (ischemic) hemispheres of CI group (83.1 ± 0.46%) were significantly higher that their own ipsilateral ones and the right hemispheres of sham group. Enalapril at dose of 0.03 mg/kg could significantly reduce ABWC of the ischemic hemispheres (PIEnT-1; 80.9 ± 0.81%). This decrease was such that ABWC of ischemic hemispheres was not significantly different from ipsilateral non-ischemic hemispheres and also sham group, whereas the ABWC of the ischemic hemispheres of PIEnT-2 (81.6 ± 0.45%) was statistically similar to ischemic hemispheres of CI group.
Fig. 6.
Brain water content of right and left hemispheres of sham, non-ischemic hemispheres (left side) and ipsilateral ischemic hemispheres (right side) in control ischemia (CI) and ischemic rats treated with enalapril during reperfusion at doses of 0.03 mg/kg (PIEnT-1) or 0.1 mg/kg (PIEnT-2). CI rats received the vehicle. Values are mean ± S.E.M. *significantly different (P<0.05) from CI, **significantly different (P<0.05) from sham.
Discussion
The results of this study reveal that post-ischemic inhibition with non-hypotensive dose of enalapril protects the brain from I/R injury. The mechanisms by which enalapril decreases ischemic neuronal damage are controversial. The damages seen during ischemia are related to the elevation of Ang II [15-17], and its receptor blockers extend the recovery of cerebral ischemia [18]. Ang II is a potent vasoconstrictor that increases vascular tone by direct stimulation of vascular smooth muscles and indirect increased release of catecholamines from sympathetic fibers [19, 20]. Some investigators believe that ischemia impairs the auto-regulatory mechanisms of cerebral vessels and CBF becomes entirely dependent of arterial blood pressure [18, 21]. Since the reduction of Ang II production after ischemia lowers cerebral vascular tone [22], we think enalapril, in the presence of normal arterial blood pressure (in PIEnT-1 group), protects the brain from I/R injury by restoring rCBF, and hypotension is the cause of failure in PIEnT-2 group. This finding is in agreement with an earlier report that arterial hypotension compromises the regulation of CBF after MCA occlusion [19].
Previous studies indicated that superoxide radicals play an important role in potentiation of delayed ischemic neuronal death after ischemia [17, 19, 23]. The maintained vascular reactivity of collateral vessels of the ischemic region is also a decisive factor for blood flow improvement after acute MCA occlusion [19]. Our observation shows that reperfusion after MCA occlusion could similarly restore the diminished rCBF both in PIEnT-1 and in CI groups (Fig. 3). However, quantitative comparison of the brain infarction failed to show such an improvement in CI rats (Fig. 5). Some investigators have specified that cerebral ischemia is associated with the excessive production of free radicals [17, 23, 24]. Reduced production of Ang II, or its receptor blockers promote(s) neuronal survival by reduction of reactive oxygen species [17, 25, 26]. Therefore, we think the protective actions of ACE inhibition after ischemia need the combination of re-establishment of normal cerebral blood flow and the reduced production of toxic free radicals, neither one alone cannot show this protection.
Brain edema is life-threatening complication of cerebral ischemia and one of the major determinants of patient’s survival after the first few hours of stroke [3]. Some investigators that showed long-term ACE inhibition has a correlation with edema and mortality rate [27-30]. This study shows that enalapril in normotensive rat reduced edema of the ischemic hemispheres independent of arterial blood pressure changes. An earlier report also showed that enalapril reduces cerebral edema in stroke-prone hypertensive rats [31] and prevents stroke dysfunctions by diminishing hypertension in salt-loaded hypertensive rats [27]. In accordance with our findings, Porritt et al. [32] showed that the beneficial effects of ACE inhibition do not correlate with changes in blood pressure. Therefore, in our study, the reduced edema in the presence of normal blood pressure besides other mechanisms probably improves the re-establishment of microcirculation of the ischemic region and helps the repair of damages done during ischemia. In normotensive rats, post-ischemic inhibition of ACE with enalapril protected the brain from ischemic reperfusion injury. This protection was coincided with normal arterial pressure blood pressure, but was absent during hypotension.
Therefore, the results of this study show that prevention of reduced arterial blood pressure is important in helping the non-hypertensive patients to cope with the stroke after treatment with ACE inhibitors.
