Abstract
Background
Angiopoietin-2 is a proinflammatory mediator of endothelial injury in animal models, and increased plasma angiopoietin-2 levels are associated with poor outcomes in patients with sepsis-associated acute lung injury. Whether angiopoietin-2 levels are modified by treatment strategies in patients with acute lung injury is unknown.
Objectives
To determine whether plasma angiopoietin-2 levels are associated with clinical outcomes and affected by fluid management strategy in a broad cohort of patients with acute lung injury.
Design, Setting, and Participants
Plasma levels of angiopoietin-2 and von Willebrand factor (a traditional marker of endothelial injury) were measured in 931 subjects with acute lung injury enrolled in a randomized trial of fluid liberal vs. fluid conservative management.
Measurements and Main Results
The presence of infection (sepsis or pneumonia) as the primary acute lung injury risk factor significantly modified the relationship between baseline angiopoietin-2 levels and mortality (p = .01 for interaction). In noninfection-related acute lung injury, higher baseline angiopoietin-2 levels were strongly associated with increased mortality (odds ratio, 2.43 per 1-log increase in angiopoietin-2; 95% confidence interval, 1.57–3.75; p < .001). In infection-related acute lung injury, baseline angiopoietin-2 levels were similarly elevated in survivors and nonsurvivors; however, patients whose plasma angiopoietin-2 levels increased from day 0 to day 3 had more than double the odds of death compared with patients whose angiopoietin-2 levels declined over the same period of time (odds ratio, 2.29; 95% confidence interval, 1.54–3.43; p < .001). Fluid-conservative therapy led to a 15% greater decline in angiopoietin-2 levels from day 0 to day 3 (95% confidence interval, 4.6–24.8%; p = .006) compared with fluid-liberal therapy in patients with infection-related acute lung injury. In contrast, plasma levels of von Willebrand factor were significantly associated with mortality in both infection-related and noninfection-related acute lung injury and were not affected by fluid therapy.
Conclusions
Unlike von Willebrand factor, plasma angiopoietin-2 has differential prognostic value for mortality depending on the presence or absence of infection as an acute lung injury risk factor. Fluid conservative therapy preferentially lowers plasma angiopoietin-2 levels over time and thus may be beneficial in part by decreasing endothelial inflammation.
Keywords: acute respiratory distress syndrome, angiopoietin-2, biomarkers, endothelial injury, pulmonary edema, von Willebrand factor
In acute lung injury (ALI) and its more severe form, the acute respiratory distress syndrome, damaged lung epithelial and endothelial barriers allow the influx of protein-rich edema fluid into the alveolar space, leading to impaired gas exchange, tissue injury, and, at worst, death (1). There is a strong interest in the role of the activated pulmonary endothelium in ALI from both biomarker and mechanistic standpoints. Once considered an inert monolayer, the pulmonary endothelium is now recognized to play an integral role in regulating permeability, tone, coagulation, and inflammation (2).
Weibel Palade bodies are storage vesicles within vascular endothelial cells that exert effects on endothelial permeability and function via their stored proteins, including von Willebrand factor (vWF) and angiopoietin-2 (Ang-2). Weibel Palade bodies can be stimulated to release their resident proteins by cAMP or elevated cytosolic Ca2+ concentrations (3). The vWF is a multimeric glycoprotein responsible for platelet adhesion in response to injury. Elevated levels of soluble vWF antigen have been associated with ALI development and mortality for more than 2 decades (4, 5). Ang-2 is a more recently described ligand for the Tie2 receptor, which (in a context dependent fashion) blocks Tie2 phosphorylation, thereby promoting vessel destabilization and inflammation (6, 7). Ang-2 is elevated in the plasma of septic subjects and has been correlated with mortality in a small ALI cohort and in patients with severe sepsis (6, 8–10). Also, genetic variation in the Ang-2 gene has been associated with increased risk for ALI after severe trauma (11). Even more importantly, Ang-2 is a mediator of ALI; knockout of Ang-2 reduces mortality in experimental models of ALI, and serum from sepsis patients with high Ang-2 levels provokes endothelial barrier disruption in in vitro models (6, 12). Whether Ang-2 levels can be modified by treatment strategy in ALI remains unknown, as does the prognostic value of Ang-2 in a large mixed cohort of ALI patients.
In this study, we measured plasma Ang-2 levels from patients enrolled in the ARDSNET FACTT trial (13, 14) and tested three main hypotheses. First, we hypothesized that elevated levels of Ang-2 at baseline and day 3 would predict mortality in a large cohort of patients with ALI from a variety of etiologies. Second, we hypothesized that persistent elevation of Ang-2 at day 3 would be associated with poor clinical outcomes. Third, given previous evidence that elevated hydrostatic pressures in the lung can enhance pulmonary inflammation (15), we hypothesized that fluid-conservative treatment would preferentially affect Ang-2 levels over time. For comparison, we also measured plasma levels of vWF, historically studied as a more traditional marker of endothelial injury with documented prognostic and pathogenetic value in sepsis and ALI (4).
