Abstract
Background: Oxidative modification of low-density lipoprotein (LDL) appears to be an early step in the pathogenesis of atherosclerosis. Meanwhile, myeloperoxidase (MPO)-catalyzed reaction is one of the potent pathways for LDL oxidation in vivo. The aim of this study was to evaluate in vitro antioxidant effects of vitamins C and E on LDL oxidation mediated by MPO. Methods: MPO was isolated from fresh plasma by sequential centrifugation using density ultracentrifugation. It was incubated with LDL and the LDL oxidation level was determined spectrophotometrically by measuring conjugated diene absorbance at 234 nm. Furthermore, vitamin C (50-200 mM) and vitamin E (10-40 mM) were added and the LDL oxidation level was determined. Results: The purity index of MPO and its enzymatic activity were 0.69 and 1127 U/mg protein, respectively. It was demonstrated that vitamin C in vitro inhibited LDL oxidation mediated by MPO; however, vitamin E was unable to act in the same way. The protection by vitamin C was concentration dependent and maximum protective effect of vitamin C was observed at 150 mM, where about 64% of the LDL oxidation was inhibited. Vitamin C increased lag time of LDL oxidation mediated by MPO up to 2.4 times. Conclusion: It can be concluded from our results that vitamin C is able to improve LDL resistance to oxidative modification in vitro. In addition, vitamin C might be effective in LDL oxidation mediated by MPO in vivo, resulting in reduction of atherosclerosis process rate.
Key Words: Antioxidant, Myeloperoxidase (MPO), Low-density lipoprotein (LDL), Vitamin E
Introduction
Myeloperoxidase (MPO), an oxidant-generating enzyme, is an abundant protein in human neutrophils [1]. When neutrophils are activated, they secrete large quantities of MPO into the extra cellular compartment. MPO at physiological conditions is capable of catalyzing the oxidation of chloride and bromide ions by hydrogen peroxide (H2O2) generating hypochlorous acid (HOCL) and hypobromous acid, respectively [2]. At physiological conditions, HOCL, which is known to be a potent oxidant, is the predominant product of the enzyme [3]. HOCL produced by MPO reacts readily with a wide variety of biomolecules such as lipoproteins [4, 5]. It has been well known that low-density lipoprotein (LDL) can be oxidized by many kinds of oxidants by different mechanisms and pathways. Some oxidants are derived from exogenous sources such as foods and smoking; however, many oxidants are produced during metabolic activities such as MPO/H2O2/Cl- system activity. There is evidence that the MPO/H2O2/Cl-system of activated phagocytes may be involved in atherosclerosis development [6]. In respect to atherogenesis, MPO binds easily to LDL and results in LDL oxidation. The oxidation of LDL by MPO leads to modifications of both lipid and protein moiety of LDL. Oxidation of tyrosyl residues on LDL converts to the 3-chlorotyrosine, a specific biomarker of HOCL [7]. However, Shao et al. [8] have shown that 3-chlorotyrosine is a specific marker of MPO-catalyzed oxidation, and is markedly elevated in lipoprotein isolated from human atherosclerotic intimae. Meanwhile, polyunsaturated fatty acids of lipid molecules in LDL are also easily oxidized and conjugated dienes are formed. Formation of conjugated diene, which is a key step in lipid per-oxidation, can be monitored as a measure of LDL oxidation [9]. Recent clinical studies in human have supported the role of MPO in atherogenesis [6]. For example, Zhang et al. [10] and also Kutter et al. [11] have shown that patients presenting MPO deficiency or a low level of blood MPO had a decreased cardiovascular risk. Two other studies have shown that the serum MPO levels predict risks in patients with acute coronary syndromes or chest pain [12, 13]. LDL oxidation mediated by MPO is one of the possible pathways for promotion of atherosclerosis, and its prevention may help patients at risk of damage. Some in vitro and in vivo investigations have shown that antioxidants reduce lipid per-oxidation, and inhibit atherogenic development [14-16]. Several studies have found antioxidants are effective in slowing LDL oxidation rate and subsequently decrease the rate of atherosclerosis [15-17]. However, the antioxidant effect on LDL oxidation mediated by MPO in vitro has not been investigated. Interestingly, the aim of this study was to evaluate the antioxidant effects of vitamin C, the most effective water-soluble antioxidant, and vitamin E (α-tocopherol), the most lipid-soluble antioxidant, on LDL oxidation mediated by MPO.
