Abstract
The process by which adult neural stem cells generate new and functionally integrated neurons in the adult mammalian brain has been intensely studied, but much more remains to be discovered. It is known that neural progenitors progress through distinct stages to become mature neurons, and this progression is tightly controlled by cell-cell interactions and signals in the neurogenic niche. However, less is known about the cell-intrinsic signaling required for proper progression through stages of adult neurogenesis. Techniques have recently been developed to manipulate genes specifically in adult neural stem cells and progenitors in vivo, such as the use of inducible transgenic mice and viral-mediated gene transduction. A critical mass of publications utilizing these techniques has been reached, making it timely to review which molecules are now known to play a cell-intrinsic role in regulating adult neurogenesis in vivo. By drawing attention to these isolated molecules (e.g. Notch), we hope to stimulate a broad effort to understand the complex and compelling cascades of intrinsic signaling molecules important to adult neurogenesis. Understanding this process opens the possibility of understanding brain functions subserved by neurogenesis, such as memory, and also of harnessing neural stem cells for repair of the diseased and injured brain.
Introduction
The birth of new neurons in the adult brain is a remarkable discovery that has gained increasing attention over the last forty years [1-3]. Research on adult neurogenesis has exploded in the past decade, with a 7-fold increase in the number of publications with keywords ‘adult neurogenesis’ between 1997 and 2007 (ISI database). Interest has intensified with the discovery of neurogenesis in the adult human brain [4-6], by findings that link adult neurogenesis to normal brain function [7-9] and disease [10-13], and by the tantalizing possibility of using adult neural stem cells in treatment of neurodegenerative and psychiatric disorders [14]. Such intense research has revealed that adult neurogenesis occurs primarily in two brain regions, the subgranular zone (SGZ) and the subventricular zone (SVZ; Fig 1) [15, 16, for other regions see 17]. The SGZ of the adult hippocampus gives rise to glutamatergic dentate gyrus granule cells, while a pathway extending from the SVZ to the olfactory bulb gives rise to inhibitory olfactory bulb granule and periglomerular cells [8, 18-22]. SGZ and SVZ neurogenesis differ in many ways, but both proceed via a remarkable “process” of adult neurogenesis, where the progeny of stem cells move through stages of proliferation, fate choice, migration and maturation (Fig 2) [23]. However, to fully understand SGZ and SVZ adult neurogenesis and their therapeutic implications for regenerative medicine, it is imperative to understand the molecular mechanisms of the process: what controls where and how adult neurogenesis occurs?
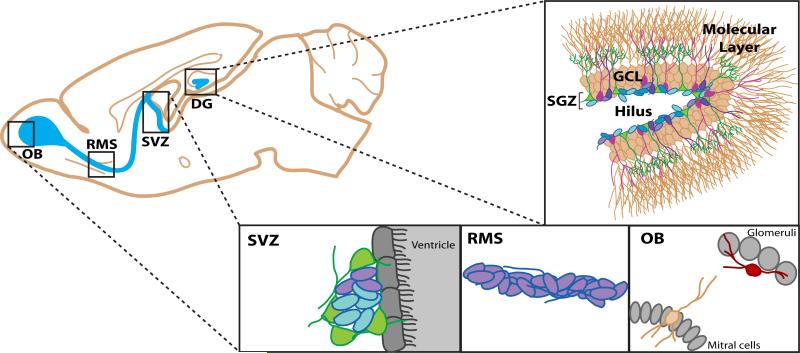
Figure 1. Adult neurogenesis occurs primarily in the subventricular zone (SVZ) and subgranular zone (SVZ).
A sagittal view of the adult mouse brain, the neurogenic regions are indicated in blue. In the SVZ, stem cells (green) reside in the wall of the lateral ventricle, just below the ependymal layer (gray), and give rise to neural progenitors (blue) and neuroblasts (purple). These neuroblasts migrate in chains along the rostral migratory stream (RMS) to reach the olfocatory bulb (OB), where they mature into functionally integrated neurons. In the SGZ of the hippocampal dentate gyrus (DG), stem cells (green) clustered near the base of the hippocampal DG granule cell layer (GCL) give rise to progenitors (light blue). These eventually give rise to immature (magenta) and mature (peach) granule cell neurons that primarily exist in the inner or hilar-half of the GCL but extend their processes out to the molecular layer to receive cortical input. Note that SVZ progenitors and their progeny migrate a relatively long distance to the OB to give rise to mature neurons, while SGZ progenitors move barely into the GCL to give rise to mature neurons.
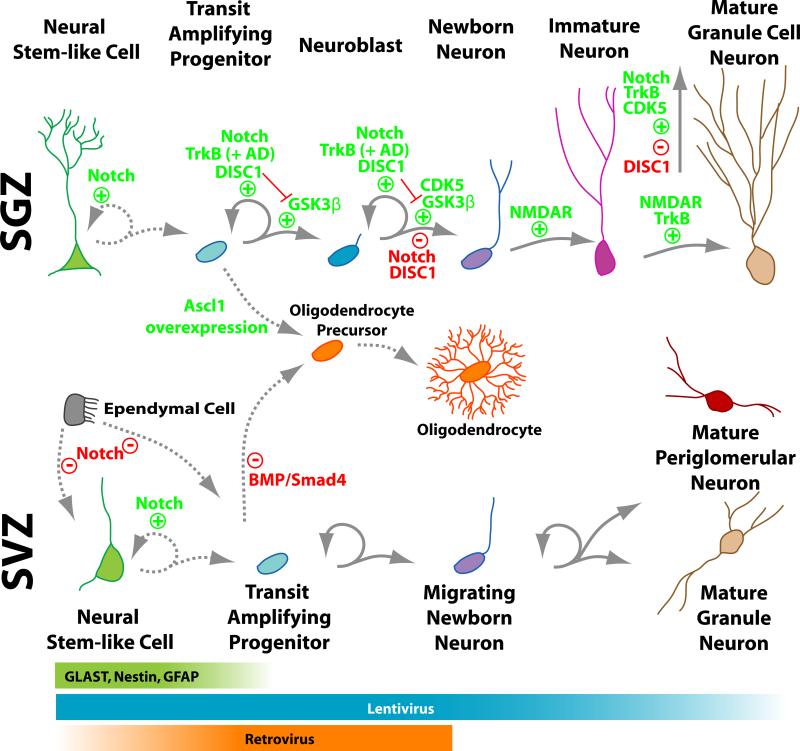
Figure 2. Adult neurogenesis is a process with distinct stages.
The process of adult neurogenesis – stem cells on the left giving rise to rapidly dividing progenitors, which in turn develop into immature and eventually mature neurons on the right – is shown in schematic form in both the hippocampal SGZ (top row) and SVZ (bottom row). In both the SVZ and SGZ, stem cells express the markers GLAST, Nestin, and GFAP (see green band across the bottom). Stem cells in both the SGZ and SVZ divide infrequently to self-renew and give rise to transit amplifying progenitors. SGZ: Self-renewal in the SGZ is dependent on Notch signaling. Transit amplifying progenitors give rise to lineage-restricted neuroblasts, both of which proliferate to expand their population. Several pathways converge to promote proliferation of these populations in the SGZ. Notch and DISC1 promote basal proliferation, while TrkB promotes proliferation in response to antidepressants (AD). DISC1 may promote proliferation by inhibiting GSK3beta and cell cycle exit. Notch signaling also negatively regulates cell cycle exit. Overexpression of Ascl1 in SGZ progenitors leads to a change in fate from neuronal to oligodendrocyte. This is specific to the SGZ. Once SGZ neuroblasts exit the cell cycle, they differentiate into neurons and extend dendrites. Maturation and survival of newborn neurons is positively regulated by many pathways, including Cdk5, NMDAR, TrkB, and Notch, while DISC1 negatively regulates maturation. SVZ: Intrinsic regulation of SVZ neurogenesis is less clear, but it is known that Notch signaling maintains ependymal cells in a differentiated state. Without Notch, these cells can contribute to SVZ neurogenesis. Smad4, a downstream target of BMP signaling, is required to inhibit oligodendrocyte differentiation of SVZ progenitors. The colored bands on the very bottom of the schematic illustrate the distinct, but overlapping, stages of neurogenesis in both the SGZ and SVZ that are transfected by lentivirus and retrovirus.
