Abstract
Background: Through its membrane and intracellular receptors, vitamin D regulates many vital functions in the body including its well known actions on musculoskeletal system. Growing body of evidences demonstrate that vitamin D undergoes some of behavioral aspects of neurocognition. The present study was designed to evaluate the effect of food regimens without vitamin D or with a supplement of 1,25(OH)2D3 on spatial performance of adult rats. Methods: The animals were trained in the Morris water maze to find a hidden platform. The time spent and the distance traveled to find the platform, speed of navigation and the percentage of unsuccessful trials were considered for assessment of the task learning. Results: Our findings indicated that the vitamin D-deprived rats had a significant lower performance compared to both the controls and the animals receiving 1,25(OH)2D3 supplementation. Concerning the unsuccessful trials, lack of vitamin D resulted in the highest failures in the maze navigation. The regimen with additional 1,25(OH)2D3 did not considerably influence learning of the maze task. Conclusion: We concluded that while vitamin D deficiency deteriorates the spatial task learning, the 1,25(OH)2D3 supplementation did not effectively underlie the maze performance.
Key Words: Vitamin D, Maze learning, Dietary supplements
Introduction
It is well known that through calcium homeostasis, bone formation and maintenance [1], vitamin D is essential for the musculoskeletal health and function [2]. During exposure to solar ultraviolet B radiation, 7-dehydrocholesterol in the skin is converted to cholecalciferol. Cholecalciferol is converted to 25-hydroxyvitamin D in the liver and then to biologically active form 1,25(OH)2D3 in the kidneys [2]. The 1,25(OH)2D3 binds to nuclear vitamin D receptors and regulates approximately 200 genes involved in cell differentiation, proliferation, and apoptosis [3]. The 1,25(OH)2D3 also promotes rapid non-genomic signaling including rapid calcium translocation through voltage-gated ion channels [4] and up-regulation of the mitogen-activated protein kinase cascade via a protein kinase C signaling pathway [5]. The vitamin D receptors are also present in many regions of human brain, including neocortex, hippocampus, thalamus, piriform cortex, hypothalamus and amygdale [6].
There are evidences demonstrating the positive effect of multivitamins and mineral supplementation on cognitive function [7]. Evidence supports the role of vitamin D in function of the developing as well as adult brain [8]. Valuable roles of vitamin D in cognitive function have been proposed [9]. Also, some reports have demonstrated the effects of vitamin D on learning [8] and behavior [10].
Spatial representation is one aspect of cognition in which the abilities of humans and animals are assessed [11]. The hippocampus is a well known area of brain involved in spatial learning and memory. Although some evidence strongly suggests that 1,25(OH)2D3 is involved in brain development and critical brain functions [12], it has been difficult experimentally to demonstrate the facts demonstrating obvious effects of the vitamin D inadequacy on cognitive or behavioral end points [13]. The present work was designed to evaluate if vitamin D deprivation influences the ability of adult rats in learning the spatial task. Also, we assessed the effect of 1,25(OH)2D3 supplementation on the maze performance of the animals.
MATERIALS AND METHODS
Animals . Adult male Wistar rats weighing 250-300 g were assigned randomly to one of the three experimental groups. The control group (CON, n = 14) were fed a normal diet receiving 15-20 µg vitamin D/kg body weight/day. The other two groups were basically given a diet similar to the control group except that one group (CON-D, n = 9) received a regimen without vitamin D and another group (CON+D, n = 11) received a supplement of 1,25(OH)2D3 (1,000 ng/100 g dry food). All groups were treated for 10 weeks. The experimental subjects were kept in a single holding room and housed in a constant temperature of 21 ± 2°C and a humidity of 55 ± 5%. The animals reared under a 12-h light/dark cycle using incandescent lighting free of ultraviolet radiation in the vitamin D action spectrum (290-315 nm). They had free access to food and water was available ad libitum. All experiments were conformed to the ethical use of animals of Kashan University of Medical Sciences (Iran).