METHODS
Clinical data and biological samples were obtained from patients enrolled in the NHLBI ARDS Network’s randomized controlled trial of a fluid-liberal vs. fluid-conservative management strategy in ALI, which found that the fluid-conservative management strategy resulted in 2.5 fewer days of assisted ventilation (13). This trial was conducted in a factorial design with a trial of central venous vs. pulmonary arterial catheter use, which demonstrated no difference in outcomes between the two groups (14). Details of both trials have been previously published in full. The trials were approved by the Institutional Review Boards at all participating hospitals, and informed consent was obtained from all participants or their surrogates, including consent to use biospecimens for future research for subjects included in this analysis. Day 0 samples for this analysis were collected before patient randomization, as was baseline clinical data. Day 3 samples were collected if patients were alive on study day 3.
Assay Procedures
Plasma biomarkers were measured using enzyme-linked immunoassay techniques (Ang-2, R&D Systems. Minneapolis, MN; vWF, Diagnostica Stago, Parsippany, NJ). All measurements were performed in duplicate. The intra-assay coefficient of variation ranged from 0.56% to 10% for Ang-2 and from 0.06% to 11% for vWF.
Statistical Methods
Statistical analysis was performed with STATA/MP 10.1 (College Station, TX). A p < .05 was considered significant for all statistical analyses. To compare baseline levels of Ang-2 and vWF in two-group comparisons, we used Mann-Whitney rank-sum tests; for multiple group comparisons, we used the Kruskal-Wallis test followed by pair-wise testing with the Bonferroni correction for multiple comparisons. Because biomarker levels were not normally distributed, we applied natural log transformation to the plasma Ang-2 and vWF levels to apply linear models. We first tested the impact of Ang-2 and vWF on mortality in unadjusted logistic regression models. After detecting an interaction between plasma Ang-2 levels and the presence of infection (either sepsis or pneumonia) as the primary risk factor for ALI, subsequent analyses were stratified by infection-related ALI. We next created multivariable logistic models to control for fluid management strategy as well as for baseline clinical features that may have affected outcomes, including age and severity of illness as reflected by Acute Physiology and Chronic Health Evaluation III score (16). These covariates were selected on the basis of previous clinical research about predictors of mortality in ALI (17). Logistic models were checked using the Hosmer-Lemeshow test and the link test (18). In addition, in a prespecified analysis, plasma Ang-2 and vWF levels were divided into quartiles to test for a nonlinear relationship with mortality, using logistic models, and ventilator-free-days, using linear models. Analysis of covariance was used to test the impact of fluid conservative management on the change in Ang-2 and vWF levels from day 0 to day 3.
RESULTS
Clinical Characteristics of Included Subjects
Table 1 summarizes the clinical characteristics of the 931 subjects who were included in the current study from the larger parent study of 1,000 patients. Sixty-nine patients were excluded because of missing or inadequate plasma samples available for study. Major clinical characteristics did not differ between included and excluded subjects.
Table 1.
Clinical characteristics of included vs. excluded patients
| Characteristic | Included Patients (n = 931) | Excluded Patients (n = 69) | p |
|---|---|---|---|
| Age, yrs | 50 ± 16 | 48 ± 17 | .38 |
| Female | 433 (47%) | 33 (48%) | .83 |
| Race or ethnicity | |||
| White | 605 (65%) | 36 (52%) | .10 |
| Black | 197 (21%) | 20 (29%) | |
| Other | 129 (14%) | 13 (19%) | |
| Risk factor for acute lung injury | |||
| Sepsis | 220 (24%) | 13 (19%) | .48 |
| Trauma | 72 (8%) | 2 (3%) | |
| Pneumonia | 433 (47%) | 38 (55%) | |
| Transfusions | 9 (1%) | 0 (0%) | |
| Aspiration | 139 (15%) | 10 (15%) | |
| Acute Physiology and Chronic Health Evaluation III | 94 ± 31 | 96 ± 31 | .55 |
| PaO2:FIO2 ratio | 132 ± 63 | 132 ± 61 | .95 |
| Fluid conservative treatment | 471 (51%) | 32 (46%) | .50 |
| Mortality at 90 days | 261 (28%) | 23 (33%) | .35 |
| Ventilator-free days | 17 (0, 23) | 9 (0, 22) | .14 |
Data expressed as mean ± SD, n (%), or median (interquartile range) as appropriate. Excluded patients had inadequate plasma for biomarker measurements.
