Abstract
Background: Helicobacter pylori, which is associated with many upper gastrointestinal diseases, is found in half of the population of the world. Several special stains and immunohistochemistry stain for H. pylori are available. The need for and usefulness of immunohistochemical (IHC) technique has been debated for years. Toluidine blue is a simple stain for microbiological studies and is easily available in laboratories. Therefore, this study was conducted to compare hematoxylin and eosin (H&E), Giemsa and toluidine blue staining with immunehistochemistry for detection of H. pylori in patients with gastritis and also to correlate the results of these staining methods with pathological grading. Methods: We reviewed 54 consecutive gastric biopsy specimens stained by H&E and Giemsa as well as by toluidine blue and immunohistochemistry stains for H. pylori. Results: H. pylori was positively identified by IHC in 43 (79.63%) patients, while positive samples were found in 18 (33.33%), 24 (44.44%) and 33 (61.11%) patients using H&E, Giemsa and toluidine blue staining methods. Our results showed that classical histological staining methods are not sensitive enough to identify low numbers or coccoid forms of organism, while toluidine blue and immunohistochemistry play an important role in detection of H. pylori infection. Conclusion: Toluidine blue has been proved to be much more reliable than H&E and Giemsa in detection of H. pylori. In addition, in post treatment biopsies and in biopsies with unexplained chronic active gastritis without histological evidence of H. pylori should have immunohistochemistry done to detect possible low density or coccoid form of organisms.
Key Words: Helicobacter pylori, Immunohistochemistry, Gastritis
Introduction
Infection with Helicobacter pylori has been established as an etiological factor in development of gastritis, peptic ulcer, gastric adenocarcinoma and Mucosal-Associated Lymphoid tissue lymphoma [1-6].
This spiral-shaped Gram-negative bacterium [7] is probably one of the most common bacterial infections throughout the world, involving 50% of population in developed countries [4, 8-11] and up to 80-90% of the population in developing countries [12]. Therefore, infection with H. pylori still constitutes a significant medical burden in less industrialized countries [13] and is common in 57-91% of Iranian population [14].
In view of this pathogenic importance and prevalence, accurate detection of H. pylori is essential for management of patients and for eradication of the bacterium following treatment [5, 6].
There are several diagnostic tests for detection of H. pylori and grouped into two categories: (1) invasive tests, such as culture, rapid urease test, histology and PCR and (2) non-invasive tests, which obviate the need for endoscopy and comprise serology, urea breath test, and fecal antigen test [15-19]. Selecting the test relies heavily upon whether a patient requires evaluation with upper endoscopy. Among biopsy-based diagnostic methods for H. pylori infection, PCR and culture are the basic techniques for determination of antibiotic sensitivities. However, they are not widely available for clinical use in Iran and therefore they cannot be routinely recommended. But histology is a common method used in Iran laboratories and has the advantage of the ability to evaluate pathogenic changes associated with H. pylori infection, such as inflammation, atrophy, intestinal metaplasia and malignancy [17]. Although the sensitivity of histology is affected by the use of medications, but guidelines for the management of H. pylori infection recommended histology for patients referred for upper endoscopy taking a proton pump inhibitor, antibiotics or bismuths [17]. There are many histochemical stains used for histological detection of H. pylori in gastric biopsies and resections, including hematoxylin and eosin (H&E), Modified Giemsa, toluidine blue and immunohisto-chemical (IHC) staining. H&E and Giemsa are routinely staining methods, which are used in pathological laboratories for detection of H. pylori. For years, different arguments have been developed about the need for and usefulness of IHC technique [20].
The present study was therefore aimed (i) to compare H&E, Giemsa and toluidine blue staining with immunohistochemistry for the detection of H. pylori in patients who are referred for upper endoscopy with or without medication history, and (ii) to compare the results of these staining methods in different groups of patients due to the degree of inflammation and activity.
MATERIALS AND METHODS
Population study. During a six-month period from April 2011 to October 2011, 54 consecutive patients diagnosed as chronic gastritis were examined. The protocol was performed in accordance with the principles of Declaration of Helsinki, and approved by the Institutional Ethics committee of Tehran University of Medical Science.