Experimental diets . The synthetic diet was formulated to contain all essential nutrients and modified AIN-93 diet (according to the reference 14 it is a special formula for making the food regimen used in this study) [14]. Each 1 kg of the synthetic diet was suspended in 1.5 liters of boiling distilled water, cooled and dried in oven under 80oC and stored at 4oC. The animals were fed based on a daily measured consumption of 15-17 g dry weight of diet/rat. Fresh diet was prepared weekly.
Analysis of serum concentration of calcium . The collected blood was centrifuged and the serum was frozen at -20oC until analysis. Using special kits (purchased from Zist Chimi Co., Iran), thawed samples were analyzed for calcium with auto-analyzer BT 300 by colorimetric method.
Apparatus. Spatial learning performance was assessed in the Morris water maze (a tank made of galvanized metal, 150 cm diameter, 70 cm depth) that was filled with water (22oC) up to 20 cm below the rim. The pool was divided into four equal quadrants named northeast, southeast, southwest and northwest. A circular platform (10 cm diameter) was submerged 1.5 cm below the water surface and located in the center of one quadrant in fixed position throughout the experiment. The testing room contained a number of extra-maze visual cues visible to the experimental subjects. A video camera and computerized tracking system were used to monitor and save the animal navigation in the water maze. Behavioral variables were quantified with the aid of customized software (Radiab7, Iran).
Behavioral testing. Each trial was started by releasing an animal into the water maze, immediately facing the perimeter, at one of the four start points (north, south, east or west). In the training phase of experiment, the animals were allowed to swim for a maximal time of 90 s to find the hidden platform. If the subject failed to find the platform within 90 s, it was guided to the platform where it remained for 15 s. Having a 15-min inter-trial interval the rat was immediately replaced in the pool. The starting location was varied in each trial. Following the completion of the trial, the rats were dried and returned to their home cage. This phase of the experiment took 5 consecutive days and each subject had four training trials per day. The parameters measured as indices of the learning of the spatial task were: the number of trials in which the animals were failed to locate the hidden platform before elapsing the maximum time of 90 s, the delay and distance to locate the platform and the speed of navigation.
Data analysis. The total time elapsed to locate the platform (in seconds), distance (in centimeters) and the speed (distance/time) of maze searching by the animal until it finds the platform (in centimeter/second) were considered as indicators of learning in the training phase of experiments. The data pooled from the training trials are presented as a mean ± SEM and analyzed by a two-way ANOVA, followed by LSD post test. The number of trials in which the subject was failed to locate the platform before finishing the maximum time (90 s) was considered as unsuccessful trials (committed errors) and statistically assessed by Cox survival regression test followed by Wald post-hoc test. The differences were considered significant with a P value of less than 0.05.
Results
The role of the active form of vitamin D on the learning and memory of the adult rats was evaluated considering the number of unsuccessful trials, the time spent, the distance traveled and the speed of steering in the Morris water maze. Also, the 1,25(OH)2D3-dependent changes of plasma calcium concentration and possibility of its role on the spatial training of the animals were considered.