MATERIALS AND METHODS
Materials. Human blood was obtained from Isfahan Blood Transfusion Service. Tetramethyl-benzidine (TMB), H2O2, cetytrimethylammonium bromide (Hexa decil trimethylammonium bromide or CTAB), vitamin C, vitamin E (α-tocopherol), and Sephadex G-150 were obtained from Sigma Chemical Co. (USA). All other chemicals were reagent grade.
Methods:
Preparation of LDL from human blood. Human serum was separated from whole blood by centrifugation at 300 ×g for 15 min. Serum LDL was precipitated by the method described by Wieland and Seidel [18]. The pH of precipitation buffer containing 64 mM trisodium citrate and 50,000 IU/L heparin was adjusted to pH 5.05 with 5N HCl. Before precipitation of LDL particles, 1 mg/ml of EDTA was added to serum samples. The precipitation reagents were allowed to equilibrate at room temperature. Then, 7 ml of the heparin citrate buffer (64 mM, pH 5.05 and 50,000 IU/L heparin) was added to 1 ml of the serum sample and mixed with a vortex mixer and the suspension was standed at ambient temperature for 10 min. The insoluble lipoproteins were centrifuged at 1000 ×g for 10 min to be sedimented. The pellet was re-suspended in 1 ml of 0.1 M sodium phosphate buffer, pH 8.0, containing 0.9% NaCl. LDL was isolated by sequential ultracentrifugation from human serum as described by Schumaker and Puppione [19]. LDL concentration was measured by a modification of the Agner’s method [20] using bovine serum albumin as standard. The LDL stock solution (1.5 mg protein/ml) was diluted with 10 mM phosphate buffer (pH 7.4) at the protein concentration required for the assay. Prior to experiments, LDL sample was dialyzed overnight against EDTA-free phosphate buffer saline (0.01 M, pH 7.4). Study of LDL oxidation reaction and antioxidant effects of vitamins C and E on this process were carried out immediately after LDL isolation.
Isolation of white blood cells . Buffy coat, the principle source of white cells, was isolated by centrifugation of citrated blood at 1000 ×g for 15 min. The layer between plasma and red cells (buffy coat) was isolated. A hypotonic solution of 155 mM NH4CL, 10 mM KHCO3 and 0.1 mM EDTA was used to lyse the remaining red cells. After centrifugation at 800 ×g for 15 min, the white cells were collected.
Myeloperoxidase isolation . MPO was isolated from white blood cells after lysing by adding 0.5% CTAB and cell debris was removed by centrifugation at 15,000 ×g at 5°C for 15 min. All subsequent centrifugations were carried out under these conditions. The supernatant was treated with solid ammonium sulfate to yield a final concentration of 50% saturation. The solution was kept at 4°C for 30 min and centrifuged to remove the precipitate. The resulting supernatant was treated with solid (NH4)2SO4 to increase the concentration to 65% saturation, and was incubated at 4°C for 30 min prior to centrifugation. This procedure, a modification of a method described by Agner [20], resulted in a pellet containing the bulk of MPO activity. Precipitated MPO was re-dissolved in a buffer containing 50 mM Tris, (pH 7.0) and 0.5% CTAB (column buffer), and chromatographed on Sephadex G-150, super fine grade. To prepare this column, Sephadex G-150 was swollen in a column buffer at room temperature for 3 days. The column (2.5 × 30 cm) was washed with several volumes of buffers before use and run at room temperature. MPO in buffer eluted from column was precipitated by the addition of solid (NH4)2SO4 to yield a final concentration of 50% saturation. After centrifugation at 8,000 ×g for 2 min, the resulted pellet was dissolved in a minimum volume of 5 mM sodium acetate buffer (pH 5.4), and then dialyzed overnight against 100 volumes of the same buffer.
Measurement of enzyme activity . MPO activity was measured according to the method of Suzuki et al. [21]. The reaction mixture consisted of 2 µg/ml MPO, 1.6 mM TMB, 0.3 mM H2O2, 80 mM sodium phosphate buffer (pH 5.4), 8% N, N dimethyl-formamide and 40% phosphate buffered saline in a total volume of 500 µL. In this assay,TMB was oxidized to a blue product that absorbs at 655 nm. The enzyme activity was measured continuously by monitoring the absorbance changes at 655 nm during 3 min. One unit of activity was determined as a change in absorption of 1.0 unit per min at 655 nm.