One answer to that question is clearly “microenvironment” or the “neurogenic niche”. A wide variety of components of the neurogenic niche have been determined, including vascular requirements, secreted factors, cilia, extrinsic cell-cell interactions, and specific innervation and release of neurotransmitter [24-26]. For example, cell-cell interactions in both the SGZ and SVZ result in intracellular signaling cascades essential to the regulation of neurogenesis, including the Cdk5 and Notch pathways [27, 28]. The signature components of the neurogenic microenvironment are clearly important in the relative restriction of adult neurogenesis to the SVZ and SGZ [29], and likely to the decreased neurogenesis seen with increasing age [30, 31]. The SVZ and SGZ microenvironment appear important for cell fate decisions, a relationship that is highly reminiscent of embryonic neurogenesis [32-34]. However, unlike embryonic neurogenesis, adult neurogenesis proceeds more slowly, with the stages of proliferation, fate-choice, migration and maturation overlapping in time, and in the case of the SGZ, in space [35]. Therefore, the process of adult neurogenesis occurs in a far more heterogeneous and complex microenvironment than in the embryo. Clearly, understanding the role of the microenvironment in the process specifically of adult neurogenesis is an area of research that warrants considerable attention [24, 36-40].
Another answer to the question ‘what controls where and how adult neurogenesis occurs?’ is “cell-intrinsic molecules”. Intrinsic factors likely provide some of the exceptionally tight control over the process of neurogenesis, regulating, for example, whether a cell returns to the cell cycle or exits it to become an immature and then a mature neuron. However, intrinsic pathways that regulate adult neurogenesis have not been thoroughly studied because many traditional constitutive knockout mice are not viable to adulthood or even to the early postnatal period [e.g. 28]. Only very recently have the tools been created to inducibly alter expression of genes specifically within adult neural stem cells and progenitors in vivo, enabling exploration of the mechanistic biology of adult neurogenesis in the natural context of its microenvironment.
Given the power of inducible techniques to dissect the intrinsic signaling cascades critical for adult neurogenesis, and given the growing number of publications that successfully use these approaches to reveal key molecules in the process of adult neurogenesis, these in vivo inducible manipulations of cell-intrinsic signaling are the focus of the next section. After a brief introduction to these techniques, we will review key cell-intrinsic signaling components revealed to be critical to adult neurogenesis by use of these techniques.
Inducible techniques to target adult neurogenesis in vivo
Since the emphasis of this review is on inducible manipulations of adult neurogenesis in vivo and the cell-intrinsic molecules identified via these manipulations, here we provide a brief overview of the two main techniques used: viral mediated gene transduction and inducible transgenic mouse lines. For sake of space and to preserve our focus on strictly in vivo approaches that investigate cell-intrinsic effects, we only mention in passing several other elegant approaches to studying cell-intrinsic effects, such as antisense oligonucleotide infusion, transplantation of stem cells from a constitutive knockout into a wildtype mouse, and co-culture of stem cells from knockout and wildtype mice [41-43].
Viral-mediated gene transfer
Viral mediated gene transduction exploits the protein-making machinery of a virus to express proteins of interest in discrete brain regions or cellular populations. Retro- and lentiviruses are particularly of interest for this review, as they allow relatively long-lasting and controlled genetic manipulations since they will insert genes into the host genome. Viral-mediated gene transfer can induce several types of genetic manipulation – including knockdown, over-expression, knockout – depending on what gene the virus is engineered to make. For example, gene knockdown or over-expression can be achieved by engineering the virus to encode a short hairpin RNA (shRNA) for the gene of interest, or to encode the gene of interest itself. Gene knockout, on the other hand, can be achieved by engineering the virus to encode Cre recombinase, a bacteriophage element that recognizes and recombines loxP sites that flank a gene of interest, or a “floxed” gene. Since viruses are typically infused into a discrete region of the brain, stereotaxic infusion of a virus encoding Cre into the brain of a floxed mouse (e.g. floxed Cdk5) allows regionally specific gene knockout without the effort of breeding bi- or trigenic mice (as discussed below). Other elegant variations of viral-mediated gene transfer exist, such as ex vivo transfection and subsequent transplantation into the brain. Since the focus of this review is on in vivo inducible techniques, the reader is referred to other excellent reviews for discussion of these other approaches [e.g. 44, 45].
When utilizing viral-mediated gene transduction, it is important to consider the virus used, as some viruses preferentially infect dividing cells, while others infect multiple cell types (Fig 2). Retroviral infusion into the brain can infect all dividing cells and, in practice, result in gene manipulation within the transit amplifying population in neurogenic regions [45, 46]. On the other hand, lentivirus targets a broader population, infecting neural stem cells and progenitor cells as well as immature [47] and sometimes mature neurons [48-50]. The distinction is important, as the ultimate outcome and interpretation differ depending on the virus. For example, manipulation of genes in a “wave” of progenitors with retrovirus leads to a discrete cohort of transfected neurons (or other progeny). In contrast, since lentivirus transduces neural stem cells – the putative source of the progenitors in the process of neurogenesis (Fig 2) – this leads to sustained output of genetically modified progeny.
Viral-mediated gene transfer has enormous benefits, including regional specificity, the ability to engineer almost any gene of interest (including those of relatively large size: 4-7 kb), and lack of need to breed bigenic animals. However, there are limitations of the technique. A major drawback is variability of titer between viral preparations, making it difficult to compare transfection efficiency and thus quantitative data across groups of animals or laboratories. Another drawback is the limited site of diffusion of the infused virus; typically infusions of 1 microliter into the brain parenchyma will produce <0.4mm penumbra of transfected region, or even less depending on white matter boundaries and other anatomical barriers to viral diffusion. This prevents transfection of large structures, like the SGZ (anterior/posterior length: ~3 mm in mouse, ~5 mm in rat) and complicates attempts to measure total hippocampal neurogenesis or the impact of decreased neurogenesis in hippocampal function. However, for the SVZ, which extends towards the OB along the rostral migratory stream, this restricted diffusion may be a positive point, allowing manipulation of one aspect of SVZ neurogenesis but not another.
Inducible mouse transgenic lines
A second technique to manipulate adult neurogenesis in vivo is the utilization of inducible transgenic mouse lines. Here we briefly describe the three components central to generation of such mouse lines: a “driver” or gene/gene promoter that has expression limited to one or more stages of adult neurogenesis (Fig 2); a genetic cassette that allows the driver to inducibly control expression of a target gene; and a response gene.
Identifying genetic components or “drivers” that are specific to discrete stages of adult neurogenesis has been challenging, but progress has been made. For example, no single marker yet exists that is specific to the stem cells themselves. Rather, adult neural stem cells express a variety of stem cell and glial markers, including Nestin, the high-affinity glial glutamate transporter GLAST, and glial fibrilary acidic protein (GFAP) [6, 51, 52]. Later stages of neurogenesis are similarly marked by discrete proteins; doublecortin (DCX), for example, is relatively selective for immature neurons [53]. The inducible aspect of using transgenic mice to target adult neurogenesis entails connecting these genes (Nestin, GLAST, GFAP for stem cells, Fig. 2) to a genetic cassette that allow the genetic manipulation to be “turned on” or “turned off”. One of the most common approaches is to link a driver gene, like Nestin or GFAP, to a genetic cassette encoding an inducible version of Cre recombinase [54], such as ERT2 or TM (tamoxifen sensitive mutant estrogen receptors) [e.g. 55, 56-58]. Another approach is to link a driver gene to a gene encoding tetracycline-transactivator (tTA) [59-61]. The resulting inducible “driver” then allows control over the response gene of interest, leading to cell-specific gene knockout, knockdown, or over-expression. For example, in the case of a Nestin- or GLASTCreERT2 driver, floxed genes can be removed specifically from Nestin- or GLAST-expressing cells after peripheral administration of tamoxifen [62, 63]. In the case of a Nestin-tTA driver, genes under control of the tetracycline operon (tet-Op) can be specifically excised from Nestin-expressing cells after cessation of doxicycline administration [64]. A “reverse” system also exists, where genes under tet-Op control can be driven in Nestin-expressing cells after administration of doxicycline [60]. Many combinations and permutations of these genetic components exist, and the reader is referred to other reviews for more details on these systems [65, 66]. However, the relevant point for this review is that these transgenic systems allow inducible control over gene expression in key stages of neurogenesis in the adult brain, making it now possible to examine the in vivo cell-intrinsic effects likely so important to the neurogenesis process.