Endoscopy and biopsy sample . Endoscopy was performed on patients by an experienced gastroenterologist after an overnight fast. One biopsy sample was obtained from Antrum during endoscopy [21, 22].
Histochemistry and immunohistochemistry . Gastric mucosa biopsies were fixed in buffered formalin and embedded in paraffin. Four-micron-thick sections were cut and mounted on slides for histochemistry and IHC. Standard histological sections were stained with H&E [23], Modified Giemsa [24] and toluidine blue. For toluidine blue staining method, deparaffinized and rehydrated sections were rinsed in distilled water and immersed in buffered toluidine blue solution for 20 minutes. Slides were washed well in water, dehydrated, cleared and then mounted. The buffered toluidine blue solution was prepared by mixing 50 ml phosphate buffer with 1 ml 1% aqueous toluidine blue. In IHC staining, acetate buffer (pH 6.0) was used as the immersion solution for heat antigen pretreatment step. Rabbit polyclonal anti-H. pylori antibody (DAKO, Bo471) and the Envision (DAKO) polymer detection system were used using Diaminobenzidine as the chromogen. Positive control sample was prepared from known H. pylori-infected tissue biopsy and negative control samples were prepared by omitting the primary antibody [25]. IHC results were considered as the gold standard in this study [6].
Detection of H. pylori and pathology. Patients with positive result in immunohistochemistry were considered to be H. pylori positive. The density of H. pylori-stained organisms was graded by using the updated Sydney system visual analog scale for normal, mild and moderate and marked with a scoring system of 0 to 3, respectively [26]. Also inflammation and activity were graded in accordance with the updated Sydney system. The degree of the gastric mucosal inflammation was classified in four grades as follows: 0 = none, 1 = mild, 2 = moderate, 3 = severe [26].
Statistical analysis . Sensitivity and specificity of each H. pylori diagnostic test as well as the positive predictive value (PPV) and negative predictive value (NPV) were used to characterize each test versus consensus.
Results
In this study, 30 male and 24 female patients were included. The mean age of the patients was 48 years with a range of 21-57 years. IHC stains showed that 43 cases were positive for H. pylori (Fig. 1). H. pylori was identified in 18 sections with H&E (Fig. 2A). Modified Giemsa permitted H. pylori identification in all cases of infection detected by the previous method and in a further six biopsies (44.4%) (Fig. 2B). In addition, nine more positive biopsies (61.1%) were disclosed by toluidine blue staining method (Fig. 2C). Detecting coccoid form of H. pylori was easier in this staining method. Twelve positive biopsies, which were not found by Giemsa staining method, had coccoid form of organisms, which were easily identified by IHC (Fig. 3A). Six positive biopsies, which were not found by Giemsa staining method, had H. pylori organism closely opposed to the epithelial cell or inside the epithelial cells, which were strikingly obvious in immunostained biopsies (Fig. 3B). Others had very scant organism. Sensitivity, specificity, PPV, and NPV of H&E, Giemsa and toluidine blue staining methods have been shown in Table 1.
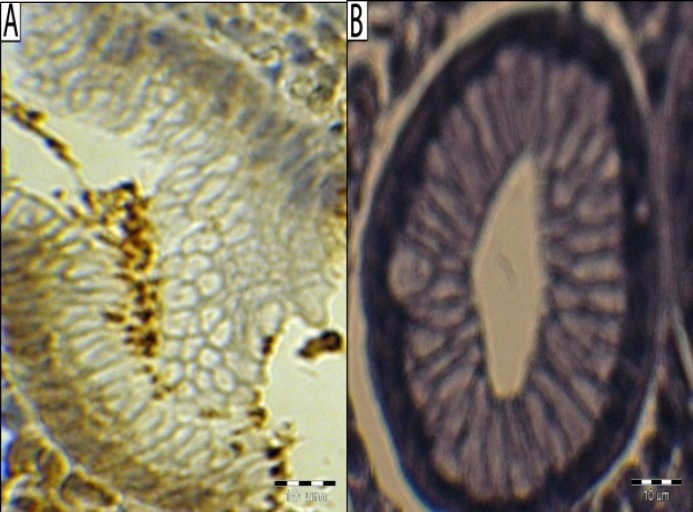
Fig. 1.
