Abstract
Antigen specific memory T cells (Tm) have shown to be an important factor in protecting hosts against subsequent infection by previously encountered pathogens. During T-cell activation, several cytokines including IL-6, IL-7 and IL-15, play crucial roles in the development of T cells into memory T cells. With the aim of generating specific Tm, we examined a strategy of sequential administration of molecular adjuvants. In this strategy a DNA vaccine encoding the VP1 capsid protein of foot and mouth disease virus (designated pcD-VP1) was co-delivered to mice along with an IL-6 expressing plasmid (pVAX-IL-6) as an initial molecular adjuvant and boosted with either an IL-7 or IL-15 expressing plasmid, (pVAX-IL-7 or proVAX-IL-15) as the secondary adjuvant. During the pcD-VP1 immunization, we demonstrated that the groups primed with IL-6 and boosted with either IL-7 or IL-15 resulted in the enhancement of cellular and humoral immune responses, maturation of dendritic cells (DCs) and macrophages, and a higher frequency of CD4+ Tm (characterized by expressing CD44highCD62Llow markers, compared with the other groups). Thus, we took advantage of the different effects of cytokines on T cell development, not only to induce a higher level of immune responses after vaccination, but also to generate a higher ratio of CD4+ Tm in this sequential cytokine prime-boost study. This would then lead to the mounting of an effective long-term antigen specific immune response.
Keywords: CD4+ T memory cell, DC maturation, FMDV DNA vaccine, IL-15, IL-6, IL-7, cytokine adjuvant, macrophage
Introduction
Following an immune response, most effector cells die but some of them survive and develop into long-lived memory cells.1 Memory T cells are critical in controlling most viral and bacterial infections, although antigen specific antibody titers often correlate well with protective immunity.2,3 The cellular basis of immunological memory has recently begun to be dissected, although observations of long-term protection against specific pathogens have been noted previously.2,4-6 T cell memory is an active process that regulates the preservation of specific memory T cell (Tm) populations in a dynamic environment.2 Naive CD4 T cells experience rapid cell divisions after activation, and following differentiate to become effectors that exert powerful activity through direct interactions with other cells or by the secretion of cytokines and/or chemokines. After antigen clearance, the majority of effectors die via apoptosis, while a small population reverts to a resting state and become memory cells.7
The size of the peripheral T cell pool is tightly controlled through cell survival, division, and death.8,9 T cells have an indispensable function in the control of most viral infections, and data have demonstrated that the maintenance of CD8-T cell memory is regulated by at least two cytokines, IL-7 and IL-15, which support survival (i.e., IL-7, IL-15) and basal homeostatic proliferation (i.e., IL-15) of specific CD8- memory T cells (TM).9,10 IL-7 is a pleiotropic cytokine which functions as a required stimuli for both survival and proliferation of peripheral T cells.9 IL-7 controls basal homeostatic proliferation, differentiation and survival of specific CD4+ Tm and has also been demonstrated to have diverse effects on differentiation, growth and function of CD8+ Tm. Memory CD4 cells become acutely dependent on IL-7 for homeostatic proliferation when TCR signaling is abolished.11 As for the mechanism, data have shown that the anti-apoptotic effects of these factors has been largely attributed to IL-7 regulation of the balance of pro-apoptotic molecules of the Bcl-2 family such as Bax, Bad, Bak and Bim vs. anti-apoptotic members such as Bcl-xL, Mcl-1 and Bcl-2.9 Data have also shown that IL-7 protects T cells from death through the induction of the anti-apoptotic proteins Bcl-212 and Mcl-1,13 and by the inhibition of pro-apoptotic proteins Bax,14 Bad,15 and Bim.16 IL-15 inhibits CD95/Fas-induced apoptosis of human CD4+ and CD8+ effector-memory T cells17 and thus stimulates the proliferation of memory T cells.18
IL-6 is a multifunctional cytokine produced by both professional APCs (i.e., B cells, macrophages, and dendritic cells) as well as non-professional APCs (epithelial, endothelial, and some tumor cells).19,20 It is most effective in inducing a robust expansion of responding naïve T cells in vitro among its many other biological functions. Thus, IL-6 plays important roles in the initiation of innate immunity and T cell-mediated immune responses.20-22
Considering the specific function of cytokine IL-6, IL-7 and IL-15 in immunity, we, in this study, hypothesized that immunized mice with IL-6 as an adjuvant and boosted with IL-7 or IL-15 as molecular adjuvants might enhance the frequency of CD4+ T memory. Therefore, the advantages of two different cytokines used sequentially are not only to enhance immune responses after viral challenge but also to enhance the frequency of CD4+ Tm resulting in the eradication of the virus. We attempted to use a eukaryotic construct encoding IL-6, IL-7 or IL-15 as molecular adjuvants to co-inoculate with the FMDV VP1 DNA vaccine and evaluated their roles in affecting the frequency of CD4+ T memory cell and anti-apoptotic molecules. We also evaluated the effects of these cytokines as adjuvants on enhancing both humoral and cellular responses in the immunized mice and the maturation of DCs and macrophages, which may correlate to innate and adaptive immune stimulations. As the results indicate, compared with other cytokine adjuvants combination groups, the groups primed with IL-6 and boosted with IL-7 or IL-15 respectively as molecular adjuvants administered with the FMDV VP1 DNA vaccine not only enhanced cellular and humoral immune responses but also induced a higher frequency of CD4+ Tm, resulting in enhanced immunity and eradication of the virus.