Inducible transgenic lines offer clear benefits over viral approaches, such as genetic control over large numbers of cells in neurogenic regions of the adult brain. This, in turn, leads to ease in quantification and in comparison across studies, and ability to assess the functional input of large numbers of adult-generated neurons to learning and memory [67-69]. One challenge of this approach is the effort that must be put into breeding and crossing the transgenic lines, and into characterizing the levels of recombination at different time points after tamoxifen administration or cessation of doxicycline. Considerations also include the mosaic nature of recombination, which leads to both recombined and wildtype cells within the same structure, limiting the utility of molecular techniques such as Western blotting or PCR. Furthermore, results can vary depending on the efficiency of the Cre-driver [57, 70, 71]. Like choice of virus, the choice of driver for Cre expression has considerable implications when interpreting results. However, because each Cre-driver targets different stages of neurogenesis (e.g. GLAST and GFAP likely targeting slightly earlier stages than Nestin) it may be possible to utilize this difference between drivers to refine our definition of the adult neural stem cell.
Cell-intrinsic molecules that regulate adult neurogenesis in vivo
The focus of the rest of this review is on the insight inducible techniques have offered into cell-intrinsic molecules important for adult neurogenesis in vivo. We first and most extensively discuss Notch, a signaling molecule involved in a plethora of cellular functions, since it is an excellent example of how these inducible approaches have helped to specifically identify a role for a molecule is discrete stages of adult neurogenesis. We then briefly discuss recent advances in understanding cell-intrinsic roles for Cdk5, neurotrophin receptors, Wnt/BMP, DISC1, glutamate and GABA receptors, and CREB. For each molecule, we highlight which stages of neurogenesis these factors are thought to regulate, and indicate new questions that have emerged.
Notch
During development, Notch1 is a universally utilized fate signal integrator in stem cells, generally allowing cells to maintain self-renewal [28]. Notch cell-intrinsic signaling typically follows this sequence of events: membrane-bound Notch binds to ligand (e.g. Delta) on an adjacent cell; gamma secretase cleaves the membrane bound Notch receptor (Fig 3); the Notch intracellular domain (NICD) is translocated to the nucleus [72, 73]; nuclear NICD interacts with mastermind-like to convert the required transcriptional cofactor of Notch, RBP-J, from a transcriptional repressor to an activator; transcription of downstream target genes, including Hes1 and Hes5, is activated [74, 75]. In neural stem cells, targets of Notch signaling work together to prevent terminal differentiation and preserve a pool of stem cells [76]. While Notch signaling is thought to maintain “stemness” and prevent exit from the cell cycle and maturation [28, 38], its role in adult neurogenesis has not been clear, primarily because Notch plays so many other roles in maturation, neuroplasticity, and even survival [77, 78]. Therefore, Notch1 was an ideal candidate for the application of inducible techniques in order to dissect its potential cell-intrinsic role in adult neurogenesis.
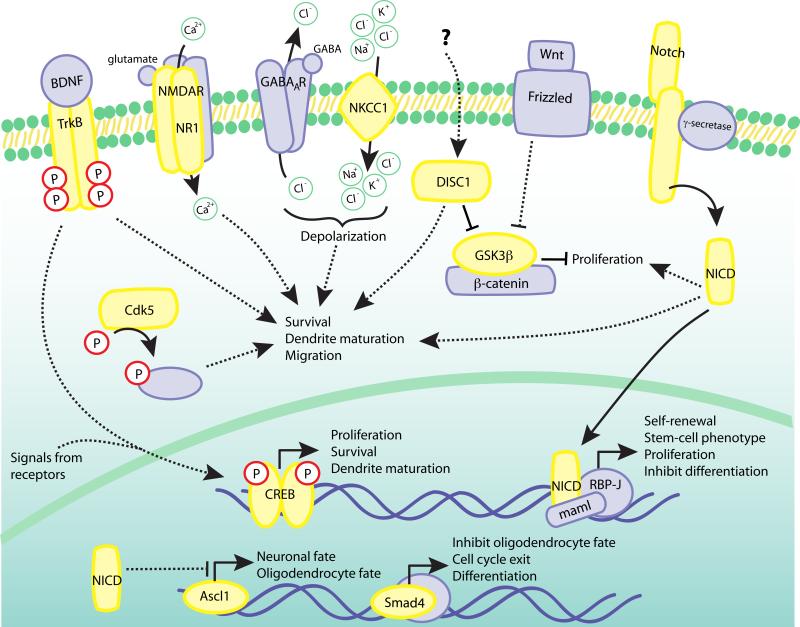
Figure 3. Potential convergence of cell-intrinsic signaling pathways for the regulation of adult neurogenesis.
This schematic of a single cell (with its bilipid membrane, cytoplasm, and nucleus containing DNA and transcriptional elements) proposes a hypothesis of how many of the individual cell-intrinsic components discussed in this review may act in concert or in opposition to regulate stages of adult neurogenesis. Components summarized in this review are indicated in yellow. Direct signaling is indicated by solid lines, while indirect signaling is indicated by dashed lines. Note that several of these proteins receive signals from outside the cell and from the neurogenic niche to regulate differentiation, proliferation, survival, and maturation.
In the first study to inducibly alter Notch1 signaling in early postnatal neural stem cells, Breunig et al. generated two lines of mice to either knock-out Notch (hGFAP-CreERT2;floxed Notch1) or over-express the active domain of Notch (hGFAP-CreERT2;floxed stop-NICD) in GFAP+ stem cells and their progeny in the SGZ [79]. Loss of Notch signaling from stem cells and their progeny shifted the cells from stem-like (GFAP+) to neuronal (DCX+), while overactivation of Notch signaling in NICD mice led to persistent GFAP+ stem cells and fewer DCX+ neurons. Loss of Notch signaling also increased cell cycle exit and decreased the progenitor pool, while overactivation of Notch signaling decreased cell cycle exit and increased the progenitor pool. Finally, loss of Notch led to stunted dendritic arbors and fewer varicosities, while overactivation of Notch signaling led to enlarged arbors and increased varicosities in DCX+ cells. Most of the data in Breunig et al. comes from manipulation of GFAP+ stem cells in the early postnatal SGZ, a region quite distinct from the adult SGZ. However they also offer data that show Notch signaling regulates cell fate in the postnatal and adult SGZ similarly. In sum, this seminal study demonstrated that Notch signaling is important not only in regulating cell cycle exit and fate choice in GFAP+ neural stem cells, but also in regulating choices at each subsequent stage of neurogenesis, even influencing maturation and survival (Fig 2).