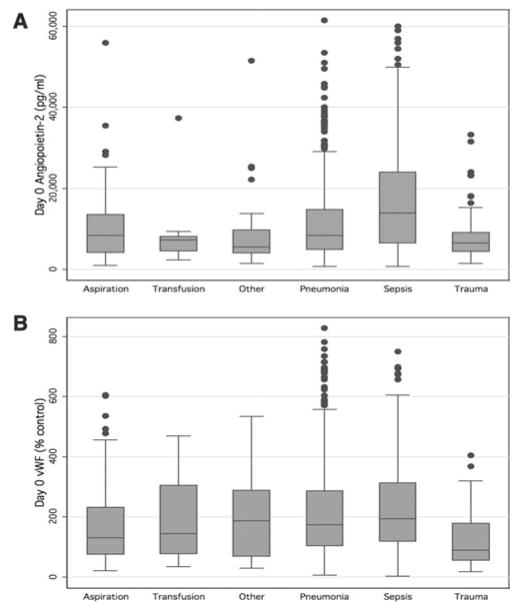
Plasma levels of both Ang-2 and vWF differed significantly depending on the clinical risk factor for ALI (Fig. 1). Specifically, levels of both Ang-2 and vWF were highest in patients with sepsis and lowest in patients with trauma after correction for multiple comparisons.
Figure 1.
Median plasma angiopoietin-2 (A) and von Willebrand factor (vWF) antigen (B) levels stratified by clinical risk factor for acute lung injury. Plasma angiopoietin-2 levels differed significantly based on risk factor for acute lung injury (p = .0001); clinical risk groups that differed significantly after correction for multiple comparisons were sepsis, trauma, and other. Plasma vWF levels also differed significantly based on risk factor for acute lung injury (p = .0001); clinical risk groups that differed significantly after correction for multiple comparisons were sepsis, trauma, and aspiration. Box plots depict median and interquartile range.
Association With Clinical Outcomes: Unadjusted Analyses
In the overall cohort of patients, elevated baseline plasma Ang-2 levels were significantly associated with mortality at 90 days (median Ang-2 in survivors 7854 pg/mL compared to nonsurvivors 10674 pg/mL; p < .0001). In an unadjusted logistic model, the odds ratio (OR) for mortality at 90 days for every 1-log increase in Ang-2 was 1.53 (95% confidence interval [CI] 1.27–1.83; p < .001). As expected, plasma vWF levels were also significantly associated with 90-day mortality (median in survivors 151% control compared to nonsurvivors 223%; p < .0001). In an unadjusted logistic model, the OR for mortality at 90 days for every 1-log increase in vWF was 1.76 (95% CI 1.43–2.16; p < .001). In a combined model including both Ang-2 and vWF as predictors of mortality, both remained independently predictive of mortality, although the effect of each was slightly attenuated (OR for 1-log increase in Ang-2, 1.35; p = .001; OR for 1-log increase in vWF, 1.60; p < .001).
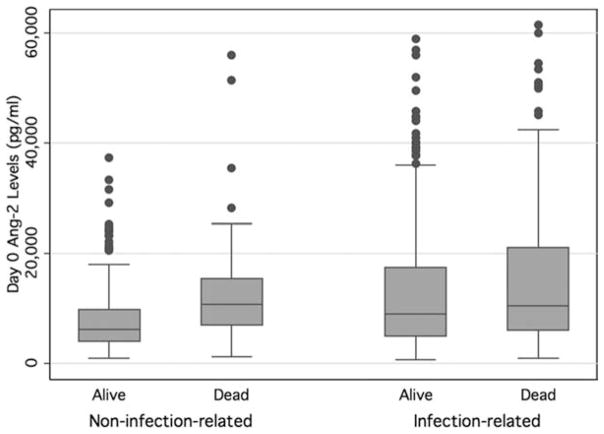
Because most of the previous evidence regarding the association between Ang-2 levels and clinical outcomes was derived from patients with infection-associated ALI (6,19,20), we tested whether the prognostic value of Ang-2 differed according to the presence of infection as the primary risk factor for ALI. In the logistic model, there was a statistically significant interaction between an infectious etiology of ALI (sepsis or pneumonia) and Ang-2 levels (p = .01 for interaction). Specifically, in patients with infection-related ALI (n = 653), the OR for mortality for every 1-log increase in Ang-2 levels was 1.30 (95% CI 1.06–1.59; p = .01), whereas in patients with noninfectious ALI (n = 278), the OR for mortality was 2.43 (95% CI 1.57–3.75, p < .001). When examined more closely, day 0 plasma Ang-2 values were similarly elevated in both survivors and nonsurvivors with infection-related ALI, whereas survivors with noninfection-related ALI had much lower levels (Fig. 2). As a result of this interaction, subsequent analyses of the relationship between Ang-2 and clinical outcomes were stratified by infection. There was no evidence of an interaction between an infectious etiology of ALI and plasma vWF levels.