Results
Expression of recombinant cytokine plasmids in BHK cells
To confirm the expression of construct pVAX-IL-7 in a eukaryotic system, the plasmid was transfected into BHK cells. Total RNA was extracted from transfected cells at 48 h and analyzed by RT-PCR in the presence of a specific set of primers. The predicted RT-PCR product was of 465 bp in size for IL-7, and was confirmed by gel electrophoresis. No specific band of a similar size was noted in any of the mRNA samples in the absence of reverse transcriptase (Fig. 1). This result indicated that the construct encoding the protein could be successfully expressed in the eukaryotic system.
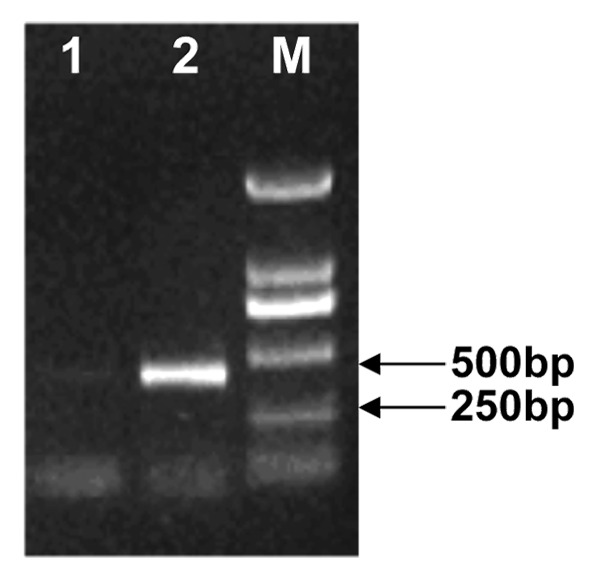
Figure 1. Eukaryotic expression of pVAX-IL-7 in BHK cells. Total RNA was extracted from BHK cells 48 h after transfection with pVAX-IL-7. Templates used for RT-PCR were as follows: lane 1, non-transfected BHK cells as a negative control; lane 2, cDNA from the transfected BHK cells with RTs; M: DNA marker DL2000.
Cytokine as molecular adjuvants increase the humoral response
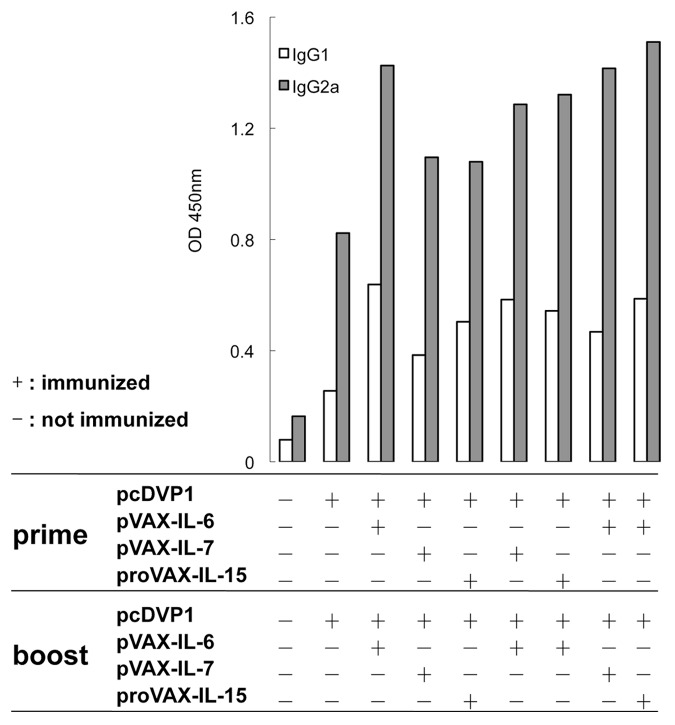
To examine the effect of molecular adjuvants on the humoral responses in mice, the pcD-VP1 was intramuscularly inoculated alone or co-inoculated either with the pVAX-IL-6, pVAX-IL-7 or proVAX-IL-15, respectively. On day 7 after the second immunization, the serum samples were collected and the amount of IgG1 and IgG2a antibodies against FMDV were detected by the ELISA. Compared with the group immunized with pcD-VP1 alone, a significantly enhanced level of IgG2a was obtained in the groups immunized with pcD-VP1 plus cytokine adjuvant(s) (Fig. 2). Among all the experimental groups, the humoral responses in the groups primed with IL-6 and then boosted either with IL-6, IL-7 or IL-15 as adjuvant were the most significantly enhanced. The data indicated that cytokine molecules in combination with DNA vaccine could improve the humoral responses in mice. The enhanced level of IgG2a suggests that the class switch of immunoglobulin is influenced by those adjuvants.