Immunohistological demonstration of H. pylori. (A) Clusters of immunolabeled organisms at the surface of pyloric mucosa of a patient infected with Helicobacter pylori, immunohistochemical staining for H. pylori (primary antibody: Bo471 DAKO, Denmark), hematoxylin counterstain, positive control reaction; (B) negative control reaction. Bar line = 10 µm
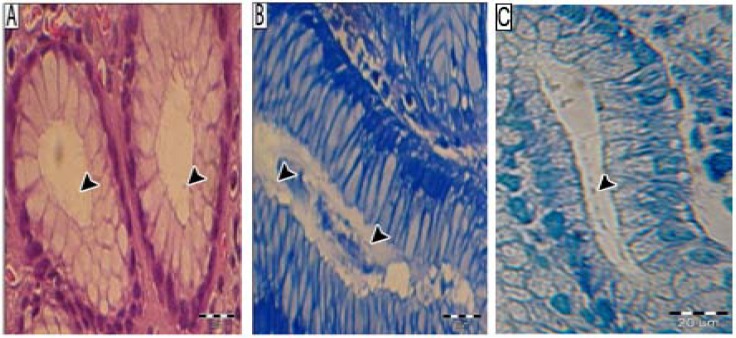
Fig. 2.
Histochemical stainning. Bacteria are visualized (A) in the lumen of antral gastric glands on H&E stain (arrows), (B) more easily in modified Giemsa stained section (arrows) and (C) in the lumen of antral gastric glands on toluidine blue stain (arrow). Bar line = 20 µm
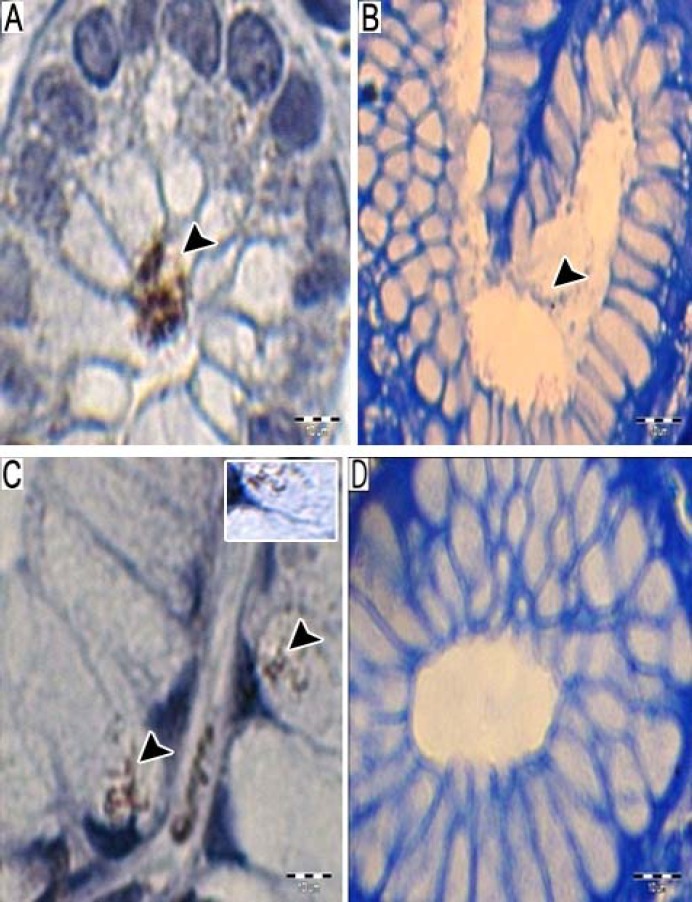
Fig. 3.
Comparative detection of H. pylori by immunohistochemical (IHC) and histochemical staining. (A) The obvious identification of cluster of modified coccoid forms of Helicobacter pylori by immunohistochemistry; (B) coccoid H. pylori on Giemsa stained section; (C) IHC stain for H. pylori shows a small area with organisms inside the epithelial cells. Inset shows individual H. pylori with characteristic elongated, slightly spiral S shaped; (D) Giemsa stained biopsy of the same sample which cannot show any H. pylori organism. Bar line = 10 µm
Table 1.