Figure 2.
Median and interquartile ranges of plasma angiopoietin-2 (Ang-2) levels stratified by the presence of infection-related acute lung injury in survivors and nonsurvivors.
Because the relationship between plasma biomarker levels and mortality may not be linear, we tested the association between quartiles of Ang-2 and vWF and mortality (Table 2). The odds of mortality increased significantly across quartiles of vWF and, in noninfection-related ALI, across quartiles of Ang-2. There was no significant relationship between quartiles of Ang-2 and mortality for patients with infection-related ALI.
Table 2.
Quartiles of baseline angiopoietin-2 and von Willebrand factor and mortality in patients with acute lung injury
| Baseline Biomarker Level | Odds Ratio for Mortality (95% Confidence Interval) | p |
|---|---|---|
| Angiopoietin-2 (infection-related acute lung injury; n = 653) | ||
| First quartile | Referent | N/A |
| Second quartile | 1.29 (0.77–2.15) | .34 |
| Third quartile | 1.46 (0.89–2.40) | .14 |
| Fourth quartile | 1.62 (1.00–2.61) | .05 |
| Test for trend across quartiles | — | .24 |
|
| ||
| Angiopoietin-2 (noninfection-related acute lung injury; n = 278) | ||
| First quartile | Referent | N/A |
| Second quartile | 1.50 (0.60–3.78) | .39 |
| Third quartile | 4.54 (1.94–10.66) | .001 |
| Fourth quartile | 5.19 (2.00–13.46) | .001 |
| Test for trend across quartiles | — | .0002 |
|
| ||
| von Willebrand factor (n = 931) | ||
| First quartile | Referent | N/A |
| Second quartile | 1.44 (0.92–2.25) | .11 |
| Third quartile | 1.76 (1.13–2.73) | .01 |
| Fourth quartile | 3.33 (2.18–5.08) | <.001 |
| Test for trend across quartiles | — | <.0001 |
N/A, not applicable.
In contrast to the mortality analyses, increasing quartiles of Ang-2 were associated with a decreasing number of ventilator-free days for both infection and noninfection-related ALI (Table 3), although the relationship was stronger in noninfection-related ALI. Similarly, the number of ventilator-free days decreased significantly across increasing quartiles of vWF (p < .001 for trend across quartiles).
Table 3.
Quartiles of baseline angiopoietin-2 and von Willebrand factor and ventilator-free days in patients with acute lung injury
| Baseline Biomarker Level | Mean Change in Ventilator-Free Days (95% Confidence Interval) | p |
|---|---|---|
| Angiopoietin-2 (infection-related acute lung injury) | ||
| First quartile | Referent | N/A |
| Second quartile | −2.2 (−4.6 to 0.16) | .07 |
| Third quartile | −2.5 (−4.8 to −0.1) | .04 |
| Fourth quartile | −4.1 (−6.3 to −1.8) | <.001 |
| Test for trend across quartiles | — | .006 |
|
| ||
| Angiopoietin-2 (noninfection-related acute lung injury) | ||
| First quartile | Referent | N/A |
| Second quartile | −2.4 (−5.4 to 0.7) | .13 |
| Third quartile | −7.1 (−10.3 to −3.9) | <.001 |
| Fourth quartile | −5.7 (−9.6 to −1.8) | .004 |
| Test for trend across quartiles | — | .0001 |
|
| ||
| von Willebrand factor | ||
| First quartile | Referent | N/A |
| Second quartile | −0.5 (−2.4 to 1.4) | .59 |
| Third quartile | −1.9 (−3.8 to −0.03) | .046 |
| Fourth quartile | −4.6 (−6.5 to −2.7) | <.001 |
| Test for trend across quartiles | — | <.0001 |
N/A, not applicable.
Association With Clinical Outcomes: Multivariable Models
In a multivariable model controlling for fluid-conservative therapy as well as previously demonstrated predictors of mortality in ALI (age and Acute Physiology and Chronic Health Evaluation III score), day 0 plasma Ang-2 levels remained significantly associated with mortality in patients with noninfection-related ALI (Table 4). In patients with infection-related ALI, the association between baseline Ang-2 levels and mortality was not significant in the multivariable model. Day 0 plasma vWF remained strongly associated with mortality after controlling for the same covariates.
Table 4.