This work establishes that Notch signaling in the early postnatal and adult SGZ recapitulates embryonic Notch signaling, but these findings open up many avenues for future work. For example, while it is intriguing that cell-intrinsic Notch signaling regulates dendritic development in the postnatal SGZ, the mechanisms underlying this regulation are unclear. One group suggests that dendritic morphology may be dependent on downstream targets of Notch, such as Hes1/5 and Neurogenenin3 [80, 81], but further investigation is needed to determine if these or other signaling events lead to Notch-induced cytoskeletal reorganization in the adult SGZ in vivo. Additionally, the GFAP driver used by Breunig et al. to target neural stem cells also targets astrocytes [55, 82, 83]. Would the results be the same if the inducible KO or over-expression of Notch was restricted to just neural stem cells and their progeny (e.g. Nestin driver), or are their results due to a cell-extrinsic effect, perhaps due to loss of Notch in SGZ astrocytes? Notch signaling is context-dependent [72], and the results of altering this pathway may be dependent upon the choice of Cre-driver, so this is an important question to consider. Furthermore, given the growing evidence that adult SGZ neurogenesis influences hippocampal behavior [67], what effect does disruption of Notch in stem cells and their progeny have on hippocampal function? Along these lines, increased neurogenesis is required for the behavioral effects of antidepressants [84]. Would animals with impaired Notch signaling increase neurogenesis in response to antidepressants or other neurogenic stimuli? Finally, we have reviewed here the literature on Notch1, when in fact there are several more members of the Notch family, Notch2-4. What distinct or redundant roles do each of the Notch family members play in adult neurogenesis?
In addition to inducible alteration of Notch signaling, studies have also used inducible methods to manipulate downstream targets of Notch. Ascl1 (previously known as Mash1) is a proneural transcription factor negatively regulated by Notch signaling [74]. One recent study used retrovirus to upregulate Ascl1 in SGZ progenitor cells [85]. Four weeks after viral injection, Ascl1 tranfection led to a remarkable fate switch in the progeny generated. While normally a small proportion of progenitor cells in the SGZ assume an oligodendrocyte fate, Ascl1 transfected progeny became oligodendrocytes at an increased frequency and at the expense of immature neurons (Fig 2). However, this fate switch was exclusive to SGZ progenitors; lateral ventricle infusion of the retrovirus did not induce a fate change in progenitors in the SVZ, perhaps because Ascl1 is normally highly expressed there, or perhaps because of the distinct microenvironment of the SVZ. This result supports the fact that regulation of downstream targets by Notch signaling is a fundamental requirement for fate choice during neurogenesis in the adult SGZ. However, this study raises questions about the potential of adult progenitors. Are progenitors in the SGZ capable of generating more cell types than those in the SVZ, or does the microenvironment dictate the potency of adult neural stem cells? Understanding how Notch signaling regulates the choices neural stem cells make during development into neurons paves the way for controlling neural stem cells in vivo, which is critical for therapeutic use. In addition, while this study did not find Ascl1 in the SGZ at baseline, this is in contrast to previous work that showed Ascl1 is expressed in relatively significant levels in the adult SGZ [86, 87]. Therefore, future work might attempt to prevent Ascl1 expression via retroviral-mediated gene transfer of shRNA, or perhaps use an inducible Ascl1-CreERT2 to selectively remove genes specifically from this fate-restricted population of cells. In this regard, NeuroD is an interesting candidate. NeuroD is another proneural transcription factor that is further downstream from Notch than Ascl1 [28]. Recent work using the Nestin-CreERT2 system has established that NeuroD is necessary and sufficient for granule cell differentiation in the adult SGZ and olfactory bulb (unpublished data, Jenny Hsieh). However, more work is needed to elucidate the cell-intrinsic mechanisms by which NeuroD drives neuronal development.
While less work has been done in the SVZ relative to the SGZ, two studies that inducibly regulated Notch in this more anterior neurogenic region are worth highlighting (Fig 2). In the first study, infusion of retrovirus expressing activated Notch into the P2 SVZ via retrovirus converted OB precursors to a stem-like state: nonmigratory, slowly dividing, and undifferentiated [88]. This work is extremely early in postnatal development and therefore cannot be considered a manipulation of adult SVZ neurogenesis. However, it is interesting to note that, as seen in SGZ neurogenesis, Notch activation appeared to maintain “stemness”. A second study used inducible manipulation of Notch to test whether ependymal cells lining the lateral ventricle are really fully differentiated “support players” under basal conditions [89]. Using lentiviral injection of FoxJ1-Cre, the authors excised the required transcriptional cofactor of Notch, RBP-J, from adult ependymal cells. The cellular specificity in this study came from the fact that adult ependymal cells are the sole expressers of FoxJ-1 in the SVZ. In the absence of Notch signaling, ependymal cells left the quiescent state and began generating granule and periglomerular neurons, which migrated and integrated into the circuitry of the olfactory bulb. Thus, in contrast to their previously appreciated support role, this study showed ependymal cells to be quiescent, but still multipotent. While striking, this study fuels the controversy surrounding the debate about neural stem cell identity. Are all radial-glial derived ependymal cells and astroglia capable of becoming mulitpotent? How does Notch signaling promote stem cell identity in one cell, while maintaining a differentiated phenotype in another cell within the same niche? Given the arsenal of tools now at our disposal to inducibly manipulate Notch signaling in discrete neurogenic populations, these questions will likely be addressed in the next few years.
Cyclin dependent kinase 5 (Cdk5)
Cdk5 is homologous to Cdk1-4, and, like other cyclin dependent kinases, it regulates aspects of the cell cycle, particularly in pathological situations [90, 91]. However, Cdk5 also appears to be extremely important in maturation and migration. Biochemical support for this comes from the fact that Cdk5 phosphorylates a host of molecules in post-mitotic neurons, including many that regulate neurite growth and synaptogenesis (Fig 3) [92, 93]. Close examination of Cdk5's role in adult neurogenesis is particularly appealing given this dual role; would KO of Cdk5 in adult-generated cells influence cell cycle/proliferation, maturation, or both? Previously, this question could not be answered because Cdk5 KO mice are not viable [94]. Therefore, exploration of whether Cdk5 played a cell-intrinsic role in adult neurogenesis awaited the development of inducible techniques.
The intrinsic role of Cdk5 in adult neurogenesis has recently been investigated using two different inducible models. Jessberger et al. used a retroviral approach to knockdown Cdk5 expression in a restricted region in the anterior SGZ. SGZ neuroblasts lacking Cdk5 had ectopically projecting dendrites, abnormal migration and dendritic spine formation, but there was only a minimal effect on survival of immature neurons [95]. These results are in contrast to those found by Lagace et al., who utilized a Nestin-CreERT2 system to ablate Cdk5 in Nestin+ stem cells and their progeny throughout the SGZ [62]. This inducible transgenic approach revealed that loss of Cdk5 from stem cells and their progeny led to decreased accumulation of immature neurons, presumably due to cell death, but no change in proliferation.
There are many differences between these groundbreaking studies that explore the cell-intrinsic role of Cdk5 in adult neurogenesis. These include differences in approach which likely result in different cell types targeted (e.g. viral-mediated gene transfer targets dividing cells vs. Nestin-CreERT2 transgenic targets stem cells and their progeny), as well as different amount of cells targeted (e.g. viral-mediated gene transfer targets specifically the anterior SGZ vs. Nestin-CreERT2 transgenic targets the entire SGZ and SVZ). Aside from these differences in methodology, it is notable that in both cases Cdk5 appears to act in relatively late stages of neurogenesis (Fig 2). Thus, both studies suggest that Cdk5's cell-intrinsic role in adult neurogenesis does not grossly influence proliferation or cell cycle progression, an exceptionally intriguing finding given the dual role of Cdk5 in cell cycle regulation and maturation in development and in vitro. Future work to reconcile these distinct but complementary results is warranted, and could focus on the hypothesis that Cdk5 mediates synapse formation, and synaptogenesis in turn mediates immature neuron survival. It will also be interesting to see if loss of Cdk5 from stem cells and their progeny disrupts hippocampal function, as has been shown for overt loss of SGZ neurogenesis [67] and cell extrinsic loss of dentate gyrus Cdk5 [96].