Figure 2. Effects of adjuvants on the level of antibodies. Balb/c mice were immunized with pcD-VP1 along with different cytokine adjuvants intramuscularly in a prime-boost fashion as indicated on the x-axis. Serum samples were collected and diluted for detection by ELISA on day 7 after the boost. VP1 antigen at 2 μg/ml was coated on each well in 96-well plate. The OD values of IgG2a and IgG1 (diluted by 50 folds) were read under wavelength of 450nm.
Adjuvant effects of cytokines on T cell proliferative response
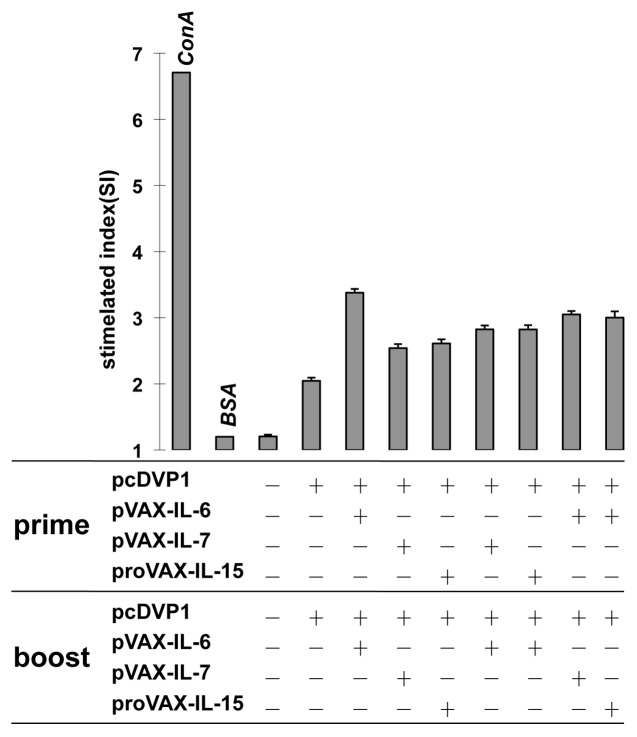
To further determine whether the expression of cytokine genes could affect cell-mediated immunity, a single-cell suspension of lymphocytes was prepared from immunized mice on day 7 after the second immunization, and re-stimulated in culture with VP1 as specific antigen, BSA as irrelevant antigen, Con A as a positive control, and medium as a negative control. As shown in Figure 3, the inoculation of the cytokine genes during immunization resulted in significantly higher levels of T cell proliferative response than that induced by pcD-VP1 alone, while the group inoculated with the IL-6 gene twice as an adjuvant was the most significantly affected. This result suggests that these cytokine adjuvants could enhance antigen-specific T cell responses.
Figure 3. Effect of adjuvants on T cell proliferations. Balb/c mice were immunized with pcD-VP1 with cytokine adjuvants intramuscularly in the prime-boost fashion as indicated on x-axis. T cells were isolated from animals (3 per group) and stimulated with VP1 protein as specific antigen, ConA as positive control, or BSA as negative control. Proliferation was analyzed by the MTT dye method and expressed as a stimulation index.