Behavioral experiments
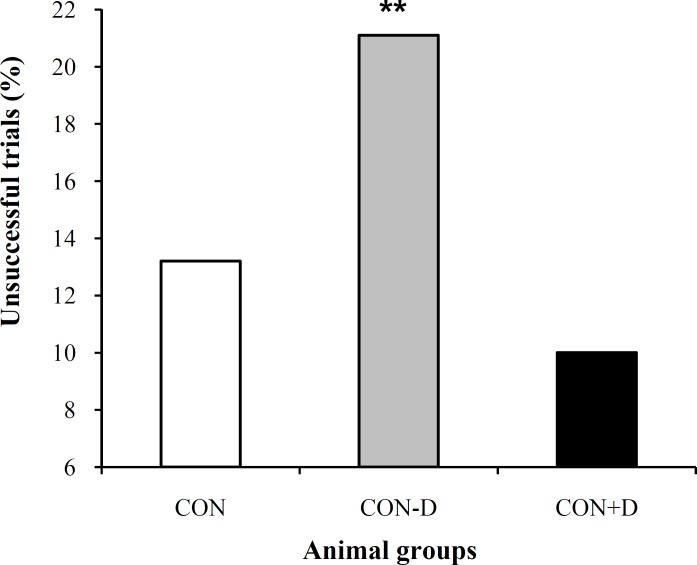
Number of the unsuccessful trials in navigation of the water maze. The experiments in which the animals were failed to finish trials before finishing the maximum time of 90 s were analyzed for the CON, CON D and CON+D groups. The experiments lasted 5 days and total trials for all animals of each group were considered for evaluation. We found that the CON rats committed 13.2% errors (37 out of 280 trials) over the experiment. The CON-D animals increased the errors to 21.1% (38 out of 180 trials). The animals receiving supplemental 1,25(OH)2D3 never access to the hidden platform in only 10% of the trials (22 out of 220 trials). Cox survival regression test appeared a considerable variation among the three groups (P = 0.005). Wald post-hoc test indicated that the differences was significant between the CON-D with both the CON and CON D animals (P = 0.004). The improved behavior of the CON+D rats compared to the CON ones was not statistically considerable. Figure 1 illustrates the errors committed by the three groups of animals.
Fig. 1.
Percentage of the unsuccessful trials in locating the hidden target during the Morris water maze searching. The 1,25(OH)2D3-deprived rats (CON-D) showed a more marked number of unsuccessful trials compared to the animals under the normal (CON) or supplemental 1,25(OH)2D3 (CON+D) food regimens. **P = 0.004 compared to the CON and CON+D groups
Training phase. In this part of experiment, the escape latency and traveled path from release of the animals in the water maze to locating the hidden platform were evaluated.
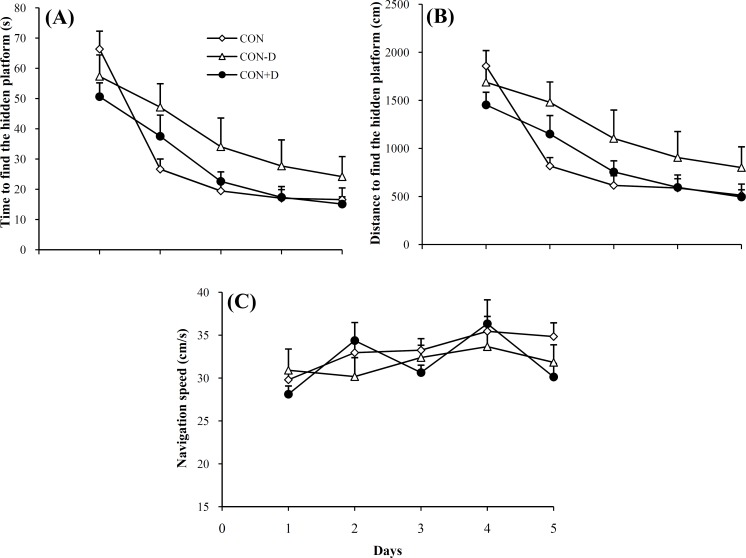
The time spent to locate the hidden platform. Analysis of variance indicated that the animals in the different groups varied in the time spent (F2, 133 = 4.753; P = 0.012) in the water maze. Spatial navigation of the CON and CON+D animals indicated that these groups analogously improved their behavior over 5 days of training. On the other hand, LSD post-hoc test indicated that the 1,25(OH)2D3-deprived rats displayed a weaker function compared to both CON (P = 0.001) and CON+D animals (P<0.0001). No difference was observable between the performance of the CON and CON+D groups. Figure 2A shows the mean latency taken to find the target in the water maze.