Multivariate analysis of baseline angiopoietin-2, baseline von Willebrand factor, and mortality
| Predictor | Odds Ratio for Mortality (95% Confidence Interval) | p |
|---|---|---|
| Angiopoietin-2 model, infection-related acute lung injury | ||
| Log angiopoietin-2 | 0.92 (0.73–1.16) | .48 |
| Age, per 1 yr | 1.02 (1.00–1.03) | .009 |
| APACHE III score, per 1 point | 1.03 (1.02–1.04) | <.001 |
| Fluid-conservative therapy | 0.76 (0.53–1.09) | .14 |
|
| ||
| Angiopoietin-2 model, noninfection-related acute lung injury | ||
| Log angiopoietin-2 | 1.94 (1.15–3.25) | .01 |
| Age, per 1 yr | 1.06 (1.03–1.08) | <.001 |
| APACHE III score, per 1 point | 1.03 (1.02–1.04) | <.001 |
| Fluid-conservative therapy | 1.53 (0.75–3.15) | .24 |
|
| ||
| von Willebrand factor model | ||
| Log von Willebrand factor | 1.51 (1.20–1.90) | <.001 |
| Age, per 1 yr | 1.03 (1.02–1.04) | <.001 |
| APACHE III score, per 1 point | 1.03 (1.02–1.03) | <.001 |
| Fluid-conservative therapy | 0.90 (0.65–1.24) | .51 |
APACHE, Acute Physiology and Chronic Health Evaluation.
Association With Clinical Outcomes: Change in Ang-2 Levels Over Time
Given previous data suggesting that a change in Ang-2 levels over time may be more prognostic than baseline levels alone (19), we compared the clinical outcomes of patients whose Ang-2 was higher on day 3 than on day 0 (termed “rising Ang-2”) to patients whose Ang-2 on day 3 was lower than on day 0 (termed “falling Ang-2”). In patients with infection-related ALI, a rising Ang-2 level was associated with more than double the odds of death (OR 2.29; 95% CI 1.54–3.43; p < .001), even after adjusting for age, Acute Physiology and Chronic Health Evaluation III score at baseline, and fluid-conservative strategy. No such association was detected in unadjusted or adjusted models for patients with noninfection-related ALI or for changes in vWF over the same time period. In addition, patients with rising plasma Ang-2 levels on day 3 had higher central venous pressures (11.7 vs. 10.1 mm Hg; p < .0001) and higher pulmonary artery diastolic pressures (22.4 vs. 20.0 mm Hg; p < .001), compared with patients with falling Ang-2 levels.
Of note, day 3 plasma Ang-2 levels were strongly associated with mortality in patients with both infection-related ALI (OR per 1-log increase, 1.64; 95% CI 1.32–2.03; p < .001) and noninfection-related ALI (OR 2.03; 95% CI 1.31–3.16; p = .002).
Effect of Fluid-Conservative Strategy
The impact of the fluid management strategy on the change in Ang-2 and vWF levels over the first 3 days of the study was measured using analysis of covariance. There were 849 patients with paired day 0 and day 3 samples available for analysis. Plasma Ang-2 levels declined over the first 3 days in both the fluid-conservative and fluid-liberal groups; however, there was a 13.2% greater decline in Ang-2 levels in the fluid-conservative group compared to the fluid-liberal group (p = .005; 95% CI 4.2%–21.3%). When this analysis was stratified by infection, based on the previously detected interaction, this effect of fluid-conservative therapy on Ang-2 levels over time was restricted to patients with infection-related ALI; specifically, in patients with infection-related ALI, there was a 15.3% greater decline in the fluid-conservative group compared to the fluid liberal group (p = .006; 95% CI 4.6%–24.8%; Table 5). The effect of fluid-conservative therapy on Ang-2 levels was particularly strong in patients who were not in shock on day 3 (and were therefore being actively managed by the fluid conservative protocol); in this group, there was a 19.9% greater decline in Ang-2 levels in patients receiving fluid-conservative therapy compared to patients receiving fluid-liberal therapy (p = .001; 95% CI 9.1%–29.4%). In contrast, the fluid management strategy had no effect on vWF levels over time (p = .15) or on Ang-2 levels over time in patients with noninfection-related ALI (p = .51).
Table 5.
Effect of fluid-conservative therapy on change in angiopoietin-2 levels over time
| Acute Lung Injury Risk Factor | Fluid Therapy | Angiopoietin-2 Day 0, pg/mL, Median (Interquartile Range) | Angiopoietin-2 Day 3, pg/mL, Median (Interquartile Range) | Effect of Fluid Conservative Therapy on Angiopoietin-2 Levels Over Timea | pa |
|---|---|---|---|---|---|
| Not infection-related | Conservative | 6824 (4107, 11628) | 5396 (2698, 9898) | −5.6% (12.1% to −20.5%) | .51 |
| Liberal | 7291 (4456, 11974) | 6360 (3493, 9648) | |||
| Infection-related | Conservative | 9640 (5230, 17594) | 7216 (3893, 13162) | −15.3% (−4.6% to −24.8%) | .006 |
| Liberal | 9541 (5539, 19052) | 8125 (4641, 15075) |
Compared to fluid-liberal therapy using analysis of covariance.