LDL oxidation mediated by MPO. Oxidation of LDL was determined based on the method described by Jerich et al. [22]. Briefly, LDL (0.25 mg/ml) was incubated with 2 mg/ml MPO and 150 mM NaCl in 10 mM phosphate buffer (pH 6) at room temperature. The oxidation reaction of LDL was started by addition of 0.5 mM H2O2, resulting in the formation of conjugated diene hydroperoxides. The absorbance was read manually at intervals of 5 min for a period of 400 min, on a Perkin-Elmer 515 UV/VIS spectrophotometer. The absorbance measurement at 234 nm can be expressed as micromoles of conjugated dienes [23] using the molar extinction coefficient of 2.95 × 104M-1cm-1 for the conjugated dienes, which is an index of LDL oxidation level [24]. The difference between the maximum absorbance measure and absorbance of blank was expressed as the level of LDL oxidation. To study inhibitory effects of vitamins C and E on LDL oxidation mediated by MPO, LDL (0.25 mg/ml) were incubated with 2 mg/ml MPO, 150 mM NaCl, and 0.5 mM H2O2 in 10 mM phosphate buffer (pH 6) in the absence (control) and presence of different concentrations of vitamin C (50, 100, 150 and 200 mM) and vitamin E (10, 20, 30, and 40 mM) followed by the absorbance determination at 234 nm as mentioned above. The lag time of LDL oxidation reaction was measured by determining the intersection point between the tangent to the slope of the curves and the horizontal axis [25].
Statistical analysis. All results were expressed as the mean ± SD and statistically interpreted by using one-way analysis of variance (ANOVA), followed by Dunnett’s test. A value of P<0.05 was considered as statistically significant.
Results
Enzyme purity. Using the method described earlier, the purity of isolated MPO was 0.69, which was calculated from absorbance ratio A430/A280 nm. In addition, enzymatic activity of the enzyme was 1127 U/mg protein.
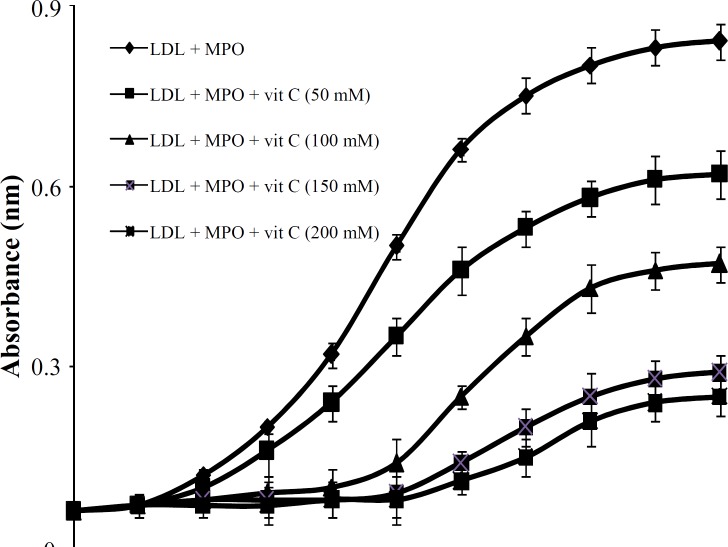
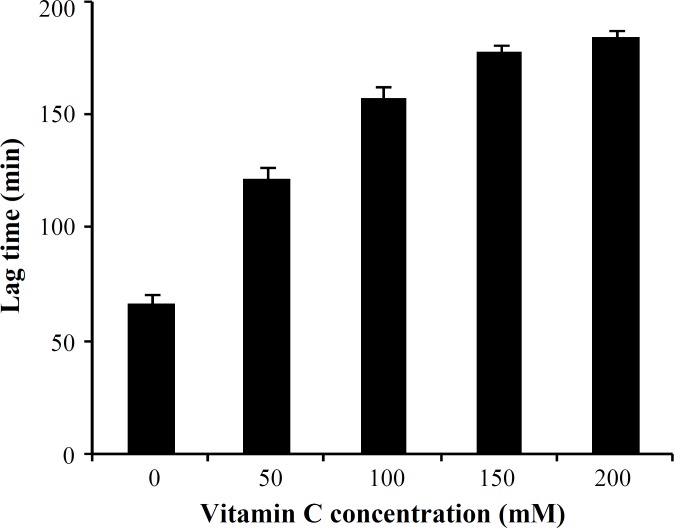
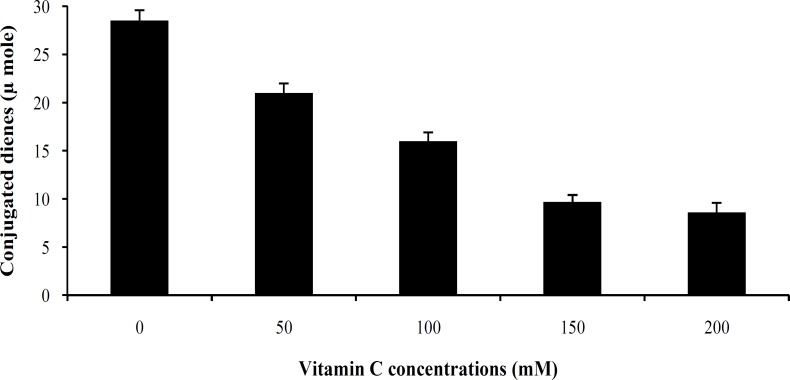