Acknowledgment
The authors cordially appreciate the joint financial supports of Vice Chancellor for Research of Shiraz University of Medical Sciences, grant no. 83-2375, and Vice Chancellor for Research of Ardabil University of Medical Sciences, grant no 88329, Iran.
References
- 1.Dirnagl U, C Iadecola, MA Moskowitz. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999 Sep;22(9):391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 2.Jones TH, Morawetz RB, Crowell RM, Marcoux FW, FitzGibbon SJ, DeGirolami Uetal. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981 Jun;54(6):773–82. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- 3.Schuier FJ, Hossmann KA. Experimental brain infarcts in cats II. Ischemic brain edema. Stroke. 1980 Nov-Dec;11(6):593–601. doi: 10.1161/01.str.11.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Panahpour H, Nekooeian AA, Dehghani GA. Inhibition of angiotensin-converting enzyme reduces cerebral infarction size in experimental-induced focal cerebral ischemia in the rat. Iran J Med Sci. 2007 Mar;32(1):12–7. [Google Scholar]
- 5.Unger T, Badoer E, Ganten D, Lang R, Rettig R. Brain angiotensin: pathways and pharmacology. Circulation. 1988 Jun;77(6 Pt 2):I40–54. [PubMed] [Google Scholar]
- 6.Culman J, Blume A, Gohlke P, Unger T. The renin-angiotensin system in the brain: possible therapeutic implications for AT(1)-receptor blockers. J Hum Hypertens. 2002 Aug;16(Suppl 3):S64–70. doi: 10.1038/sj.jhh.1001442. [DOI] [PubMed] [Google Scholar]
- 7.Brdon J, Kaiser S, Hagemann F, Zhao Y, Culman J, Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J Hypertens. 2007 Jan;25(1):187–96. doi: 10.1097/01.hjh.0000254376.80864.d3. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Yamakawa H, Bregonzio C, Terrón JA, Falcón-Neri A, JM Saavedra. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002 Sep;33(9):2297–303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi MT, Shid-Moosavi SM, Dehghani GA. Contribution of nitric oxide synthase (NOS) in blood-brain barrier disruption during acute focal cerebral ischemia in normal rat. Pathophysiology. 2010 Feb;19(1):13–20. doi: 10.1016/j.pathophys.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989 Jan;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 11.Panahpour H, Dehghani GA. Inhibition of central angiotensin-converting enzyme with enalapril protects the brain from ischemia/reperfusion injury in normotensive rat. Daru. 2010;18(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Vakili A, Nekooeian A, Dehghani GA. Aminoguanidine reduces infarct volume and improves neurological dysfunction in transient model of focal cerebral ischemia in rat. DARU. 2006;14(1):26–30. [Google Scholar]
- 13.Gerriets T, Stolz E, Walberer M, Muller C, Kluge A, Bachmann A, et al. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke. 2004 Feb;35(2):566–71. doi: 10.1161/01.STR.0000113692.38574.57. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadi MT, Shid MoosaviSM, Dehghani GA. Contribution of nitric oxide synthase (NOS) activity in blood-brain barrier disruption and edema after acute ischemia/reperfusion in aortic coarctation-induced hypertensive rats. Iran Biomed J. 2011;15(1-2):22–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari R, Cargnoni A, Curello S, Ceconi C, Boraso A, Visioli O. Protection of the ischemic myocardium by the converting-enzyme inhibitor zofenopril: insight into its mechanism of action. J Cardiovasc Pharmacol. 1992;19(5):694–704. [PubMed] [Google Scholar]
- 16.Kohara K, Mikami H, Okuda N, Higaki J, Ogihara T. Angiotensin blockade and the progression of renal damage in the spontaneously hypertensive rat. Hypertension. 1993 Jun;21(6 Pt2):975–9. doi: 10.1161/01.hyp.21.6.975. [DOI] [PubMed] [Google Scholar]