Statistical analysis according to standard test
| Method |
Sensitivity
(%) |
Specificity
(%) |
PPV
(%) |
NVP
(%) |
|---|---|---|---|---|
| H&E | 41.86 | 100.00 | 62.07 | 69.44 |
| Modified Giemsa | 53.49 | 95.24 | 69.70 | 66.60 |
| Toluidine blue | 76.74 | 100.00 | 75.00 | 47.62 |
PPV, positive predictive value; NPV, negative predictive value; H&E, hematoxylin and eosin
The presence of chronic gastritis was confirmed in all gastric biopsy specimens as follows: 16 cases with moderate chronic active gastritis, 5 cases with moderate chronic inactive gastritis, 9 cases with mild chronic active gastritis and 24 cases with mild chronic inactive gastritis. Table 2 shows test results compared to the standard test in different groups of patients. In patients with mild chronic inactive gastritis, IHC revealed H. pylori organisms in 15 cases, while H&E, modified Giemsa and toluidine blue found H. pylori in 1, 5 and 9 cases, respectively. The highest rate of false-negative results was observed in this group of patients, whereas the lowest was observed in patients with moderate chronic active gastritis. In this group of patients, H&E, modified Giemsa and toluidine blue could not find organism just in 3, 2 and 1 infected patients, respectively.
Degree of H. pylori colonization on immunostained resections was as follows: 22 cases with mild colonization, 14 cases with moderate colonization, and 7 cases with severe bacterial colonization. Table 3 shows classical staining results in three groups of patients due to the bacterial colonization assessed. Marked infections with numerous organisms were detected more easily in histochemical stained sections, whereas the highest negative results were obtained in patients with mild bacterial colonization.
Discussion
Since the discovery of H. pylori, several diagnostic methods have been become available for determining the presence of H. pylori infection [19]. However, there is no established method to provide a definitive or standard diagnosis of H. pylori infection. Laboratories should choose a test or tests that are appropriate for their own conditions, patients' numbers and costs, and they have to prepare their own diagnostic method [5]. As Iran is a developing country with a high background rate of H. pylori infection [14], study on accuracy of these diagnostic methods is needed.
Several studies have demonstrated that IHC stains are the most sensitive histological method to detect H. pylori [6, 27, 28]. For example, Jonkers and colleagues [28] showed that a polyclonal IHC stain (DAKO, B471, Denmark) was highly specific and had a low interobserver variation when compared to a modified Giemsa and a Warthin-Starry stain. However, the necessity for IHC stains has been debated in recent years and it is institution and laboratory dependent [20]. In this study, we evaluate the value of IHC and toluidine blue for detection of H. pylori in comparison with Giemsa and H&E stains.
The value of H&E stain compared with other special stains in the identification of H. pylori has been discussed in other articles [28, 29]. Pity et al. [26] concluded that, H&E had no clinical value in detection of H. pylori in gastric biopsies. Their findings was in contrast with that of Wang et al. [30], which showed that routine H&E staining method was sufficient for identification of the organism. In our study, H&E had the sensitivity of 41.86%, specificity of 100% and PPV of 62.07%. Therefore, H&E stain did not possess the sensitivity needed for an adequate screening test.
The most striking finding of this study is the unexpectedly low reliability of Giemsa staining in detection of H. pylori. In a study in 2002 by Wabinga [21] including 48 patients and using IHC as a gold standard, the performance of Giemsa staining was evaluated. Giemsa showed sensitivity of 85% and specificity of 89% in the referred study. Also, in a study by Rotimi et al. [6], Giemsa showed a sensitivity of 87%, and their results were comparable to those of the study by Hartman and Owens [31]. In our study, this method exhibited a sensitivity of 53.74% and specificity of 95.24%. This finding may be related to differences among the studies with regard to the medication history of patients included. Contrary to the most previous studies [32, 33], we did not exclude patients with medication history, because in daily practice, histology is widely used in this group of patients who are referred for upper endoscopy.
Moreover, we found that toluidine blue sensitivity and specificity was 76.74% and 100%, respectively. In addition, the statistical analysis showed a PPV of 75% and NPV of 47.62% compared to IHC. Toluidine blue proved to be much more sensitive than H&E and modified Giemsa. Our laboratory has found that this method is cheap and easy to use and produces more reliable results than H&E/modified Giemsa methods.
Table 2.