Neurotrophin receptors
Neurotrophins, such as brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF), and their membrane-bound receptors, TrkB and p75, have long been presumed to regulate adult neurogenesis [97], but the extent to which this regulation was cell-intrinsic or cell-extrinsic has not been clear. Research suggests that cells in discrete (especially early) stages of SGZ or SVZ neurogenesis do not make or release neurotrophins. However, neurogenic cells do express the receptors for the neurotrophins (Fig 3), suggesting a cell-intrinsic role for these receptors. p75, a receptor with roughly equal affinity for the neurotrophins, and TrkB, a receptor with greatest relative affinity for BDNF, are both expressed on dividing SVZ cells [98, 99]. In the SGZ, p75 is expressed on dividing SGZ cells [100], but TrkB has dynamic expression across the stages of SGZ neurogenesis [101]. Interestingly, TrkB is expressed on a large proportion of stem cells, but its expression then drops in proliferating cells, only to increase again a large proportion as the progeny mature [101]. Of course, presence of the receptor is not definitive evidence of its cell-intrinsic effect. It is only recently through the use of inducible techniques that a cell-intrinsic role for neurotrophin receptors in adult neurogenesis has become clear. Here we will focus on two recent papers that used transgenic systems to remove TrkB from stem cells and their progeny in the adult SGZ.
In the first study, Li et al. used a Nestin-CreERT2 system to genetically remove floxed TrkB from stem cells and their progeny in order to determine the long-term and functional consequences of TrkB signaling in SGZ neurogenesis [68]. Loss of TrkB from stem cells and their progeny did not dirupt basal neurogenesis, but these mice failed to show an increase in neurogenesis upon administration of antidepressants, a treatment that increases proliferation and the number of immature neurons in wild-type animals [102]. This induced decrease in TrkB expression and signaling in Nestin+ cells also blocked the behavioral effects of antidepressant treatment in two tests of anxiety/depression, the novelty-supressed feeding and tail-suspension tests.
A second study by Bergami et al. utilized the GLAST-CreERT2 system to ablate TrkB signaling in adult neural stem cells and their progeny [63], although GLAST is known to drive gene expression in astrocytes as well as stem cells. This group showed a decrease in survival of neurons after tamoxifen-induced deletion of TrkB. By analyzing immature neurons at an earlier time point, they were able to demonstrate that a compromised dendritic morphology (complexity, dendritic length) may have led to this decreased survival. This phenotype also correlated with a decrease in number of protrusions (potential synapses) along dendrites, as well as in the ability of recombined cells to develop long-term potentiation after tetanic stimulation. In this study as well, loss of TrkB signaling in adult-generated cells had functional consequences on the behavior of the animal. Ablation of TrkB specifically in adult neurogenic cells led to a marked increase in measures of anxiety on two different paradigms, the open field and elevated plus maze tests. Together these studies demonstrated requirement of TrkB signaling in SGZ neurogenesis and hippocampal function. Specifically, TrkB in neuroblasts appears important for normal proliferation in response to pharmacological stimulation, and in immature neurons for proper integration and survival of newly born neurons (Fig 2). Functionally, TrkB in SGZ neuroblasts may be important for mood-related behavior.
The role of TrkB and other neurotrophin receptors in the SVZ is less certain than in the SGZ. While these receptors are expressed in the SVZ [98, 99], recent studies suggest that the TrkB ligand BDNF does not stimulate SVZ neurogenesis [42] as it does in the SGZ [103]. However, given that a genetic variant of BDNF can influence SVZ neurogenesis and olfaction [104] and that neurotrophin receptors may regulate neurogenesis under pathological conditions [105], additional work to explore a role for neurotrophin receptors in SVZ neurogenesis and to more thoroughly understand its role in SGZ neurogenesis is warranted. Inducible techniques will be especially important to such research given that multiple ligands are known to interact with each receptor, and that the complexity of receptor interactions, isoforms, splice variants, and signaling cascades grows each year [106-108].
Wnt/BMP
Wnt and bone morphogenic protein (BMP) are key regulators of neurogenesis during embryonic development. Their role in adult neurogenesis might have been predicted based on the expression of many Wnt and BMP signaling components in the adult SVZ and SGZ [109] and because Wnt and mediators of Wnt signaling, like β -catenin, are upregulated when neurogenesis is increased experimentally [e.g. 110, 111, 112]. In addition, a tremendous number of papers have utilized elegant in vitro approaches to further surmise a role for Wnt and BMP signaling in neurogenesis [e.g. 113, 114]. However, only recently have the inducible approaches that are the focus of this review been applied to the question of whether these signaling molecules are important in adult neurogenesis in a cell-intrinsic manner. One study built a large basis of in vitro support for the role of Wnt signaling in adult hippocampal neurogenesis, but also included loss and gain of function data from infusions of lentiviruses encoding either a signaling inhibitor (Wnt1/dnWnt) or stimulator (Wnt3) [43]. Blocking Wnt signaling suppressed, while stimulating Wnt signaling enhanced, SGZ neurogenesis, as indicated by changes in DCX+/BrdU+ cell number. Intriguingly, the same group used the same approach to show an effect of Wnt signaling on neurogenesis and learning and memory [115]. These studies emphasize that interference with Wnt signaling can disrupt not only early and late stages of neurogenesis, but can also have functional consequences in terms of the animal's behavior.
Downstream targets in the BMP pathway, such as Smad4, are also critical for normal neurogenesis. A recent study showed that Smad4 is expressed exclusively in progenitors of the SVZ and not in the SGZ [116]. This study then utilized the GLAST-CreERT2 system to demonstrate a requirement for Smad4 in adult neural progenitors. Deletion of Smad4 in SVZ GLAST+ stem cells decreased the number of neuroblasts generated and increased the number of Olig2+ oligodendrocyte precursors that migrated to the corpus callosum. Inducible deletion of Smad4 from either stem cells (using lentivirus) or transit amplifying cells and neuroblasts (using retrovirus) showed that Smad4 signaling was only required at the earliest stage of SVZ neurogenesis for proper neuronal development (Fig 2). This work demonstrates that a single element of a signaling cascade reduced in a discrete cell type in an adult animal can result in a dynamic change in the fate of cells generated, and suggests BMP signaling is essential to adult neurogenesis.
DISC1
Mutations in the gene “disrupted in schizophrenia 1” (DISC1) have been shown to increase susceptibility of an individual to become schizophrenic [117]. DISC1 is an intracellular signaling molecule whose upstream regulators and downstream targets are still rather enigmatic. Two recent studies have attempted to elucidate the function of DISC1 in adult-generated neurons by utilizing inducible genetic manipulations [118, 119]. Duan et al. infused a retrovirus encoding a shRNA to knock down expression of DISC1 in the dentate gyrus adult animals, and found that the process of neurogenesis was actually enhanced. Neuronal progeny of stem cells with decreased DISC1 signaling had longer dendrites and more dendritic crossings, enhanced spine formation and synaptogenesis, and increased excitability. However, these enhancements were also accompanied by ectopic dendrites and cell bodies, emphasizing a likely balance between too much and too little DISC1 signaling. This study concludes that DISC1 negatively regulates the progression of maturation of adult-generated neurons (Fig 2).
The relationship between DISC1 and neurogenesis was further advanced by Mao et al., who also provided a possible downstream target for DISC1 signaling [119]. After characterizing an interaction between DISC1 and GSK3β and β-catenin in vitro, they stereotaxically infused DISC1 shRNA lentivirus into the dentate gyrus to knockdown DISC1. Five weeks later SGZ proliferation was decreased, with no change in cell death. Intriguingly, a GSK inhibitor prevented the decrease in SGZ proliferation, suggesting that DISC1 regulates proliferation through interaction with GSK3β. Additionally, animals with reduced DISC1 cell-intrinsic signaling showed behavioral abnormalities associated with schizophrenia endophenotyes, including hyperactivity in an open field, novelty-induced hyperlocomotion and decreased latency to immobility in a forced swim test. GSK3β-signaling inhibition in DISC1 knockdown animals also prevented the development of these behavioral phenotypes. While the behavioral interpretations of this paper may be viewed as controversial (e.g. forced swim test is more associated with depression than schizophrenia, and testing it in hyperactive animals may be confounding), in general this body of evidence strongly supports the conclusion that DISC1 negatively regulates GSK3β to maintain normal adult SGZ neurogenesis (Fig 2, 3).