Adjuvant effects on DC maturation
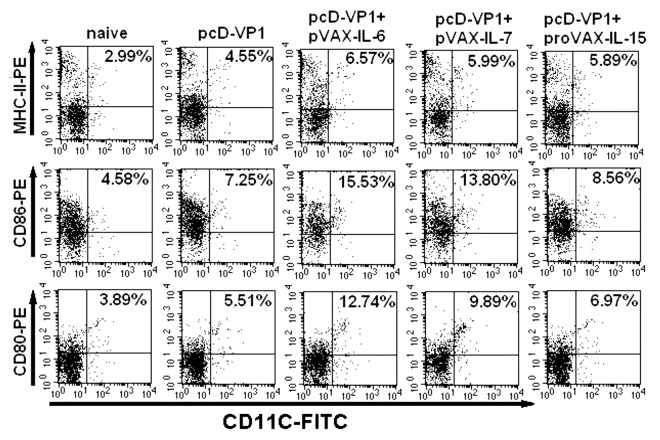
The maturation or activation of DC cell plays a critical role in the initiation of both innate and adaptive immune responses and have a significant impact on the naive T cell responses to microbial pathogens. Thus, we sought to determine the effect of cytokines (IL-6, IL-15 and IL-7) on the DCs maturation by detecting the expression of the surface co-stimulatory molecules at 48 h after the immunization. Compared with other groups, mice immunized with pcD-VP1 plus pVAX-IL-6 induced the highest levels of CD80, CD86 and MHC-II expression on DC cells (Fig. 4A and B), suggesting that IL-6 induces maturation of DC cells more efficiently, which might initiate the innate system and mount the subsequent adaptive immune responses more effectively.
Figure 4. Analysis of antigen-specific cytokine production in T cells by FACS. (A) T cells isolated from the spleen of Balb/c mice of the different groups at 48 h after the first immunization. Intracellular stainings for CD80+CD11c+, CD86+CD11c+ and MHC-II+CD11c+ cells were performed. The percentages of cytokine-positive cells are shown in each dot-plot and the gates were set on CD11c+ T cells or events. Data shown are representative from three independent experiments. (B) Total CD11c+ positive cells expressing CD80, CD86 and MHC-II are summarized.
Effects of adjuvants on cytokine profile of macrophages
Macrophages play a crucial role in innate and adaptive immune responses. To detect the effects, macrophages were isolated and analyzed for the expression of cytokines IFN-γ,IL-6,TNF-α,TGFβ,IL-10 and co-stimulatory molecule CD86 from mice 48h after the first immunization. Compared with the DNA vaccine alone, the group administered the cytokine adjuvants upregulated the levels of mRNA for IFN-α,IL-6,TNF-α and CD86 and slightly downregulated the level of mRNA for cytokines IL-10 and TGFβ (Fig. 5). Interestingly, the group co-immunized with pcD-VP1 and pVAX-IL-6 made the most significant changes in the profile mentioned above. These results suggested that inoculations with IL-7 or IL-15 expressing plasmids could activate macrophages, however, the addition of IL-6 gene not only potently induced the maturation and activation of macrophages, but could also mount an anti-suppressive effect against anti-inflammatory responses.
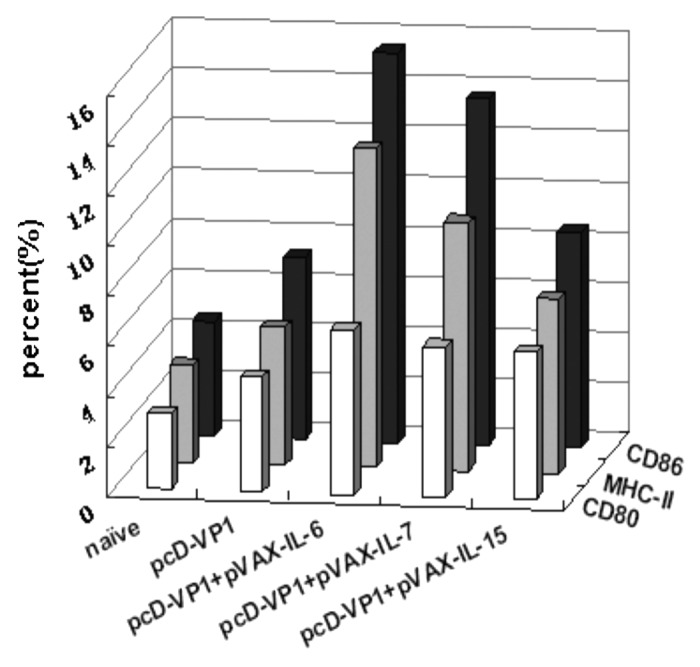
Figure 5. Effect of adjuvants on the cytokine expressions in macrophages. RNAs were extracted macrophages derived from immunized mice at the 48h after the first immunization. The expression of the cytokines IFN-α, IL-6, TNF-α, TGFβ, IL-10 and co-stimulatory molecule CD86 were analyzed by RT-PCR and run on 1% agarose gel (left panel) and relative concentrations to HPRT were plotted (right panel).