Detection of Helicobacter pylori using H&E, Giemsa, toluidine blue and IHC staining methods in correlation with degree of inflammation and activity.
| Degree of inflammation ( no. of cases) |
H&E
|
Giemsa
|
Toluidine blue
|
IHC
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| +No. (%) | -No. (%) | +No. (%) | -No. (%) | +No. (%) | -No. (%) | +No. (%) | -No. (%) | ||||
| Mild chronic inactive gastritis (24) |
1 (4.20) | 23 (95.8) | 5 (20.8) | 19 (79.2) | 9 (37.50) | 15 (62.50) | 15 (62.50) | 9 (37.5) | |||
| Moderate chronic inactive gastritis (5) |
2 (40.00) | 3 (60.0) | 3 (60.0) | 2 (40.0) | 3 (60.00) | 2 (40.00) | 4 (80.00) | 1 (20.0) | |||
| Mild chronic active gastritis (9) |
2 (22.20) | 7 (77.8) | 2 (22.2) | 2 (22.2) | 6 (66.66) | 3 (33.30) | 8 (88.88) | 1 (11.1) | |||
| Moderate chronic active gastritis (16) |
13 (81.25) | 3 (18.75) | 14 (87.5) | 14 (87.5) | 15 (93.75) | 1 (6.25) | 16 (100.00) | 0 (0.0) | |||
| Total (54) | 18 (33.30) | 36 (66.7) | 24 (44.4) | 24 (44.4) | 33 (61.11) | 18 (33.30) | 43 (79.63) | 11 (20.1) | |||
H&E, hematoxylin and eosin, IHC, immunohistochemical
Table 3.
Detection of Helicobacter pylori using H&E, Giemsa, toluidine blue staining methods in different groups of patients due to degree of bacterial colonization.
| Bacterial colonization (no. of cases) |
H&E
|
Giemsa
|
Toluidine blue
|
|||||
|---|---|---|---|---|---|---|---|---|
| +No. (%) | -No. (%) | +No. (%) | -No. (%) | +No. (%) | -No. (%) | |||
| Mild (22) | 2 (9.90) | 2 (90.9) | 5 (22.72) | 17 (77.3) | 14 (63.63) | 8 (36.4) | ||
| Moderate(14) | 10 (71.43) | 4 (26.8) | 11 (78.57) | 3 (21.4) | 12 (85.71) | 2 (14.3) | ||
| Marked(7) | 6 (85.71) | 1 (14.3) | 7 (100.00) | 0 (0.0) | 7 (100.00) | 0 (0.0) | ||
| Total(43) | 18 (41.86) | 25 (58.1) | 24 (55.81) | 24 (55.8) | 33 (76.74) | 10 (23.3) | ||
H&E, hematoxylin and eosin, IHC, immunohistochemical
High frequency of false-negative result in traditional stained sections with mild chronic inactive gastritis suggested that the presence of this status may result in a pathologist s failure to detect H. pylori in some cases. As a result, when mild chronic inactive gastritis is found, a careful search for organisms with an IHC is needed to diminish exposure to the side effect of false-negative result for patients. In addition, H&E did not find organism in 3 positive cases with moderate chronic active gastritis. This finding indicated that when no H. pylori can be found with histochemical stains, IHC should be applied to prevent false-negative results.
In conclusion, toluidine blue is very straightforward, inexpensive and it is more reliable than H&E/modified Giemsa in detection of H. pylori. The major disadvantage of this method is that there is little contrast between organisms and tissues.
Immunohistochemistry is a reliable technique for detection of H. pylori. Coccoid forms of the organisms, which may not be amenable to other staining methods, were seen easily on immunostained sections. Also, H. pylori antigen in the lamina propria and beneath the surface epithelial is detectable by IHC, while it can be hardly detectable by histochemical stains. On the other hand, the method is fairly expensive, especially because a negative control needs to be used with every slide. Therefore, it is not clearly practical and economical to perform H. pylori immunohistochemistry on every gastric biopsy specimen. However, in cases with inactive gastritis or low degree of inflammation, and cases with chronic active gastritis with negative histological result, IHC stain should be superior to histochemical stains in detection of H. pylori.