DISCUSSION
In this large multicenter cohort of patients with ALI attributable to a variety of causes, rising Ang-2 levels over time were strongly and independently predictive of poor outcomes in patients with infection-related ALI. Similarly, a fluid-conservative management strategy preferentially decreased Ang-2 levels over time in patients with infection-related ALI, suggesting that one mechanism of benefit of a fluid-conservative strategy may be decreased endothelial injury. Interestingly, higher baseline plasma Ang-2 levels were associated with poor clinical outcomes primarily in patients with noninfection-related ALI. This finding contrasts sharply with previous data in which baseline Ang-2 was strongly associated with outcomes in sepsis-related ALI.
Given the extensive published data describing high Ang-2 levels in patients with infection and specifically sepsis, we decided a priori to examine whether infection as a primary risk factor for ALI impacted the prognostic value of these markers. Whereas we found that the presence of infection as the primary risk factor for ALI affected the prognostic value of Ang-2, the direction of this effect was contrary to both our original hypothesis and to the majority of the published literature in this area, in that the prognostic value of Ang-2 was much stronger in noninfection-related ALI. Closer examination of the raw data reveals that baseline levels of Ang-2 are higher in patients with infection-related ALI compared to their noninfected counterparts but in both survivors and nonsurvivors (Fig. 2). Thus, baseline Ang-2 may have less prognostic value in this group because it is elevated regardless of the eventual clinical outcome. Of note, the association between baseline Ang-2 levels and the composite outcome of ventilator-free days (combining time on the ventilator with mortality) is statistically significant even in infection-related ALI, emphasizing that plasma Ang-2 does have some prognostic significance, albeit weaker, in this group of patients.
Why might our results differ so significantly from previous work, suggesting that baseline Ang-2 has significant prognostic value in patients with sepsis-related ALI? Several explanations are possible. First, Ang-2 may be similar to an acute phase reactant in infectious ALI, elevated but neither a mechanistic driver of illness nor a prognostic of outcome. Although possible, this explanation seems unlikely. Work by Parikh et al (6) shows that the Ang-2 protein itself can cause systemic and pulmonary capillary leak in animal models of sepsis. Furthermore, animals deficient in Ang-2 are resistant to hyperoxic lung injury (12). Such evidence supports the idea of Ang-2 as a mediator of illness as opposed to a marker of severity. A second possibility is that previous studies simply did not test for an interaction between the etiology of ALI and the prognostic value of Ang-2. The previous studies reporting an association between baseline Ang-2 levels and poor clinical outcomes in patients with ALI of mixed etiology do not report testing for interactions with either sepsis or infection as causes of ALI (10, 20, 21). A third possibility is that spectrum bias may be partially responsible for the differing results in our cohort compared to these previous studies; that is, previous studies that selected patients based on the presence of sepsis or septic shock may encompass a different severity of disease than patients in our study who were selected based on ALI. Finally, differences in the timing of plasma samples may have affected the prognostic value of Ang-2 levels in our cohort compared to previous studies. Day 3 Ang-2 levels were strongly predictive of clinical outcomes in patients with infection-related ALI in our cohort, as was an increasing Ang-2 level over time. Thus, it may be that our day 0 samples captured patients in a different stage of evolution of their illness compared with previous studies and thus have different prognostic value.
The finding that rising Ang-2 levels on day 3 more than doubled the odds of death in patients with infection-related ALI is particularly intriguing. The association between persistently high Ang-2 levels and increased mortality has been previously reported in a septic shock cohort (19). The finding that changes in levels of Ang-2 over time are both associated with higher intravascular pressures and highly predictive of outcomes could indicate that Ang-2 is a sensitive indicator of persistent endothelial injury unresponsive to conventional care. Whether serial Ang-2 levels might be used to risk-stratify ALI patients into responders and nonresponders to best care, help guide enrollment in clinical trials of novel therapeutics, or perhaps even serve as a surrogate outcome in therapeutic trials remains to be tested.
The finding that Ang-2 levels were preferentially reduced by a fluid-conservative management strategy confirmed one of our initial hypotheses and has important implications for future research. This finding suggests that the fluid-conservative management strategy is beneficial not only by its presumed primary effect of reducing lung water but also by decreasing endothelial injury. In addition, this result matches well with previous experimental work demonstrating that increases in hydrostatic pressure in the pulmonary vasculature can cause endothelial inflammation (15). Alternatively, decreases in pulmonary edema in the fluid-conservative group may have decreased transcapillary pressure in the alveoli, resulting in less endothelial injury. The finding that the effect of fluid-conservative therapy on Ang-2 levels over time was restricted to infection-related ALI is biologically plausible, because infection-related ALI is characterized by more profound endothelial activation with higher baseline Ang-2 and vWF levels compared to noninfection-related ALI. Thus, if fluid management exerts effects on endothelial injury via pulmonary hydrostatic pressure, then these effects would be expected to be most prominent in infection-related ALI.