Taken together, as depicted in Figures 4 and 5, both DCs and macrophages are activated by the co-administrations with cytokine adjuvants pVAX-IL6, pVAX-IL7 and proVAX-IL15, of which will elicit potent aforementioned immune responses.
Adjuvant effects of cytokine molecules on the generation of CD4+ memory
T cell specific memory responses have been demonstrated as an important factor in protecting hosts against subsequent infection by previously exposed pathogens. We next directly assessed the number of memory CD4+ T cells bearing the CD44high CD62Llow markers. As depicted in Figure 6, 100 d after the second immunization, the group immunized with pVAX-IL-6 and boosted with pVAX-IL-7 as adjuvants induced the highest expression level of CD44highCD62Llow in the CD4+ T cells among all the groups, followed by the group immunized with pVAX-IL-6 and boosted with pVAX-IL-15 as adjuvants. In addition to a comparison to the group immunized with pcD-VP1 alone, we observed the higher expressing level of CD44highCD62Llow within CD4+ T cells was from the groups co-administrated with pcD-VP1 prime-boost with the heterologous cytokine adjuvants. These results are consistent with our hypothesis that the administration of pVAX-IL-6 during primary inoculation and boosting with heterologous adjuvant pVAX-IL-7 or proVAX-IL-15 could be more potentially facilitate antigen-specific recalled immune responses and thus to induce a greater protection.
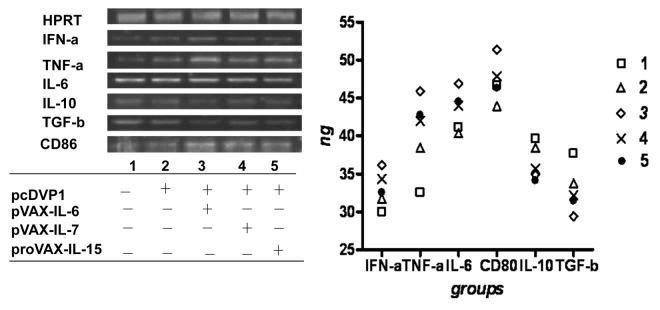
Figure 6. Effect of adjuvants on the generation of CD4+ T memory cells. (A) analysis of the number of CD4+ T memory cells bearing the CD44highCD62Llow markers from mice at 100 d after the second immunization by FACS. The percentages of cytokine-positive cells are shown after the gates set on CD4+ T cells from the total splenocytes. (B) the percentage of CD44highCD62Llow T cells in total CD4 cells are summarized and shown.
Expression of memory T cell associated genes
Having demonstrated the population of antigen specific memory CD4+ T cells in the adjuvant groups, we next tested the expression of memory T cell associated genes by RT-PCR 100 d after the second immunization. As shown in Figure 7, the group immunized with pVAX-IL-6 and boosted with the heterologous adjuvant pVAX-IL-7 or proVAX-IL-15 induced the highest level of mRNAs for BCL-2, Spi2A, IL-15Rα, IL-7-Ra and BCL-XL when compared with other groups. It suggests that cytokines enhanced the frequency of CD4+ Tm cells was in part through the upregulation of anti-apoptotic gene expressions.
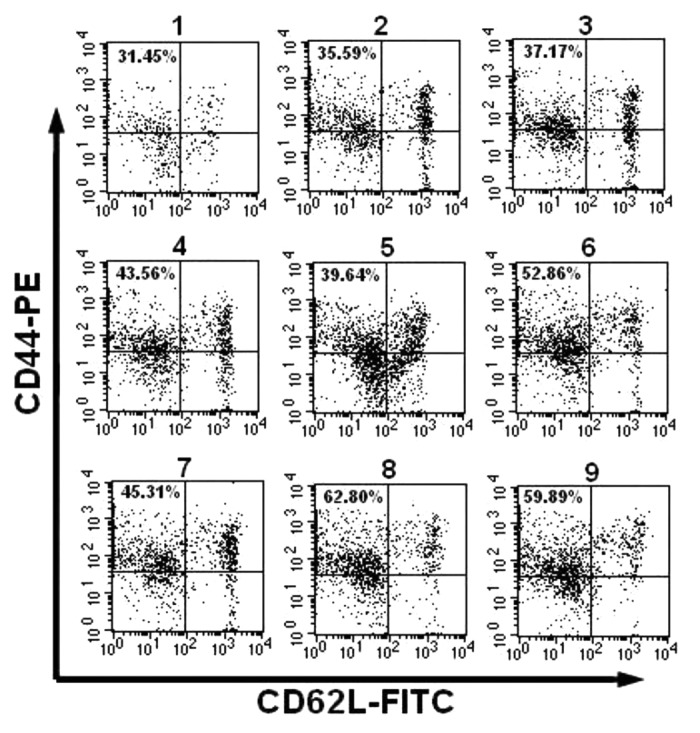
Figure 7. The expression of memory T cell associated genes (anti-apoptotic genes) by RT-PCR. RNAs were extracted from splenocytes of mice on day 100 after the second immunization and used to perform RT-PCR with specific primers. The RT-PCR results were analyzed by running on 1% agarose gel (left panel) and relative concentrations to HPRT were plotted (right panel).