As a corollary, one study has recently shown a requirement of the GSK3β interactor β-Catenin in POMC-expressing immature neurons [120]. This study induced a specific knockout of β-Catenin in early postnatal dentate gyrus immature neurons and found a deficit in dendritic morphology. It will be interesting to see if this result is replicated utilizing inducible techniques in the more mature neurogenic niche, as β-Catenin is an interesting point of convergence between many pathways (Wnt, cadherin, TrkB) and DISC1.
Glutamate receptors
Glutamate is a well-known regulator of adult neurogenesis [121, 122], but only recently have studies begun to dissect a cell-intrinsic role for glutamate receptors. The NMDA glutamate receptor (NMDAR) is a crucial integrator of excitation and physiological changes that can lead to potentiation of synapses and long-term changes in neuronal circuits. Its role in the process of adult SGZ neurogenesis has been investigated by use of retroviral knockdown of the NR1 subunit of the receptor [123]. Maturing neurons lacking functional NMDAR had a decreased density in SGZ at 3 weeks. Decrease in number of maturing neurons was also accompanied by an increase in cell death. The authors suggest the existence of a critical period in the life of a newborn neuron, in which synaptic activity mediated by NMDAR is ultimately required for new neuron survival (Fig 2, 3). However, many questions remain unanswered. For example, pharmacological and biochemical studies in vivo and in vitro strongly suggest a role for NMDAR in other stages of neurogenesis, such as proliferation and fate choice [e.g. 124, 125, 126]. Therefore, more studies are needed to investigate whether NMDAR is a cell-intrinsic signal for these early stages of neurogenesis. In addition, evidence suggests that other glutamate receptors (kainate, AMPA, mGluR) are also potential cell-intrinsic regulators of SGZ neurogenesis [e.g. 127, 128, 129], and this is worthy of additional investigation using the inducible tools now available.
Even less is known about the cell-intrinsic role of NMDA or any glutamate receptor subtype in the SVZ. Recent studies suggest certain glutamate receptors are expressed and functional in discrete stages of SVZ neurogenesis [121], while others show that global manipulation of glutamate receptors leads to altered SVZ neurogenesis [130]. The paucity of information about the importance of glutamate receptor subtypes in SGZ and SVZ neurogenesis likely will change given the current availability of the tools to inducibly manipulate these proteins in the adult neurogenic regions.
GABA receptors
Another neurotransmitter receptor essential to neurogenesis in general is the GABAA receptor (GABAAR) [131-133]. In fact, GABAergic signaling has been charged with dictating the “tempo” with which cells progress through stages of adult neurogenesis. Work in embryonic development and in vitro work in juvenile SGZ and SVZ suggest potential roles for GABAAR in proliferation, migration and maturation [121, 134]. GABAAR is an ionotropic receptor that passes chloride upon GABA binding. The chloride potential in maturing neurons depends upon expression of the chloride transporters NKCC1 and KCC2 [135]. Until KCC2 is expressed and begins to extrude chloride, the chloride reversal potential is dependent on NKCC1, such that GABAAR activation causes depolarization [136]. GABAergic excitation promotes maturation of adult neural stem cells in the SGZ in vitro [137], thus coupling neuronal activity to progression through stages of neurogenesis [138]. This phenomena of being excited by GABA, a neurotransmitter that typically inhibits mature neurons, is one of many factors that led to adult-generated progenitors being referred to as “young and excitable” [139] (Fig 3).
Much work has been done on the role of GABA in regulating stages of adult neurogenesis in the SVZ and SGZ [121, 132, 140, 141]. Here we highlight a seminal study that inducibly advanced the “switch” to inhibitory transmission of the GABAAR and examined the effect on SGZ adult neurogenesis in vivo [142]. This study used retrovirus expressing shRNA to knock down NKCC1 expression in the adult SGZ, while leaving the level of KCC2 expression unchanged. This cell-intrinsic manipulation resulted in a lower intracellular chloride concentration and hyperpolarization of the cell upon GABA application. It was discovered that immature neurons that are prematurely hyperpolarized by GABA showed reduced formation of both GABAergic and glutamatergic synapses. Electrophysiological measures of synapse formation were accompanied by structural deficits in maturation of adult generated neurons. Newborn neurons with an early switch to hyperpolarization had decreased complexity as measured by total dendritic length, branch number, and Sholl analysis of number of crossings per unit distance from the soma. This study shows that inducible manipulation of a single type of chloride transporter can significantly alter the rate at which new neurons in the adult SGZ mature (Fig 2). Taken together with cell extrinsic studies showing that GABA can influence proliferation, migration, and fate in adult neurogenesis [132, 133, 143], additional studies are needed to explore if GABAAR contributes similarly to all these stages, or whether distinct components of the GABAergic signaling pathway regulate distinct phases of adult neurogenesis.
CREB
One essential integrator of neuronal activity and gene transcription is cyclic AMP response element binding protein (CREB). Neuronal activity can result in calcium dependent as well as cAMP/PKA dependent phosphorylation of CREB and subsequent regulation of genes that lead to long-term changes in activity and morphology (Fig 3) [144]. While little has been done to inducibly regulate CREB in a cell-intrinsic manner to study adult neurogenesis in vivo, intriguing evidence suggests that such studies are necessary. For example, CREB is activated in differentiating neurons as they move from the SVZ to the OB and deletion of CREB in early postnatal animals decreases survival of OB granule cells [145]. In addition, immature SGZ neurons present the phosphorylated form of CREB, and pharmacological activation of PKA/CREB signaling enhances neuronal proliferation [146].
One study that has manipulated CREB signaling in SVZ adult neurogenesis in vivo used a Nestin-tTA transgenic mouse that overexpressed a mutant form of CREB (mCREB) in stem cells and their progeny [64]. In contrast to their hypothesis that CREB disruption would alter adult SVZ neurogenesis, disruption of CREB DNA binding via mCREB overexpression did not alter proliferation or fate in the SVZ. It is possible that mCREB overexpression was not sufficient to test the cell-intrinsic role of CREB; in this study, mCREB acted as a competitive antagonist for CREB binding to DNA, but did not directly affect either the expression or phosphorylation state of CREB. Therefore, additional studies are needed that apply the growing knowledge of how to interfere with CREB signaling in vivo [147, 148]. Given the relatively clear expression and activation of CREB in distinct stages of adult neurogenesis [145, 146], growing evidence that cell extrinsic CREB mediates neurogenesis [e.g. 149], and the ability of CREB to both integrate numerous intracellular signals and mediate transcription (Fig 3; [150]), it will be extremely surprising if CREB does not emerge in the next five years as a major player in cell-intrinsic regulation of neurogenesis in vivo.
Conclusion
The studies summarized here are among the first to inducibly alter expression of a single molecule specifically in adult neural stem cells and progenitors in vivo. Taken as a whole, it is intriguing to hypothesize that this wide variety of pathways may converge within individual cells to regulate multiple aspects of adult neurogenesis (Fig 3). Of course, the results of these studies are biased by the fact that the inducible techniques currently available require manipulation through the early stages of neurogenesis in order to look at later stages of maturation. Future studies will likely employ inducible techniques using promoters that drive expression selectively in immature neurons, such as POMC, PSA-NCAM or DCX, and will focus on signaling molecules and cascades ripe for analysis, such as CREB, mentioned above, adhesion molecules [151, 152] and other growth factor cascades [153-156]. As more inducible systems are developed and more signaling pathways interrogated, the cell-intrinsic requirements for stages of adult neurogenesis will be established. Ultimately, intimate knowledge of the signaling cascades that regulate adult neurogenesis could provide precise pharmacological and cellular interventions to manipulate the process, resulting in specific treatments to repair the damaged brain.
Acknowledgements
We are grateful to our colleagues, and Dr. Jenny Hsieh in particular, in the UT Southwestern Stem Cells in Neuroscience Community for helpful discussions and feedback about this review. This work was supported by grants from the National Institutes of Health (DA016765, DA023701, DA007290, and DA023555 to A.J.E.) and NASA. MAJ is supported by the Basic Science Training Program in Drug Abuse (NIDA T32-DA7290, PI: Amelia J. Eisch, Ph.D.), and JLA was supported by the Basic Science Training Program in Mental Health (NIMH T32-MH076690, PI: Carol Tamminga, M.D.) and the Medical Scientist Training Program at UT Southwestern.