The effect of vitamin C on human LDL oxidation. Exposure of LDL to MPO/H2O2/Cl- system in the presence of increasing concentrations of vitamin C (50, 100, 150 and 200 mM) resulted in 64% inhibition of LDL susceptibility to oxidation when compared with the control sample (Fig. 1). Maximum inhibitory effect of vitamin C was observed at 150 mM concentration of vitamin C, and did not significantly increase with increasing concentration of vitamin C up to 200 mM (Fig. 2). The protection effect of vitamin C was dose-dependent between 50 to 150 mM (Fig. 2). The lag time of LDL oxidation reaction and conjugated dienes produced during LDL oxidation were calculated from the curves of the Figure 1 and have been shown in Figures 2 and 3, respectively. This lag time was increased up to 2.4 times by vitamin C.
Fig. 1.
Effect of different concentrations of vitamin C on LDL oxidation induced by myeloperoxidase (MPO) system. Low-density lipoprotein (LDL) was incubated in the absence and presence of 50-200 mM of vitamin C and changes in absorbance at 234 nm were read at intervals of 5 min for a period of 400 min. values represents mean SD of three independent experiments. vit, vitamin
Fig. 2.
Effects of different concentrations of vitamin C on lag time of low-density lipoprotein (LDL) oxidation induced by myeloperoxidase system. Values were obtained from LDL oxidation curves. Values represent mean SD of three independent experiments
Fig. 3.
Effect of different concentrations of vitamin C on conjugated dienes induced by myeloperoxidase-system. Conjugated dienes produced were calculated from the maximum increase of the absorbance and molar absorbance for conjugated dienes of 2.95 × 104M-1cm-1.
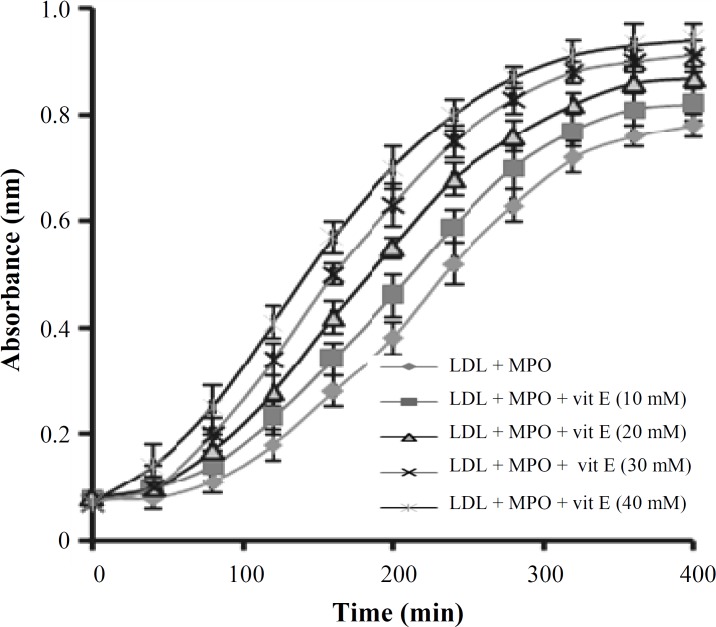
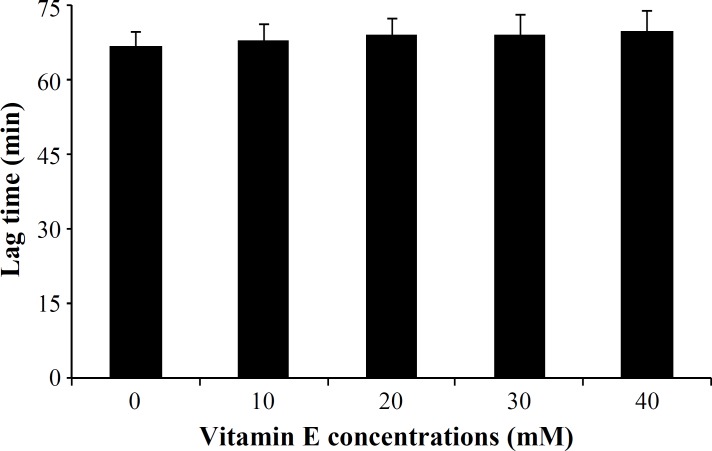
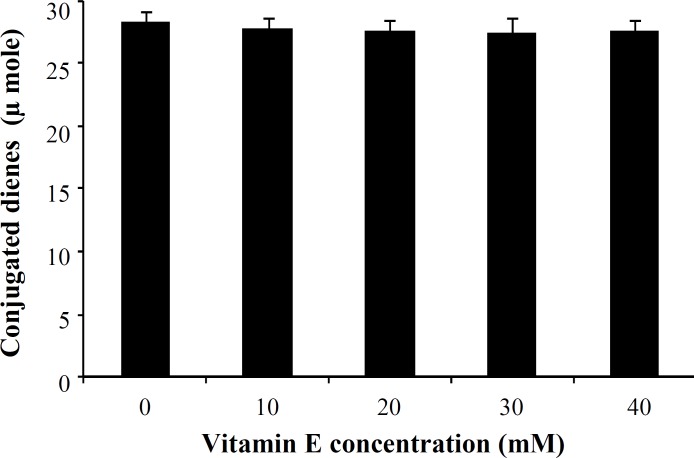
The effect of vitamin E on human LDL oxidation. Incubation of LDL with MPO/H2O2/Cl- system in the presence of different concentrations of vitamin E (10, 20, 30 and 40 mM) showed no significant difference between LDL oxidation rate in the presence or absence of vitamin E (Fig. 4). The lag time of LDL oxidation reaction and conjugated dienes produced during LDL oxidation was calculated from the curves of the Figure 4 and has been shown in Figures 5 and 6, respectively.