Discussion
T cell memory has been demonstrated as an important factor in protecting the hosts against subsequent infection of previously encountered pathogens. It has been demonstrated that cytokines are crucial factors for T-cell development, survival and activation. IL-6, as a pleotropic cytokine modulates many aspects of immune responses,20,21 hemopoiesis and is a cofactor for T cell proliferation as well as mediating the prevention of cell death. IL-7, identified as a dominant cytokine, and IL-15, an accessory cytokine, regulate basal homeostatic proliferation and survival of antiviral CD4+T memory cells.2 Considering each specific function for IL-6, IL-7 and IL-15 in the immune system, we hypothesized that primary inoculation with IL-6 and boosting with heterologous adjuvant IL-7 or IL-15 during the DNA vaccinations should not only augment immune responses, but also enhance the ratio of CD4+ Tm. The results of this study demonstrates that higher humoral and cellular immune responses were achieved, and importantly, the higher ratio of CD4+ T memory cells were observed with the association of such cytokine prime-boost protocol.
The enhanced adaptive responses are likely linked to the better activation and maturation of DC cells and macrophages during the initial IL-6 facilitation, since we have demonstrated that IL-6 upregulated the level of mRNA for cytokines IFN-α, IL-6, TNF-α and CD86 (Fig. 5). Interestingly, the upregulation of innate immune signals was in a reciprocal relationship with the level of IL-10 and TGFβ expressions. IL-10 and TGFβ have been recently characterized to be anti-inflammatory cytokines and exert suppressive functions against elicited immune responses.28 Their levels are tightly associated with the generation of Treg cells and induction of immunotolerance in the periphery.29 The IL-6 counteracts tolerogenic responses derived from IL-10 and TGFβ and therefore, facilitates innate immune responses.
Since IL-7 and IL-15 have been demonstrated as important factors during the expansion and development of effector T cells into Tm cells, it is logical to use them in sequential applications during the DNA vaccinations by initiating innate immunity with IL-6 at the primary inoculation and boost with IL-7 or IL-15 to assist in the development and expansion of effector T cells. This hypothesis appears to be correct as we observed that the group immunized with pVAX-IL-6 and boosted with pVAX-IL-7 produces the highest expression of CD44high CD62Llow in activated CD4+ T cells in all the groups, followed by the group immunized with pVAX-IL-6 and boosted with pVAX-IL-15. Furthermore, this enhanced ratio of Tm cells is further supported by the observation of induction of higher level of expressions for BCL-2, Spi2A, IL-15Rα, IL-7-Ra and BCL-XL within the T effectors in these groups on days 100 after the second immunization (Fig. 7). The upregulation of anti-apoptotic factors Spi2A, BCL-2 and BCL-XL have been demonstrated to play pivotal role in survives of CD4+T memory cells.30 In general, activated effector cells are highly susceptible to apoptosis, including triggering of both activation-induced cell death (AICD) and passive mechanisms induced by withdrawal of growth/survival factor or nutrients referred to as programmed cell death (PCD).31 Since IL-7 and IL-15 share a common receptor to activate STAT5 via Jak3, the activated STAT5 is strongly implicated in cell survival via the control of the Bcl2 family members.32,33 The result demonstrated in Figure 7 suggests that improved survival might be ascribed to an anti-apoptotic function owning to the expression of inoculated cytokines. Therefore, these cytokines play a role in regulating T effector cell survival by counteracting the pro-apoptotic members of the Bcl2 family.