The distance traveled to locate the hidden platform. We evaluated the traveled path by the subjects introduced to the training phase of experiments. The behavior of the three tested groups for the spatial learning was statistically different (F2, 133 = 5.79; P = 0.004). As illustrated in the Figure 2B, the CON-D animals needed a longer swim distance to find the maze target when compared to either CON (P<0.0001) or CON+D (P<0.0001) rats. The difference observed between the vehicle and the 1,25(OH)2D3 treated rats was not significant.
The speed of maze navigation during the spatial task learning. To confirm if vitamin D deprivation and, in turn, decreased level of plasma calcium underlie locomotion of the animals, the navigation speed was also analyzed. Analysis of variance demonstrated that the speed of maze navigation by the CON, CON-D and CON+D animals was almost similar (F2, 133 = 0.585; P = 0.711) (Fig. 2C).
Fig. 2.
Performance of the control (CON, open squares), receiving additional vitamin D (CON+D, solid circles) and vitamin D-deprived (CON-D, open triangles) rats during 5 days of the water maze training. Curves show (A) the escape latency, (B) the distance traveled and (C) the speed of navigation in the three tested group of rats. Each data point indicates mean ± SEM summed results of four daily trials.
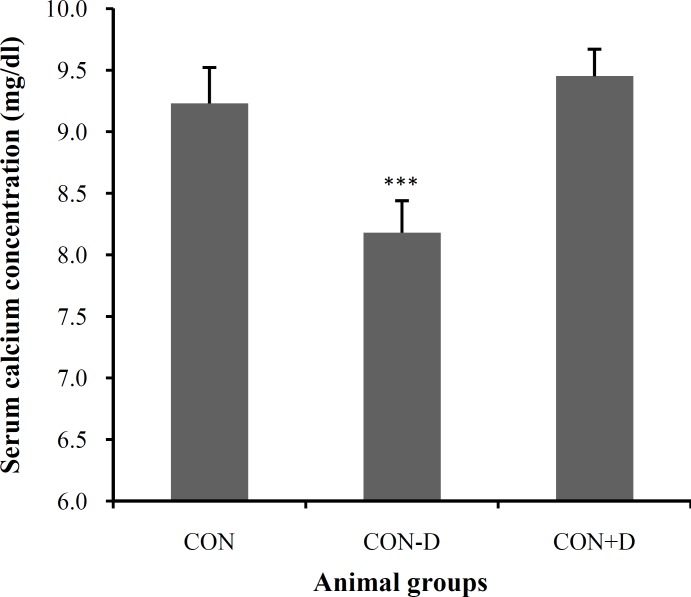
Plasma concentration of calcium. The biochemical assessment in our study uncovered that the plasma concentration of calcium varies in the animals under different regimens (F2, 25 = 28.276; P = 0.0001). The post test showed that the vitamin D-deprived rats have a plasma level of calcium significantly less than both CON (P<0.0001) and CON+D (P<0.0001) groups. However, the animals receiving additional 1,25(OH)2 D3 displayed no noticeable changes in the serum calcium when compared to the CON group. Figure 3 represents the plasma levels of calcium.
Discussion
Findings of the present study uncovered that a food regimen empty of vitamin D gives rise to a pronounced lower performance in the spatial task. The CON-D rats never located the maze target in about 21% of trials; one third more than the CON animals. In addition, they required a longer time to learn the place of the hidden platform compared to the controls. On the other hand, vitamin D supplementation did not significantly influence the maze performance in learning the maze task.
The vitamin D receptor and catalytic enzymes are co-localized in the areas of the brain involved in complex planning, processing, and formation of new memories [9]. In embryonic animal models, vitamin D deficiency results in morphological brain changes [15] and subtle and discrete learning and memory impairments in adulthood [8]. Human studies have also considered the effect of vitamin D on the cognitive function of brain. Consistent to our results, Dean et al. [16] reported that vitamin D supplementation does not influence cognitive or emotional functioning in healthy adults.