Fig. 4.
Effect of different concentrations of vitamin E ( -tocopherol) on low-density lipoprotein (LDL) oxidation induced by myeloperoxidase (MPO)/H2O2/Cl- system. LDL was incubated in the absence and presence of 10-40 mM of vitamin E, and changes in absorbance at 234 nm were read at intervals of 5 min for a period of 400 min. Values represents mean SD of three independent experiments. Vit, vitamin
Discussion
Oxidation of LDL has been widely accepted to be an early step in atherosclerosis process [26]. Meanwhile, various enzymes may contribute to oxidation, such as MPO, lipoxygenases, NADPH oxidases, and nitric oxide synthetases. Among these enzymes, neutrophil's MPO is the most abundant enzyme in human body and its biochemical pathway is one of the known potent pathways for LDL oxidation in vivo. One of the most important strategies to prevent atherosclerosis is aimed at reducing oxidative stress. Numerous studies have indicated that some compounds with antioxidant properties in vivo prevent atherosclerosis through decreasing oxidized LDL level in the body [15-17]. Therefore, investigation of substances that can inhibit LDL oxidation in vivo may be able to effectively help to prevent atherosclerosis. The most persuasive evidence has shown that supplementation of some animal models with antioxidants slow atherosclerosis. However, less information was presented to show the effect of these antioxidants in vitro. In this study, we evaluate in vitro antioxidant effects of vitamins C and E on LDL oxidation mediated by MPO. Vitamin C, the most effective water-soluble antioxidant, and vitamin E, highly efficient lipid-soluble antioxidant, were chosen in this study because of their possible use as dietary supplement.
At first, MPO was isolated from white blood cells to a purity index and to enzymatic activity of 0.69 and 1127 U/mg protein, respectively. The purity index of MPO was similar to another study published earlier [27].
Fig. 5.