Although we demonstrated that the cytokine sequential prime-boost strategy with the IL-6 in combination with IL-7 or IL-15 could enhance the ratio of CD4+ T memory cells, it is unclear if such a high ratio of CD4+ T memory cells could lead to an enhanced protection against viral challenge Therefore, further investigation on this issue is warranted.
Materials and Methods
Animal, cell and peptide
Female Balb/c mice aged 6–8 weeks were purchased from the Animal Institute of Chinese Medical Academy and were provided with free access to food and water ad libitum. The FMDV VP1 peptide of a T cell epitope (aa133–147, SSKYGDTSTNNVRGD) was synthesized by GL Biochem Co., Ltd.
Antibodies and fluorescent dye
Fluorescent conjugated rat anti-mouse monoclonal antibodies including anti-IL-4-PE, anti-IFN-γ-FITC, anti-CD4-FITC, anti-CD4-PE, anti-CD4-APC, anti-CD44-PE, anti-CD8-PE, anti-CD11c-FITC, anti-CD80-PE, anti-CD86-PE, anti-MHC-II-PE, anti-CD62L- FITC (eBiosciences, Inc.) and isotype controls were purchased from BD PharMingen.
Plasmid constructions
The plasmid pcD-VP1 encoding for the FMDV VP1 protein was constructed in our lab as described previously.23 Mouse IL-6, IL-15 and TNF-α genes were constructed and described previously.22 The mouse IL-7 gene, including a signal sequence, was amplified by RT-PCR from mRNA isolated from spleen cells (GeneBank accession number NM_008371 for IL-7) and cloned into a pMD18-T vector (TaKaRa). The cDNA was further subcloned into a pVAX1 vector (Invitrogen), and designated as pVAX-IL7. All the plasmids were maxi-prepared by the alkaline method and purified by Qiagen Maxi prep according to the manufacturer’s instruction (QIAGEN China Co., Ltd.), subsequently diluted in saline solution.
Transfection of the BHK cell lines
The purified plasmid pVAX-IL7 was transfected into BHK cells with lipofectamine according to the manufacturer’s instructions (Invitrogen). The transfected cells were harvested after 48 h and total cellular RNA was extracted according to a previously described protocol.25 The expression of cytokine molecules was detected by RT-PCR with a specific primer set for the IL-7 gene as listed: IL-7, forward: 5′-CGGGATCCATGGAGTGCCACATTAAAG-3′, reverse: 5′-GCCGTCGACTGTCCTGTTTATATACT-3′. The PCR product was examined electrophorectically for proper size on a 1% agarose gel.
Immunization
Female mice at 6–8 weeks old were randomly divided into 9 groups, 12 animals per group. They were immunized intramuscularly with pcD-VP1 alone or co-administered with the respective molecular adjuvant(s) listed in Table 1. Administration of plasmids was performed on days 0 and 14. Sera samples were collected before and after immunizations.
Table 1. Immunization groups.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prime |
pcDVP1 |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| pVAX-IL-6 |
- |
- |
+ |
- |
- |
- |
- |
+ |
+ |
|
| pVAX-IL-7 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
| proVAX-IL-15 |
- |
- |
- |
- |
+ |
- |
+ |
- |
- |
|
| Boost | pcDVP1 |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| pVAX-IL-6 |
- |
- |
+ |
- |
- |
+ |
+ |
- |
- |
|
| pVAX-IL-7 |
- |
- |
- |
+ |
- |
- |
- |
+ |
- |
|
| proVAX-IL-15 | - | - | - | + | - | - | - | + |
Note: Immunizations were performed on days 0 and 14 with 100ug of vaccine or adjuvant per mouse intramuscularly. +, immunized; -, not immunized.
Detection of anti-FMDV antibody
Mice were bled on day 7 after the second immunization. An ELISA was performed following a previously published procedure. The optical density (OD) values of IgG2a and IgG1 were detected by ELISAs as previously described25; Peroxidase-labeled rat anti-mouse IgG1 and IgG2a (Southern Biotechnology Associates) were used for the enzymatic reaction and detections.
T cell proliferation
Three mice from each group were sacrificed and single lymphocyte suspensions were prepared from the spleen on day 7 after the second injection as described previously,26 and in vitro stimulation was used for 48 hrs with 10 μg/ml of Con A (positive control), 10 μg/ml of the VP1 protein (specific antigen stimulation), 5 μg/ml of BSA (irrelevant antigen) or no antigen (negative control). T cell proliferation was evaluated by Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay according to the manufacturer’s instruction (Promega). MTT solution (10 μl each well, 5 mg/ml) was added to each well for color development. After 4 h of incubation, the OD values of plates were read at 595 nm (Magellan, Tecan Austria GmbH). Data were expressed as stimulation index (SI), calculated as the mean reading of triplicate wells of antigen stimulation, divided by the mean reading of triplicate wells of the negative control.