Why changes in Ang-2 over time, and not vWF, track with outcome and fluid-conservative therapy in ALI remains an interesting biological question. Both are elevated at baseline when released from Weibel Palade bodies. Perhaps after initial release of preformed stores, their ongoing synthesis and release are differentially regulated in response to endothelial stress. Our work on this uncoupling of the vWF and Ang-2 levels when followed serially is consistent with the pattern observed in severe sepsis identified by van der Heijen (20). A better understanding of regulation of ongoing endothelial cell exocytosis in response to sepsis and ALI is needed to move beyond observation toward mechanistic understanding.
In conclusion, whereas higher levels of Ang-2 and vWF are predictive of higher mortality and fewer ventilator-free days in this large multicenter ALI cohort, the prognostic value of Ang-2 levels in patients with ALI differs based on the presence of infection. Furthermore, the prognostic value of Ang-2 also differs considerably based on the timing of Ang-2 measurement; rising levels of Ang-2 over time are a particularly poor prognostic sign in patients with infection-related ALI. Finally, our findings indicate that fluid-conservative therapy may be beneficial in part by decreasing endothelial inflammation in patients with infection-related ALI. Future studies should focus on whether serial Ang-2 levels may be able to serve as a surrogate outcome in clinical trials of novel therapies for ALI.
Acknowledgments
Supported, in part, by contracts (NO1-HR 46046-64 and NO1-HR-16146-54) with the National Heart, Lung, and Blood Institute (NHLBI). Dr. Calfee was supported by HL090833, by the Flight Attendant Medical Research Institute, and by KL2RR024130 from the NCRR, a component of the NIH. Dr. Matthay was supported by HL 51856.
We thank the National Heart, Lung, and Blood Institute, A.L. Harabin, D. Gail, P. Lew, and M. Waclawiw.
Footnotes
The remaining authors have not disclosed any potential conflicts of interest.
For information regarding this article, carolyn.calfee@ucsf.edu
References
The following persons and institutions participated in the FACTT trial:
Steering Committee Chair: G.R. Bernard; Clinical Coordinating Center: D.A. Schoenfeld, B.T. Thompson, N. Ringwood, C. Oldmixon, F. Molay, A. Korpak, R. Morse, D. Hayden, M. Ancukiewicz, A. Minihan; Protocol-Review Committee—J.G.N. Garcia, R. Balk, S. Emerson, M. Shasby, W. Sibbald; Data Safety and Monitoring Board: R. Spragg, G. Corbie-Smith, J. Kelley, K. Leeper, A.S. Slutsky, B. Turnbull, C. Vreim; ARDS Clinical Trials Network Consultant: P. Parsons; Clinical Centers are as follows: University of Washington, Harborview: L. Hudson, K. Steinberg, M. Neff, R. Maier, K. Sims, C. Cooper, T. Berry-Bell, G. Carter, L. Andersson; University of Michigan: G.B. Toews, R.H. Bartlett, C. Watts, R. Hyzy, D. Arnoldi, R. Dechert, M. Purple; University of Maryland: H. Silverman, C. Shanholtz, A. Moore, L. Heinrich, W. Corral; Johns Hopkins University: R. Brower, D. Thompson, H. Fessler, S. Murray, A. Sculley; Cleveland Clinic Foundation: H.P. Wiedemann, A.C. Arroliga, J. Komara, T. Isabella, M. Ferrari; University Hospitals of Cleveland: J. Kern, R. Hejal, D. Haney; MetroHealth Medical Center: A.F. Connors; University of Colorado Health Sciences Center: E. Abraham, R. McIntyre, F. Piedalue; Denver Veterans Affairs Medical Center: C. Welsh; Denver Health Medical Center: I. Douglas, R. Wolkin; St. Anthony Hospital: T. Bost, B. Sagel, A. Hawkes; Duke University: N. MacIntyre, J. Govert, W. Fulkerson, L. Mallatrat, L. Brown, S. Everett, E. VanDyne, N. Knudsen, M. Gentile; University of North Carolina: P. Rock, S. Carson, C. Schuler, L. Baker, V. Salo; Vanderbilt Universit: A.P. Wheeler, G. Bernard, T. Rice, S. Bozeman, T. Welch; University of Pennsylvania: P. Lanken, J. Christie, B. Fuchs, B Finkel, S. Kaplan, V. Gracias, C.W. Hanson, P. Reilly, M.B. Shapiro, R. Burke, E. O’Connor, D. Wolfe; Jefferson Medical College: J. Gottlieb, P. Park, D.M. Dillon, A. Girod, J. Furlong; LDS Hospital: A. Morris, C. Grissom, L. Weaver, J. Orme, T. Clemmer, R. Davis, J. Gleed, S. Pies, T. Graydon, S. Anderson, K. Bennion, P. Skinner; McKay-Dee Hospital: C. Lawton, J. d’Hulst, D. Hanselman; Utah