Effect of different concentrations of vitamin E on lag time of the low-density lipoprotein (LDL) oxidation mediated by myeloperoxidase. Values were obtained from the LDL oxidation curves of Figure 4. Values represent mean SD of three independent experiments.
Our results presented here revealed a significant reduction (P<0.02) in the susceptibility of LDL to oxidation mediated by MPO in the presence of vitamin C. The protection of LDL oxidation by vitamin C at concentrations between 50 mM to 150 mM was concentration dependent, showing the maximum effect at 150 mM in which about 64% inhibition was occurred. There are a lot of reports regarding the antioxidant properties of various substances and their effect on LDL susceptibility to oxidation [28-30]. However, most of them have investigated the process in the presence of oxidizing cations such as Cu. Das et al. [31] investigated the in vitro oxidation of LDL isolated from normal and hypercholesterolemic subjects by Cu2+ ions in the presence of different concentrations of vitamin C. They have also reported a significant inhibition in the susceptibility of LDL to oxidation in the presence of 40 mmol/L of vitamin C in normal subjects. When using LDL isolated from hypercholesterolemic subjects, they had to use 80 mmol/L vitamin C to be able to oxidize LDL. It has been suggested that mechanisms by which oxidized form of LDL can affect many components of the atherogenic process, including thrombosis, leads to tissue damages [26]. Therefore, it is believed that inhibition of LDL oxidation can lead to the prevention of atherosclerosis and its complications. In addition, two other important indices of LDL oxidation were also investigated in this study. First, the lag time of the LDL oxidation reaction. The lag time of the LDL oxidation mediated by MPO in the absence of vitamin C was 67 ± 3.2 min, and it was significantly lengthened up to 183 ± 4.1 min, showing an increase of 2.73 times. It means that susceptibility of the LDL oxidation mediated by MPO had decreased up to 64% in the presence of vitamin C. Second, conjugated dienes, the most abundant intermediate, produced during LDL oxidation. It was decreased from 28.5 ± 1.3 μmole in the absence of vitamin C to 8.3 ± 0.41 μmole in the presence of vitamin C (200 mM). Protection of LDL oxidation by vitamin C was concentration dependent showing the maximum effect of vitamin C at 150 mM. At dose of 400 mg vitamin C/day, plasma is completely saturated, and steady-state plasma vitamin C concentration will be approximately 80 mM [32]. For this reason, vitamin C concentration in our in vitro study was chosen in the normal plasma concentration range. As mentioned above, it seems that vitamin C at dose of around 400 mg/day might be maximally improved the lag time of the LDL oxidation reaction in vivo and it is useful for decreasing atherosclerosis process rate. LDL incubation with MPO system in the presence or absence of vitamin E (α-tocopherol) did not show any significant differences in LDL oxidation rate. In addition, there were no significant differences between the amounts of conjugated dienes produced during LDL oxidation mediated by MPO in the absence or presence of vitamin E. The similar results were obtained for lag time of LDL oxidation in the presence or absence of vitamin E.
In numerous studies, antioxidant properties of vitamin E and some other substances which have antioxidant activity, have been shown to reduce LDL oxidation in vivo [33-35]. There might be various mechanisms by which vitamin E is able to show its preventive role in atherosclerosis, although the mechanism of action still remains unclear. For example, Ricciarelli et al. [36] in their experience have found that vitamin E reduces the uptake of oxidized form of LDL by inhibiting CD36 scavenger receptor (a specific receptor for oxidized LDL) expression. Furthermore, Teupesr et al. [37] showed that vitamin E reduces cholesterol esterification process rate and uptake of esterified LDL. They also suggested that all effects of vitamin E are due to the down-regulation of CD36 scavenger receptor. According to the data presented here, vitamin E is unable to reduce the reaction rate of LDL oxidation mediated by MPO.
Fig. 6.
Effect of different concentrations of vitamin E on the conjugated dienes produced during low-density lipoprotein (LDL) oxidation mediated by myeloperoxidase. Values were obtained from the LDL oxidation curves of Figure 4. Values represent mean SD of three independent experiments.
However, there might be some mechanisms to show vitamin E may be able to inhibit the oxidation reaction of LDL. However, in vitro LDL oxidation mediated by MPO have been less studied, and for better understanding of vitamin E effect on the LDL oxidation mediated by MPO, more kinetic information about MPO is needed.
In conclusion, we may suggest that vitamin C has measurable effects on LDL oxidation indices and might be beneficial in prevention damages resulted in atherosclerosis.