Acknowledgment
This research was supported by a grant from Tehran University of Medical Science. The authors would like to thank staff of the Pathology Department of Rasoul Akram Hospital for their cooperation in collecting gastric specimens. We would also like to thank Dr. Banafshe Esmaeilzade for the assistance in preparation of IHC manuscript.
References
- 1.Akanda MR, Rahman AN. Comparative Study of Different Methods for Detection of Helicobacter Pylori in Gastric Biopsies. Dinajpur Med Col J. 2011 Jan;4(1):1–6. [Google Scholar]
- 2.Koumi A, Filippidis T, Leontara V, Makri L, Panos MZ. Detection of Helicobacter pylori: A faster urease test can save resources. World J Gastroenterology. 2011 Jan;17(3):349–53. doi: 10.3748/wjg.v17.i3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SI, Fowora MA, Otegbayo JA, Abdulkareem FB, Omonigbehin EA, Adegboyega A, et al. Comparison of PCR with other diagnostic techniques for the detection of H pylori infection in patients presenting with gastroduodenal symptoms in Nigeria. Int J Mol Epidemiol Genet. 2011 May;2(2):178–84. [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10(4):720–41. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aktepe OC, Çiftçi IH, Şafak B, Uslan T, Dilek FH. Five methods for detection of Helicobacter pylori in the Turkish population. World J Gastroenterol. 2011 Dec;17(47):5172–6. doi: 10.3748/wjg.v17.i47.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotimi O, Cairns A, Gray S, Moayyedi P, Dixon MF. Histological identification of Helicobacter pylori: comparison of staining methods. J Clin Pathol. 2000 Oct;53:756–9. doi: 10.1136/jcp.53.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paniagua GL, Monroy E, Rodríguez R, Arroniz S, Rodríguez G, Cortés JL, et al. Frequency of vacA, cagA and babA2 virulence markers in Helicobacter pylori strains isolated from Mexican patients with chronic gastritis. Ann Clin Microbiol Antimicrob. 2009 Apr;30:8–14. doi: 10.1186/1476-0711-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold BD, Colletti RB, Abbott M, Czinn SJ, Elitsur Y, Hassall E, et al. Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000 Nov;31:490–7. doi: 10.1097/00005176-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Talebkhan Y, Mohammadi M, Khalili G, Sheykholeslami A, Rakhshani N, Mahboudi F, et al. Detection of Helicobacter pylori infection by imported IgG ELISA kits in comparison with Iranian homemade kit. Govaresh. 2006;11(2):120–5. [Google Scholar]
- 10.Douraghi M, Mohammadi M, Shirazi MH, Oghalaie A, Saberi KashaniS, Mohagheghi MA, et al. Simultaneous detection of Caga and Cage of Helicobacter pylori strains recovered from Iranian patients with different gastroduodenal diseases. Iranian J Publ Health. 2009;38(2):98–105. [Google Scholar]
- 11.Pacifico L, Anania C, Osborn JF, Ferraro F, Chiesa C. Consequences of Helicobacter pylori infection in children. World J Gastroenterol. 2010 Nov;16(41):5181–94. doi: 10.3748/wjg.v16.i41.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doosti A, ghasemi-dehkordi P. Helicobacter pylori vacA genotypes in Shahrekordian (Iran) H pylori positive patients. Res J Bio Sci. 2009;4(1):11–5. [Google Scholar]
- 13.Duynhoven YT, Jonge R. Transmission of Helicobacter pylori: a role for food? Bull World Health Organ. 2001;79(5):455–60. [PMC free article] [PubMed] [Google Scholar]
- 14.Hashemi MR, Rahnavardi M, Bikdeli B, Dehghani ZahedaniM.H. pylori infection among 1000 southern Iranian dyspeptic patients. World J Gastroenterol. 2006 Sep;12(34):5479–82. doi: 10.3748/wjg.v12.i34.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glupczynski Y. Microbiological and serological diagnostic tests for Helicobacter pylori: an overview. Br Med Bull. 1998;54(1):175–86. doi: 10.1093/oxfordjournals.bmb.a011668. [DOI] [PubMed] [Google Scholar]