References
- 1.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126(3):337–89. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 2.Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57(6):737–49. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- 3.Kempermann G. In: Adult Neurogenesis: Stem Cells and Neuronal Development in the Adult Brain. Kempermann G, editor. Oxford University Press; USA: 2005. p. 448. [Google Scholar]
- 4.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 5.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–9. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 6.Roybon L, et al. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE. 2009;4(3):e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempermann G. Stem cells in the healthy and diseased adult brain. Nervenheilkunde. 2004;23(2):90–93. [Google Scholar]
- 8.Ortega-Perez I, Murray K, Lledo PM. The how and why of adult neurogenesis. J Mol Histol. 2007;38(6):555–62. doi: 10.1007/s10735-007-9114-5. [DOI] [PubMed] [Google Scholar]
- 9.Ko HG, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2(1):1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisch AJ. Adult neurogenesis: implications for psychiatry. Prog Brain Res. 2002;138:315–42. doi: 10.1016/S0079-6123(02)38085-3. [DOI] [PubMed] [Google Scholar]
- 11.Eisch AJ, et al. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28(46):11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenbosch R, et al. Adult neurogenesis and the diseased brain. Curr Med Chem. 2009;16(6):652–66. doi: 10.2174/092986709787458371. [DOI] [PubMed] [Google Scholar]
- 13.Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21(3):290–5. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- 14.Ormerod BK, Palmer TD, Caldwell MA. Neurodegeneration and cell replacement. Philos Trans R Soc Lond B Biol Sci. 2008;363(1489):153–70. doi: 10.1098/rstb.2006.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85(2):523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 17.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8(6):481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 18.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331(1):243–50. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 19.Imayoshi I, et al. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009 doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 20.Quinones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 21.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 22.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10(6):727–34. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G, et al. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27(8):447–52. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 25.Breunig JJ, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105(35):13127–32. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15(1):20–7. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maccioni RB, et al. The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology. Eur J Biochem. 2001;268(6):1518–27. doi: 10.1046/j.1432-1033.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8(6):709–15. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 29.Gage FH, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92(25):11879–83. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2(10):894–7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 31.Luo J, et al. The aging neurogenic subventricular zone. Aging Cell. 2006;5(2):139–52. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2(4):287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 33.Temple S. The development of neural stem cells. Nature. 2001;414(6859):112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 34.Gotz M, Barde YA. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46(3):369–72. doi: 10.1016/j.neuron.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Duan X, et al. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18(1):108–15. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan JD, et al. Cellular niches for endogenous neural stem cells in the adult brain. CNS Neurol Disord Drug Targets. 2007;6(5):336–41. doi: 10.2174/187152707783220866. [DOI] [PubMed] [Google Scholar]
- 37.Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363(1489):123–37. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basak O, Taylor V. Stem cells of the adult mammalian brain and their niche. Cell Mol Life Sci. 2009;66(6):1057–72. doi: 10.1007/s00018-008-8544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez-Buylla A, et al. The Heterogeneity of Adult Neural Stem Cells and the Emerging Complexity of Their Niche. Cold Spring Harb Symp Quant Biol. 2008 doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- 40.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan XT, et al. Antisense Noggin oligodeoxynucleotide administration decreases cell proliferation in the dentate gyrus of adult rats. Neurosci Lett. 2004;366(1):107–11. doi: 10.1016/j.neulet.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 42.Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28(50):13368–83. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 44.Suhonen J, et al. Ex vivo and in vivo gene delivery to the brain. Curr Protoc Hum Genet. 2006 doi: 10.1002/0471142905.hg1303s51. Chapter 13: p. Unit 13 3. [DOI] [PubMed] [Google Scholar]
- 45.Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc. 2006;1(6):3049–55. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- 46.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Hooijdonk LW, et al. Lentivirus-mediated transgene delivery to the hippocampus reveals sub-field specific differences in expression. BMC Neurosci. 2009;10:2. doi: 10.1186/1471-2202-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Consiglio A, et al. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci U S A. 2004;101(41):14835–40. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geraerts M, et al. Lentiviral vectors mediate efficient and stable gene transfer in adult neural stem cells in vivo. Hum Gene Ther. 2006;17(6):635–50. doi: 10.1089/hum.2006.17.635. [DOI] [PubMed] [Google Scholar]
- 50.Kafri T, et al. Lentiviral vectors: regulated gene expression. Mol Ther. 2000;1(6):516–21. doi: 10.1006/mthe.2000.0083. [DOI] [PubMed] [Google Scholar]
- 51.Namba T, et al. The fate of neural progenitor cells expressing astrocytic and radial glial markers in the postnatal rat dentate gyrus. Eur J Neurosci. 2005;22(8):1928–41. doi: 10.1111/j.1460-9568.2005.04396.x. [DOI] [PubMed] [Google Scholar]
- 52.Filippov V, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23(3):373–82. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 53.Brown JP, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 54.Feil S, Valtcheva N, Feil R. Inducible cre mice. Methods Mol Biol. 2009;530:1–21. doi: 10.1007/978-1-59745-471-1_18. [DOI] [PubMed] [Google Scholar]
- 55.Mori T, et al. Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia. 2006;54(1):21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 56.Hirrlinger PG, et al. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54(1):11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- 57.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27(46):12623–9. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganat YM, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26(33):8609–21. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, et al. Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol. 1998;54(3):495–503. doi: 10.1124/mol.54.3.495. [DOI] [PubMed] [Google Scholar]
- 60.Yu TS, et al. Temporally regulated expression of Cre recombinase in neural stem cells. Genesis. 2005;41(4):147–53. doi: 10.1002/gene.20110. [DOI] [PubMed] [Google Scholar]
- 61.Sprengel R, Hasan MT. Tetracycline-controlled genetic switches. Handb Exp Pharmacol. 2007;(178):49–72. doi: 10.1007/978-3-540-35109-2_3. [DOI] [PubMed] [Google Scholar]
- 62.Lagace DC, et al. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105(47):18567–71. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergami M, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105(40):15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beech RD, et al. Nestin promoter/enhancer directs transgene expression to precursors of adult generated periglomerular neurons. J Comp Neurol. 2004;475(1):128–41. doi: 10.1002/cne.20179. [DOI] [PubMed] [Google Scholar]
- 65.Sun Y, Chen X, Xiao D. Tetracycline-inducible expression systems: new strategies and practices in the transgenic mouse modeling. Acta Biochim Biophys Sin (Shanghai) 2007;39(4):235–46. doi: 10.1111/j.1745-7270.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Z, et al. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol. 2002;13(2):121–8. doi: 10.1016/s1084-9521(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 67.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang CL, et al. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 70.Carlen M, et al. Genetic visualization of neurogenesis. Exp Cell Res. 2006;312(15):2851–9. doi: 10.1016/j.yexcr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Imayoshi I, et al. Temporal regulation of Cre recombinase activity in neural stem cells. Genesis. 2006;44(5):233–8. doi: 10.1002/dvg.20212. [DOI] [PubMed] [Google Scholar]
- 72.Radtke F, Schweisguth F, Pear W. The Notch ‘gospel’. EMBO Rep. 2005;6(12):1120–5. doi: 10.1038/sj.embor.7400585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 74.Kageyama R, et al. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306(2):343–8. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 75.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 76.Alexson TO, et al. Notch signaling is required to maintain all neural stem cell populations--irrespective of spatial or temporal niche. Dev Neurosci. 2006;28(1-2):34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 77.Carlson ME, Conboy IM. Regulating the Notch pathway in embryonic, adult and old stem cells. Curr Opin Pharmacol. 2007;7(3):303–9. doi: 10.1016/j.coph.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Corbin JG, et al. Regulation of neural progenitor cell development in the nervous system. J Neurochem. 2008;106(6):2272–87. doi: 10.1111/j.1471-4159.2008.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breunig JJ, et al. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(51):20558–63. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salama-Cohen P, et al. NGF controls dendrite development in hippocampal neurons by binding to p75NTR and modulating the cellular targets of Notch. Mol Biol Cell. 2005;16(1):339–47. doi: 10.1091/mbc.E04-05-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salama-Cohen P, et al. Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J Neurochem. 2006;97(5):1269–78. doi: 10.1111/j.1471-4159.2006.03783.x. [DOI] [PubMed] [Google Scholar]