Acknowledgment
This work was supported by the Research Council of Isfahan University of Medical Sciences, Isfahan, Iran (Research No. 79294).
References
- 1.Ranguelova K, Rice AB, Khajo A, Triquigneaux M, Garantziotis S, Magliozzo RS, et al. Formation of reactive sulfite-derived free radicals by the activation of human neutrophils: an ESR study. Free Radic Biol Med. 2012 Apr;52(8):1264–71. doi: 10.1016/j.freeradbiomed.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane AE, Tan JT, Hawkins CL, Heather AK, Davies MJ. The myeloperoxidase–derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signaling via the mitogen–activated protein kinase (MAPK) pathway in macrophages. Biochem J. 2010 Aug;430(1):161–9. doi: 10.1042/BJ20100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Cao Z, Moore DR, Jackson PL, Barnes S, Lambeth JD, et al. Microbicidal activity of vascularperoxidase 1 in human plasma via generation of hypochlorous acid. Infect Immun. 2012 Jul;80(7):2528–37. doi: 10.1128/IAI.06337-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Soud HM, Maitra D, Byun J, Souza CE, Banerjee J, Saed GM, et al. The reaction of HOCl and cyanocobalamin: corrin destruction and the liberation of cyanogens chloride. Free Radic Biol Med. 2012;Feb(3):616–25. doi: 10.1016/j.freeradbiomed.2011.10.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resch U, Semlitsch M, Hammer A, Susani-Etzerodt H, Walczak H, Sattler W, et al. Hypochlorite-modified low-density lipoprotein induces the apoptotic machinery in Jurkat T-cell lines. Biochem Biophys Res Commun. 2011 Jul;410(4):895–900. doi: 10.1016/j.bbrc.2011.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karakas M, Koenig W, Zierer A, Herder C, Rottbauer W, Baumert J, et al. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/Kora Augsburg study. J Intern Med. 2012 Jan;271(1):43–50. doi: 10.1111/j.1365-2796.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 7.Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, et al. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology (Oxford) 2012 Oct;51(10):1796–803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- 8.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-1, the major HDL protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem. 2012 Feb;287(9):6375–86. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satchell L, Leake DS. Oxidation of low-density lipoprotein by iron at lysosomal pH: inplications for atherosclerosis. Biochemistry. 2012 May;51(18):3767–75. doi: 10.1021/bi2017975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2012 Nov;286(17):2136–42. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 11.Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequence of total and subtotal myeloperoxidase deficiency: risk or benefit. Acta Haematol. 2000;104(1):10–5. doi: 10.1159/000041062. [DOI] [PubMed] [Google Scholar]
- 12.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Münzel T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003 Sep;108(12):1440–5. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 13.Brennan ML, Penn MS, Van LenteF, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003 Oct;349(17):1595–604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 14.Turchi G, Alagona G, Lubrano V. Protective activity of plicatin B againest human LDL oxidation induced in metal ion-dependent and independent processes Experimental and theoretical studies. Phytomedicine. 2009 Nov;16(11):1014–26. doi: 10.1016/j.phymed.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 15.López-Alarcón C, Speisky H, Lissi E. Antioxidant effect of 5-aminosalicylic acid on copper mediated LDL oxidation. Biol Res. 2007;40(2):155–62. doi: 10.4067/s0716-97602007000200006. [DOI] [PubMed] [Google Scholar]
- 16.Bleys J, Miller ER3rd, Pastor-Barriuso R, Appel LJ, Guallar E. Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006 Oct;84(4):880–7. doi: 10.1093/ajcn/84.4.880. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar EC, Jascolka TL, Teixeira LG, Lages PC, Ribeiro AC, Vieira EL. il-rich diet on the development of atherosclerosis: balance between antioxidant and hyperlipidemic properties. Braz J Med Biol Res. 2012 Jul;45(7):601–9. doi: 10.1590/S0100-879X2012007500074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. J Lipid Res. 1383 Jul;24(7):904–9. [PubMed] [Google Scholar]