Valley Regional Medical Center: K. Sundar, T. Hill, K. Ludwig, D. Nielson; University of California, San Francisco: M.A. Matthay, M. Eisner, B. Daniel, O. Garcia; San Francisco General: J. Luce, R. Kallet; University of California, San Francisco, Fresno: M. Peterson, J. Lanford; Baylor College of Medicine: K. Guntupalli, V. Bandi, C. Pope; Baystate Medical Center: J. Steingrub, M. Tidswell, L. Kozikowski; Louisiana State University Health Sciences Center: B. deBoisblanc, J. Hunt, C. Glynn, P. Lauto, G. Meyaski, C. Romaine; Louisiana State University Earl K. Long Center: S. Brierre, C. LeBlanc, K. Reed; Alton-Ochsner Clinic Foundation: D. Taylor, C. Thompson; Tulane University Medical Center: F. Simeone, M. Johnston, M. Wright; University of Chicago: G. Schmidt, J. Hall, S. Hemmann, B. Gehlbach, Vinayak, W. Schweickert; Northwestern University: J. Dematte D’Amico, H. Donnelly; University of Texas Health Sciences Center: A. Anzueto, J. McCarthy, S. Kucera, J. Peters, T. Houlihan, R. Steward, D. Vines; University of Virginia: J. Truwit, A.F. Connors, M. Marshall, W. Matsumura, R. Brett; University of Pittsburgh: M. Donahoe, P. Linden, J. Puyana, L. Lucht, A. Verno; Wake Forest University: R.D. Hite, P. Morris, A. Howard, A. Nesser, S. Perez; Moses Cone Memorial Hospital: P. Wright, C. Carter-Cole, J. McLean; St. Paul’s Hospital, Vancouver: J. Russell, L. Lazowski, K. Foley; Vancouver General Hospital: D. Chittock, L. Grandolfo; Mayo Foundation: M. Murray.
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Ochoa CD, Wu S, Stevens T. New developments in lung endothelial heterogeneity: Von Willebrand factor, P-selectin, and the Weibel-Palade body. Semin Thromb Hemost. 2010;36:301–308. doi: 10.1055/s-0030-1253452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romani de Wit T, Rondaij MG, Hordijk PL, et al. Real-time imaging of the dynamics and secretory behavior of Weibel-Palade bodies. Arterioscler Thromb Vasc Biol. 2003;23:755–761. doi: 10.1161/01.ATV.0000069847.72001.E8. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DB, Wiener-Kronish JP, Murray JF, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flori HR, Ware LB, Milet M, et al. Early elevation of plasma von Willebrand factor antigen in pediatric acute lung injury is associated with an increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2007;8:96–101. doi: 10.1097/01.PCC.0000257097.42640.6F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 8.Orfanos SE, Mavrommati I, Korovesi I, et al. Pulmonary endothelium in acute lung injury: From basic science to the critically ill. Intensive Care Med. 2004;30:1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- 9.Ricciuto DR, Dos Santos CC, Hawkes M, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39:702–710. doi: 10.1097/CCM.0b013e318206d285. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29:656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer NJ, Li M, Feng R, et al. ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med. 2011;183:1344–1353. doi: 10.1164/rccm.201005-0701OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhandari V, Choo-Wing R, Lee CG, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiedemann HP, Wheeler AP, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler AP, Bernard GR, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 15.Kuebler WM, Ying X, Singh B, et al. Pressure is proinflammatory in lung venular capillaries. J Clin Invest. 1999;104:495–502. doi: 10.1172/JCI6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 17.Eisner MD, Thompson T, Hudson LD, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 18.Vittinghoff E, Glidden D, Shiboski SC, et al. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. NewYork: Springer Science+Business Media; 2005. [Google Scholar]
- 19.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, et al. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med. 2009;35:1567–1574. doi: 10.1007/s00134-009-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63:903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 21.Ong T, McClintock DE, Kallet RH, et al. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med. 2010;38:1845–1851. doi: 10.1097/CCM.0b013e3181eaa5bf. [DOI] [PMC free article] [PubMed] [Google Scholar]