- 82.Schousboe A, Waagepetersen HS. Glial modulation of GABAergic and glutamat ergic neurotransmission. Curr Top Med Chem. 2006;6(10):929–34. doi: 10.2174/156802606777323719. [DOI] [PubMed] [Google Scholar]
- 83.Hartfuss E, et al. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229(1):15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 84.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 85.Jessberger S, et al. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11(8):888–93. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000;20(16):6095–105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim EJ, et al. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27(47):12764–74. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chambers CB, et al. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128(5):689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- 89.Carlen M, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12(3):259–67. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 90.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25(42):9658–68. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J, Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle. 2008;7(22):3487–90. doi: 10.4161/cc.7.22.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie Z, Samuels BA, Tsai LH. Cyclin-dependent kinase 5 permits efficient cytoskeletal remodeling--a hypothesis on neuronal migration. Cereb Cortex. 2006;16(Suppl 1):i64–8. doi: 10.1093/cercor/bhj170. [DOI] [PubMed] [Google Scholar]
- 93.Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol. 2008;28(3):351–69. doi: 10.1007/s10571-007-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93(20):11173–8. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jessberger S, et al. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6(11):e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sananbenesi F, et al. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10(8):1012–9. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36(2):267–86. [PubMed] [Google Scholar]
- 98.Tonchev AB, et al. Expression of angiogenic and neurotrophic factors in the progenitor cell niche of adult monkey subventricular zone. Neuroscience. 2007;144(4):1425–35. doi: 10.1016/j.neuroscience.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 99.Giuliani A, et al. p75(NTR)-immunoreactivity in the subventricular zone of adult male rats: expression by cycling cells. J Mol Histol. 2004;35(8-9):749–58. doi: 10.1007/s10735-004-9609-2. [DOI] [PubMed] [Google Scholar]
- 100.Okano HJ, Pfaff DW, Gibbs RB. Expression of EGFR-, p75NGFR-, and PSTAIR (cdc2)-like immunoreactivity by proliferating cells in the adult rat hippocampal formation and forebrain. Dev Neurosci. 1996;18(3):199–209. doi: 10.1159/000111408. [DOI] [PubMed] [Google Scholar]
- 101.Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008;18(5):435–9. doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 104.Bath KG, et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28(10):2383–93. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sotthibundhu A, et al. Abeta(1-42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11(3):272–80. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 107.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7(1):46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 109.Li G, Pleasure SJ. Morphogenesis of the dentate gyrus: what we are learning from mouse mutants. Dev Neurosci. 2005;27(2-4):93–9. doi: 10.1159/000085980. [DOI] [PubMed] [Google Scholar]
- 110.Morris DC, et al. Wnt expression in the adult rat subventricular zone after stroke. Neurosci Lett. 2007;418(2):170–4. doi: 10.1016/j.neulet.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Madsen TM, et al. Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biol Psychiatry. 2003;54(10):1006–14. doi: 10.1016/s0006-3223(03)00700-5. [DOI] [PubMed] [Google Scholar]
- 112.Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2008;13(3):285–92. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- 113.Lim DA, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–26. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 114.Bonaguidi MA, et al. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28(37):9194–204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jessberger S, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Colak D, et al. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28(2):434–46. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Millar JK, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–23. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 118.Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mao Y, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–31. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao X, et al. Conditional knock-out of beta-catenin in postnatal-born dentate gyrus granule neurons results in dendritic malformation. J Neurosci. 2007;27(52):14317–25. doi: 10.1523/JNEUROSCI.3206-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38(6):602–10. doi: 10.1007/s10735-007-9153-y. [DOI] [PubMed] [Google Scholar]
- 122.Vicini S. The role of GABA and glutamate on adult neurogenesis. J Physiol. 2008;586(16):3737–8. doi: 10.1113/jphysiol.2008.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tashiro A, et al. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 124.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15(6):4687–92. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 126.Joo JY, et al. Activation of NMDA receptors increases proliferation and differentiation of hippocampal neural progenitor cells. J Cell Sci. 2007;120(Pt 8):1358–70. doi: 10.1242/jcs.002154. [DOI] [PubMed] [Google Scholar]
- 127.Gandhi R, et al. Group I mGluR5 metabotropic glutamate receptors regulate proliferation of neuronal progenitors in specific forebrain developmental domains. J Neurochem. 2008;104(1):155–72. doi: 10.1111/j.1471-4159.2007.04955.x. [DOI] [PubMed] [Google Scholar]
- 128.Ye GL, et al. AMPA and NMDA receptor-mediated currents in developing dentate gyrus granule cells. Brain Res Dev Brain Res. 2005;155(1):26–32. doi: 10.1016/j.devbrainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 129.Suzuki M, et al. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24(3):645–53. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- 130.Di Giorgi-Gerevini V, et al. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12(8):1124–33. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- 131.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3(9):715–27. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 132.Markwardt S, Overstreet-Wadiche L. GABAergic signalling to adult-generated neurons. J Physiol. 2008;586(16):3745–9. doi: 10.1113/jphysiol.2008.155713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang DD, Kriegstein AR. Defining the Role of GABA in Cortical Development. J Physiol. 2009 doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ge S, et al. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30(1):1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 135.Lee H, et al. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur J Neurosci. 2005;21(9):2593–9. doi: 10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kriegstein AR, Owens DF. GABA may act as a self-limiting trophic factor at developing synapses. Sci STKE. 2001;2001(95):PE1. doi: 10.1126/stke.2001.95.pe1. [DOI] [PubMed] [Google Scholar]
- 137.Tozuka Y, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47(6):803–15. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 138.Deisseroth K, Malenka RC. GABA excitation in the adult brain: a mechanism for excitation- neurogenesis coupling. Neuron. 2005;47(6):775–7. doi: 10.1016/j.neuron.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 139.Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15(1):121–8. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 140.Overstreet Wadiche L, et al. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94(6):4528–32. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 141.Ge S, et al. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586(16):3759–65. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Akiba Y, et al. gamma-Aminobutyric acid-mediated regulation of the activity-dependent olfactory bulb dopaminergic phenotype. J Neurosci Res. 2009 doi: 10.1002/jnr.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 145.Giachino C, et al. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J Neurosci. 2005;25(44):10105–18. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakagawa S, et al. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002;22(22):9868–76. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahn S, et al. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18(2):967–77. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mioduszewska B, Jaworski J, Kaczmarek L. Inducible cAMP early repressor (ICER) in the nervous system--a transcriptional regulator of neuronal plasticity and programmed cell death. J Neurochem. 2003;87(6):1313–20. doi: 10.1046/j.1471-4159.2003.02116.x. [DOI] [PubMed] [Google Scholar]
- 149.Gur TL, et al. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27(29):7860–8. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89(1):121–45. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim JH, et al. Retrovirally transduced NCAM140 facilitates neuronal fate choice of hippocampal progenitor cells. J Neurochem. 2005;94(2):417–24. doi: 10.1111/j.1471-4159.2005.03208.x. [DOI] [PubMed] [Google Scholar]
- 152.Seki T. Hippocampal adult neurogenesis occurs in a microenvironment provided by PSA-NCAM-expressing immature neurons. J Neurosci Res. 2002;69(6):772–83. doi: 10.1002/jnr.10366. [DOI] [PubMed] [Google Scholar]
- 153.Kuhn HG, et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–9. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mudo G, et al. The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain. J Neural Transm. 2009 doi: 10.1007/s00702-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 155.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 156.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–52. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]