- 19.Schumaker VN, Puppione DL. Sequential flotation ultrcentrifugation. Methods Enzymol. 1986;128:155–70. doi: 10.1016/0076-6879(86)28066-0. [DOI] [PubMed] [Google Scholar]
- 20.Agner K. Crystalline myeloperoxidase. Acta Chem Scand. 1958;12:89–94. [Google Scholar]
- 21.Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for yeloperoxidase in human poly-morphonuclear leukocytes. Anal Biochem. 1983 Jul;132(2):345–52. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 22.Jerlich A, Horakova L, Fabjan JS, Giessauf A, Jürgens G, Schaur RJ. Correlation of low density lipoprotein modification by myeloperoxidase with hypochlorous acid formation. Int J Clin Lab Res. 1999;29(4):155–61. doi: 10.1007/s005990050083. [DOI] [PubMed] [Google Scholar]
- 23.Carnevale R, Pignatelli P, Lenti L, Buchetti B, Sanguigni V, Di SantoS, et al. LDL are oxidatively modified by platelets via GP91 phox and accumulate in human monocytes. FASEB J. 2007 Mar;21(3):927–34. doi: 10.1096/fj.06-6908com. [DOI] [PubMed] [Google Scholar]
- 24.Puhl H, Waeg G, Esterbauer H. Methods to determine oxidation of low-density lipoproteins. Methods Enzymol. 1994;233:425–41. doi: 10.1016/s0076-6879(94)33049-2. [DOI] [PubMed] [Google Scholar]
- 25.Barre DE, Mizier-Barre A, Griscti O, Hafez K. No gender associated differences in LDL oxidation in response to a CuSO4 challenge in a population of Caucasians with well- controlled type 2 diabetes. Int J Diabetes Metab. 2009;17(3):81–85. [Google Scholar]
- 26.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–92. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olorunniji FJ, Iniaghe MO, Adebayo JO, Malomo SO, Adediran SA. Mechanism-based inhibition of my eloperoxidase by hydrogen peroxide: enhancement of inactivation rate by organic donor substrates. Open Enzym Inhib J. 2009;2:28–35. [Google Scholar]
- 28.Özsoy MB, Pabuçcuoğlu A. The effect of acetaminophen on oxidative modification of low- density lipoproteins in hypercholesterolemic rabbits. J Clin Biochem Nutr. 2007 Jul;41(1):27–31. doi: 10.3164/jcbn.2007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenson RS. Statins in aterosclerosis: lipid- lowering agents with antioxidant capabilities. Atherosclerosis. 2004 Mar;173(1):1–12. doi: 10.1016/S0021-9150(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 30.Jin W, Thuong PT, Su ND, Min BS, Son KH, Chang HW, et al. Antioxidant activity of cleomiscosins A and C isolated from Acerokamotoanum. Arch Pharm Res. 2007 Mar;(3):275–81. doi: 10.1007/BF02977606. [DOI] [PubMed] [Google Scholar]
- 31.Das S, Snehlata , Das N, Srivastava LM. Role of ascorbic acid on in virto oxidation of low- density lipoprotein derived from hypercholesterolemic patients. Clin Chim Acta. 2006 Oct;372(1-2):202–5. doi: 10.1016/j.cca.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natal Acad Sci USA. 2001 Aug;98(17):9842–6. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzukawa M, Ishikawa T, Yoshida H, Nakamura H. Effect of in vivo supplementation with low dose vitamin E on susceptibility of low density lipoprotein to oxidative modification. J Am Coll Nutr. 1995 Feb;14(1):46–52. doi: 10.1080/07315724.1995.10718472. [DOI] [PubMed] [Google Scholar]
- 34.Burkitt. M. A critical overview of the chemistry of copper- dependent low density lipoprotein oxidation: role of lipid hydroperoxides, α-tocopherol, thiols and ceruloplasmin. Arch Biochem Biophys. 2001;394:117–35. doi: 10.1006/abbi.2001.2509. [DOI] [PubMed] [Google Scholar]
- 35.Janisch KM, Williamson G, Needs P, Plumb GW. Properties of quercetin conjugates: modulation of LDL oxidation and binding to human serum albumin. Free Radic Res. 2004 Aug;38(8):877–84. doi: 10.1080/10715760410001728415. [DOI] [PubMed] [Google Scholar]
- 36.Ricciarelli R, Zingg JM, Azzi A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–7. doi: 10.1161/01.cir.102.1.82. [DOI] [PubMed] [Google Scholar]
- 37.Teupser D, Thiery J, Seidel D. Alpha-tocopherol down-regulates scavenger receptor activity in macrophages. Atherosclerosis. 1999 May;144(1):109–15. doi: 10.1016/s0021-9150(99)00040-4. [DOI] [PubMed] [Google Scholar]