Further, McGrath et al. [17] observed an inverse association between 25(OH)D3 and a test of learning and memory in older adults (60-90 years). What are the mechanisms by which the 1,25(OH)2D3 undergoes the behavioral aspects of the neurocognition? Neuro-protection through anti-oxidative, immune-modulation [18], neuronal calcium regulation, enhanced nerve conduction and detoxification [19] could be probable mechanisms.
The 1,25(OH)2D3 up-regulates gamma glutamyl transpeptidase [20] increasing antioxidant glutathione. Additionally, similar to the benefits of traditional antioxidant nutrients, 1,25(OH)2D3 inhibits inducible nitric oxide syntheses [21] that serves to protect the brain from free radicals-induced damage. The 1,25(OH)2D3 has been also shown to attenuate the neurotoxicity of 6-hydroxydopamine exposure in rats [22]. If these neuroprotective effects influence the cognitive function of the brain needs to be illustrated.
Neurotrophins are proteins necessary for neuronal survival in aging and neuropathological conditions [23]. Decreased synthesis of neurotrophin leads to a compromised spatial navigation influencing the hippocampus, which is involved in spatial learning and memory [23]. 1,25(OH)2D3 up-regulates neurotrophin factors such as neurotrophin-3 [18]. Neurotrophin-3 is found in the hippocampus, which is especially sensitive to age or pathology-related degeneration [23] and neocortex and protects nerve transmission and synaptic plasticity [19]. The protein also increases signal transmission in the hippocampal cells, which are known to have high levels of the vitamin D receptor mRNA [6]. Therefore, the positive effect of 1,25(OH)2D3 on the spatial task may be contributed to the neuronal protective roles of the several factors regulated by 1,25(OH)2D3.
Fig. 3.
The plasma concentration (mg/dl) of calcium in the CON, CON-D and CON+D groups. The values indicate mean ± SEM. ***P<0.0001, the difference between the CON-D group with the CON and CON+D groups.
Concerning the serum concentration of calcium, our findings indicated that the CON-D animals have a lower level of plasma calcium compared to the other two groups; interestingly comparable to the results of maze performances. The vitamin D endocrine system plays a primary role in the maintenance of calcium homeostasis and the stringent regulation of extracellular fluid calcium concentration within narrow limits is essential to support normal nerve and muscle function [24]. Studies have shown that vitamin D confers regulatory benefits in neuronal calcium homeostasis and protects neurons from excess calcium entry in the brain [25]. It has been also shown that excessive calcium levels are deleterious for memory formation and cognitive function [26]. Multiple calcium-dependent processes can be regulated by 1,25(OH)2D3 in neurons with particular impact on reducing age-related changes associated with calcium disregulation [27]. One potential target for 1,25(OH)2D3 may be L-type voltage-sensitive calcium channel (L-VSCC) [4]. L-VSCC appear to play important roles in regulation of some calcium-dependent neuronal processes, including neuronal excitability [28] and experimentally induced form of synaptic plasticity and long-term potentiation (LTP) [29].
Several studies have found that levels of calbindin as well as other calcium binding proteins (parvalbumin and calretenin) increase in specific brain areas after treatment with 1,25(OH)2D3 [30], which could have implications for neuroprotection [31]. Although excessive calcium levels are deleterious for memory formation and cognitive function [26], parvalbumin, a high-affinity cytoplasmic calcium-binding protein, expressed in specific neurons to maintain a fast firing rate by reducing the calcium-dependent potassium outflow [32]. The performance of the experimental subjects in this study could not be associated with the effect of the 1,25(OH)2D3 on the level of plasma calcium. Instead, it seems that some 1,25(OH)2D3-dependent modifications such as regulation of the calcium-binding proteins, intracellular calcium and L-VSCC in cellular level undergo the cognitive behavior of the animals. Conclusively, the vitamin D-deprived rats show a poorer function than either the CON or CON+D rats. It is worthwhile to note that according to the data presented here, the lower concentration of plasma calcium in the CON-D group does not significantly influence locomotion of the animals during the maze steering. Therefore, the impaired performance of the CON-D rats was not associated to disability in swimming.