- 16.Micu G, Staniceanu F, Zurac S, Popp C, Bastian A, Gramada E, et al. Diagnosisand treatment in Helicobacter pylori infection. Rom J Itern Med. 2010;48(3):239–47. [PubMed] [Google Scholar]
- 17.Chey WD, Wong BCY. American College of Gastroenterology Guideline on the Management of Helicobacter pylori Infection. Am J Gastroenterol. 2007 Aug;102(8):1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 18.Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998 Dec;93(12):2330–8. doi: 10.1111/j.1572-0241.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 19.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van derMerweS. Helicobacter pylori in developing countries. world gastroenterology organisation global guidelines. J Gastrointestin Liver Dis. 2010 Sep;20(3):299–304. [PubMed] [Google Scholar]
- 20.Smith SB, Snow AN, Perry RL, Qasem SA. Helicobacter pylori: to stain or not to stain? Am J Clin Pathol. 2012 May;137(5):733–8. doi: 10.1309/AJCP8DGTAVG7MBMT. [DOI] [PubMed] [Google Scholar]
- 21.Wabinga HR. Comparison of immunohistochemical and modified Giemsa stains for demonstration of Helicobacter pylori infection in an African population. Afr Health Sci. 2002 Aug;2(2):52–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Tzeng JE, Lin YL, Chung SM, Chu YT. Comparison of four diagnostic methods for Helicobacter pylori. Tzu Chi Med J. 2005;17(5):339–43. [Google Scholar]
- 23.Avwioro G. Histochemcal uses of Hematoxylin- a review. JPCS. 2011 Apr-Jun;1:24–34. [Google Scholar]
- 24.Gray SF, Wyatt JI, Rathbone BJ. Simplified techniques for identifying Campylobacter pyloridis. J Clin Pathol. 1986 Nov;39(11):1279–80. doi: 10.1136/jcp.39.11.1279-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss J, TsangTK , Meng X, Zhang H, Kilner E, Wang E, et al. Detection of Helicobacter pylori gastritis by PCR correlation with inflammation scores and immunohistochemical and CLOtest findings. Am J Clin Pathol. 2008 Jan;129(1):89–96. doi: 10.1309/APMPEP54G7PN958G. [DOI] [PubMed] [Google Scholar]
- 26.Pity IS. Identification of Helicobacter pylori in gastric biopsies of patients with chronic gastritis: histopathological and immunohistochemical study. Duhok Med J. 2011;5(1):69–77. [Google Scholar]
- 27.Dixon MF, Genta RM, Yardley JH, Correa P. Classification andgrading of gastritis: the updated sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1994 Oct;20(10):1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Jonkers D, Stobberingh E, de BruineA, Arends JW, Stockbrügger R. Evaluation of immunohistochemistry for the detection of Helicobacter pylori in gastric mucosal biopsies. J infect. 1997 Sep;35(2):149–54. doi: 10.1016/s0163-4453(97)91611-x. [DOI] [PubMed] [Google Scholar]
- 29.Koenig M, Schofield JB, Warren BF, Shepherd NA. The routine use of histochemical stains in gastrointestinal pathology: a UK-wide survey. Histopathology. 2009 Aug;55(2):214–7. doi: 10.1111/j.1365-2559.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang XI, Zhang S, Abero F, Thomas J. The role of routine immunohistochemistry for Helicobacter pylori in gastric biopsy. Ann Diagn Pathol. 2010;14(4):256–9. doi: 10.1016/j.anndiagpath.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Hartman DJ, Owens SR. Are routine ancillary stains required to diagnose Helicobacter infection in gastric biopsy specimens? An institutional quality assurance review. Am J of Clin Pathol. 2012 Feb;137(2):255–60. doi: 10.1309/AJCPD8FFBJ5LSLTE. [DOI] [PubMed] [Google Scholar]
- 32.Sultana A, Badruddoza SM, Rahman F. Correlation between endoscopic and histological findings in different gastroduodenal lesion and its association with Helicobacter Pylori. AKMMC J. 2011;2(2):6–10. [Google Scholar]
- 33.Chomvarin C, Kulsuntiwong P, Mairiang P, Sangchan A, Kulabkhow C, Chau-in S, et al. Detection of H. pylori in dyspeptic patients and correlation with clinical outcomes. Southeast Asian J Trop Med Public Health. 2005 Jul;36(4):917–922. [PubMed] [Google Scholar]