- 17.Ravati A, Junker V, Kouklei M, Ahlemeyer B, Culmsee C, Krieglstein J. Enalapril and moexipril protect from free radical-induced neuronal damage in vitro and reduce ischemic brain injury in mice and rats. Eur J Pharmacol. 1999 May;373(1):21–33. doi: 10.1016/s0014-2999(99)00211-3. [DOI] [PubMed] [Google Scholar]
- 18.Groth W, Blume A, Gohlke P, Unger T, Culman J. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J Hypertens. 2003 Nov;21(11):2175–82. doi: 10.1097/00004872-200311000-00028. [DOI] [PubMed] [Google Scholar]
- 19.Shima T, Hossmann K, Date H. Pial arterial pressure in cats following middle cerebral artery occlusion. Relationship to blood flow, regulation of blood flow and electrophysiological function. Stroke. 983 Sep-Oct;14(5):713–9. doi: 10.1161/01.str.14.5.713. [DOI] [PubMed] [Google Scholar]
- 20.Dzau VJ. Vascular renin-angiotensin system in hypertension. New insights into the mechanism of action of angiotensin converting enzyme inhibitors. Am J Med. 1988 Apr;84(4A):4–8. doi: 10.1016/0002-9343(88)90463-9. [DOI] [PubMed] [Google Scholar]
- 21.Haas DC, Anderson GHJr, Streeten DH. Role of angiotensin in lethal cerebral hypoperfusion during treatment of acute hypertension. Arch Intern Med. 1985 Oct;145(10):1922–4. [PubMed] [Google Scholar]
- 22.Speth RC, Harik SI. Angiotensin II receptor binding sites in brain microvessels. Proc Natl Acad Sci USA. 1985 Sep;82(18):6340–3. doi: 10.1073/pnas.82.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calaqui B, et al. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998 Oct;18(20):8292–9. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991 Dec;88(24):11158–62. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugawara T, Kinouchi H, Oda M, Shoji H, Omae T, Mizoi K. Candesartan reduces superoxide production after global cerebral ischemia. Neuroreport. 2005 Mar;16(4):325–8. doi: 10.1097/00001756-200503150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Zhou F, Xiang Z, Feng WX, Zhen LX. Neuronal free Ca2+ and BBB permeability and ultrastructure in head injury with secondary insult. J Clin Neuroscience. 2001 Nov;8(6):561–3. doi: 10.1054/jocn.2001.0980. [DOI] [PubMed] [Google Scholar]
- 27.Stier C, Benter IF, Ahmad S, Zuo H, Selig N, Roethel S, et al. Enalapril prevents stroke and kidney dysfunction in salt-loaded stroke-prone spontaneously hypertensive rats. Hypertension. 1989 Feb;13(2):115–21. doi: 10.1161/01.hyp.13.2.115. [DOI] [PubMed] [Google Scholar]
- 28.Stier CJr, Chander P, Gutstein W, Levine S, Itskovitz H. Therapeutic benefit of captopril in salt-loaded stroke-prone spontaneously hypertensive rats is independent of hypotensive effect. Am J Hypertens. 1991 Aug;4(8):680–7. doi: 10.1093/ajh/4.8.680. [DOI] [PubMed] [Google Scholar]
- 29.Vacher E, Fornes P, Domergue V, Richer C, Bruneval P, Giudicelli JF. Quinapril prevents stroke both during and after the treatment period in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 1993 Nov;6(11 Pt 1):951–9. doi: 10.1093/ajh/6.11.951. [DOI] [PubMed] [Google Scholar]
- 30.Inada Y, Ojima M, Itoh K, Shino A, Nishikawa K. Effects of delapril on stroke, kidney dysfunction and cardiac hypertrophy in stroke-prone spontaneously hypertensive rats. Drugs Exp Clin Res. 1995;21(2):41–9. [PubMed] [Google Scholar]
- 31.Blezer ELA, Nicolay K, Koomans HA, Joles JA. Losartan versus enalapril on cerebral edema and proteinuria in stroke-prone hypertensive rats. Am J Hypertens. 2001 Jan;14(1):54–61. doi: 10.1016/s0895-7061(00)01231-0. [DOI] [PubMed] [Google Scholar]
- 32.Porritt MJ, Chen M, Rewell SSJ, Dean RG, Burrell LM. Howells DW, ACE inhibition reduces infarction in normotensive but not hypertensive rats: correlation with cortical ACE activity. J Cereb Blood Flow Metab. 2010 Aug;30(8):1520–6. doi: 10.1038/jcbfm.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]