It is believed that high vitamin foods reduce probability of dementia [33]. Beside vitamin D, the role of other vitamins in cognitive phenomena as well as LTP, a suggested mechanism in processing learning and memory, has also been considered. Evidence indicates that some other vitamins such as vitamin B12 [34] and alpha-tocopherol [35], positively influence production of LTP.
Although increased level of intracellular calcium through calcium channels or NMDA receptors is a necessity for production of LTP, it is a destructive factor for cell protection as well as cognitive performances. Therefore, further study is needed to clear such a paradox.
Acknowledgment
This work was funded by grant No. 7753 from Department of Nutrition and Biochemistry, Tehran University of Medical Sciences to A. D Jazayery. We wish to gratefully thank Dr. GH. Hamidi for his excellent technical assistance. We also appreciate Physiology Research Center, Kashan University of Medical Sciences (Iran) for association in performing the experiments.
References
- 1.Heaney RP, Weaver CM. Calcium and vitamin D. Endocrinol Metab Clin North Am. 2003 Mar;32(1):181–94. doi: 10.1016/s0889-8529(02)00063-4. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged >= 60 y. Am J Clin Nutr. 2004 Sep;80(3):752–58. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 3.Segaert S, Bouillon R. Vitamin D and regulation of gene expression. Curr Opin Clin Nutr Metab Care. 1998 Jul;1(4):347–54. doi: 10.1097/00075197-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Meszaros JG, Karin NJ, Akanbi K, Farach CarsonMC. Down-regulation of L-type Ca2+ channel transcript levels by 1,25-dihydroxyvitamin D3 - Osteoblastic cells express L-type alpha(1C) Ca2+ channel isoforms. J Biol Chem. 1996 Dec;271(51):32981–85. doi: 10.1074/jbc.271.51.32981. [DOI] [PubMed] [Google Scholar]
- 5.Boyan BD, Wang L, Wong KL, Jo H, Schwartz Z. Plasma membrane requirements for 1 alpha,25(OH)(2) D-3 dependent PKC signaling in chondrocytes and osteoblasts. Steroids. 2006 Apr;71(4):286–90. doi: 10.1016/j.steroids.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the Vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005 Jan;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am J Clin Nutr. 2007 Oct;86(4):1082–93. doi: 10.1093/ajcn/86.4.1082. [DOI] [PubMed] [Google Scholar]
- 8.Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res. 2005 Jun;161(2):306–12. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: Preventing "D"ecline? Mol Aspects Med. 2008 Dec;29(6):415–22. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annweiler C, Schott AM, Rolland Y, Blain H, Herrmann FR, Beauchet O. Dietary intake of vitamin Dand cognition in older women: a large population-based study. Neurology. 2010 Nov;75(20):1810–6. doi: 10.1212/WNL.0b013e3181fd6352. [DOI] [PubMed] [Google Scholar]
- 11.Poucet B. Spatial cognitive maps in animals-new hypotheses on their structure and neural mechanisms. Psychol Rev. 1993 Apr;100(2):163–82. doi: 10.1037/0033-295x.100.2.163. [DOI] [PubMed] [Google Scholar]
- 12.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction. FASEB J. 2008 Apr;22(4):982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 13.McGrath JJ, Feron FP, Burne THJ, Mackay-Sim A, Eyles DW. Vitamin D-3-implications for brain development. J Steroid Biochem Mol Biol. 2004 May;89-90(1-5):557–60. doi: 10.1016/j.jsbmb.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 14.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997 May;127(5):838S–41S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 15.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D-3 and brain development. Neuroscience. 2003 May;118(3):641–53. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 16.Dean AJ, Bellgrove MA, Hall T, Phan WM, Eyles DW, Kvaskoff D, McGrath JJ. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults--a randomised controlled trial. PLoS One. 2011;6(11):e25966. doi: 10.1371/journal.pone.0025966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-Hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007 Sep;29(1-2):49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- 18.Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1,25- dihydroxyvitamin D-3,regulates NT-3,NT-4 but not bonf messenger-RNA in astrocyes. Neuroreport. 1994 Dec;6(1):124–6. doi: 10.1097/00001756-199412300-00032. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Schuman EM. Intracellular Ca2+ signaling is required for neurotrophin-induced potentiation in the adult rat hippocampus. Neurosci Lett. 2000 Mar;282(3):41–44. doi: 10.1016/s0304-3940(00)00893-4. [DOI] [PubMed] [Google Scholar]
- 20.Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem. 1999 Aug;73(2):859–66. doi: 10.1046/j.1471-4159.1999.0730859.x. [DOI] [PubMed] [Google Scholar]
- 21.Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalo-myelitis. Brain Res Mol Brain Res. 1997 May;45(2):255–67. doi: 10.1016/s0169-328x(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, et al. Vitamin D-3 attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001 Jun;904(1):67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 23.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000 Sep;33(2-3):199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 24.Anderson PH, May BK, Morris HA. Vitamin d metabolism: new concepts and clinical implications. Clin Biochem Rev. 2003;24(1):13–26. [PMC free article] [PubMed] [Google Scholar]
- 25.Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001 Jan;21(1):98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res. 2003;110(2):193–202. doi: 10.1016/s0169-328x(02)00643-5. [DOI] [PubMed] [Google Scholar]
- 27.Brewer LD, Porter NM, Kerr DS, Landfield PW, Thibault O. Chronic 1 alpha,25-(OH)(2) vitamin D-3 treatment reduces Ca2+-mediated hippocampal biomarkers of aging. Cell Calcium. 2006;40(3):277–86. doi: 10.1016/j.ceca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Landfield PW, Thibault O, Mazzanti ML, Porter NM, Kerr DS. Mechanisms of neuronal death in brain aging and Alzheimers-disease-role of endocrine-mediated calcium dyshomeostasis. J Neurobiol. 1992 Nov;23(9):1247–60. doi: 10.1002/neu.480230914. [DOI] [PubMed] [Google Scholar]
- 29.Kapur A, Yeckel MF, Gray R, Johnston D. L-type calcium channels are required for one form of hippocampal mossy fiber LTP. J Neurophysiol. 1998 Apr;79(4):2181–90. doi: 10.1152/jn.1998.79.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexianu ME, Robbins E, Carswell S, Appel SH. 1 alpha,25 dihydroxyvitamin D-3-dependent up-regulation of calcium-binding proteins in motoneuron cells. J Neurosci Res. 1998 Jan;51(1):58–66. doi: 10.1002/(SICI)1097-4547(19980101)51:1<58::AID-JNR6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc Natl Acad Sci USA. 1990 Jun;87(11):4078–82. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celio MR. Calbindin-D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35(2):375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 33.Taghizadeh M, Djazayery A, Salami M, Eshraghian MR, Zavareh SA. Vitamin-D-free regimen intensifies the spatial learning deficit in Alzheimer's disease. Int J Neurosci. 2011 Jan;121(1):16–24. doi: 10.3109/00207454.2010.523132. [DOI] [PubMed] [Google Scholar]
- 34.Chen WH, Wang M, Yu SS, Su L, Zhu DM, She JQ, et al. Clioquinol and vitamin B12 (cobalamin) synergistic-ally rescue the lead-induced impairments of synaptic plasticity in hippocampal dentate gyrus area of the anesthetized rats in vivo. Neuroscience. 2007 Jul;147(3):853–64. doi: 10.1016/j.neuroscience.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Xie Z, Sastry BR. Induction of hippocampal long-term potentiation by alpha-tocopherol. Brain Res. 1993 Feb;604(1-2):173–9. doi: 10.1016/0006-8993(93)90365-t. [DOI] [PubMed] [Google Scholar]