Co-stimulatory molecules staining of DC cells
Three mice from each group were sacrificed at 48h after the first immunization. Single cell suspensions from the spleens were made at 2 × 106 cells/200 μl and were blocked with 2 μl of FcγmAb (0.5 μg/ml) for 30 min at 4°C. After washing with PBS, cells were stained with isotype controls, or double stained with anti-CD11c-FITC and anti-CD80-PE, or anti-CD11c-FITC and anti-CD86-PE, or anti-CD11c-FITC and anti-MHC-II-PE. The fluorescent intensities were measured by the FACS Calibur and analyzed by Cell Questpro software (BD Biosciences).
Determination of cytokine expression by RT-PCR
The expression level of cytokines was determined by semi-quantitative RT-PCR as previously described.25 Total RNA was extracted from T cells of mice on day 100 after the second immunization or from macrophages 48h after the first immunization. cDNA was synthesized using AMV reverse transcriptase and a (dT)18 primer which served as a template for the subsequent PCR reaction, which was performed with optimized primers specific for hypoxanthine phosphoribosyl transferase (HPRT), a housekeeping gene, or for cytokine genes.27 The sequences of the primers and conditions for PCRs are listed in Table 2 and Table 3, respectively. The PCR products were resolved on 2% agarose gels and visualized by ethidium bromide (EtBr) staining under UV light. Bands were analyzed by image process software (Alphamager 2200 winzk, Bio-Rad).
Table 2. Target anti-apoptotic gene primers and parameters of the PCR.
| Target gene | Primers | Parameters |
|---|---|---|
| HPRT |
5′: 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ 3′: 5′-GAGGGTAGGCTGGCCTATGGCT-3′ |
94°C 30s, 60°C 30s, 72°C 40s |
| Spi2A |
5′: 5′-CCCCTTTGACCCGAATGA-3′ 3′: 5′-CTTCCTGAGTTGGGCATC-3′ |
94°C 30s, 53.5°C 30s, 72°C 40s |
| BCL-XL |
5′: 5′-CGATGAGTTTGAACTGCG-3′ 3′: 5′-CACCTAGAGCCTTGGATCC-3′ |
94°C 30s, 55°C 30s, 72°C 40s |
| BCl-2 |
5: 5′-GGCTACGAGTGGGATGCT-3′ 3′: 5′-GGGTCATGTGTGTGGAGAG-3′ |
94°C 30s, 55°C 30s, 72°C 40s |
| IL-7Ra |
5”: 5′-CCGATCCATTCCCCATAACG-3′ 3′: 5′-ATGAGGTGAAAGGCGTTGAA-3′ |
94°C 30s, 56°C 30s, 72°C 40s |
| IL-15Ra | 5′: 5′-GCTCCAGGCTGACACCATC-3′ 3′: 5′-TCTCAGCCGTGCCGTGT-3′ |
94°C 30s, 62.5°C 30, 72°C 40s |
Table 3. Target cytokines and co stimulatory gene primers and parameters of the PCRs.
| Target gene | Primers | Parameters |
|---|---|---|
| HPRT |
5′: 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ 3′: 5′-TCGGTATCCGGTCGGATGGGAG-3′ |
94°C 30s, 60°C 30s, 72°C 40s |
| IFN-α |
5′: 5′-TGTCTGATGCAGCAGGTGG-3′ 3′: 5′- AAGACAGGGCTCTCCAGAC-3′ |
94°C 30s, 58°C 30s, 72°C 40s |
| CD86 |
5′: 5′-CAGTTACTGTGGCCCTCCTC-3′ 3′: 5′-ACTCTGCATTTGGTTTTGCT-3′ |
94°C 30s, 57°C 30s, 72°C 40s |
| TNF-α |
5′: 5′-GGCAGGTCTACTTTGGAGTCATTGC-3′ 3′: 5′- ACATTCGAGGCTCCAGTGAATTCGG-3′ |
94°C 30s, 58°C 30s, 72v 40s |
| TGFβ |
5′: 5′-CGCAACAACGCCATCTAT-3′ 3′: 5′-ACTTCCAACCCAGGTCCTT-3′ |
94°C 30s, 58°C 30s, 72°C 40s |
| IL-10 |
5′: 5′-ACCTGGTAGAAGTGATGCCCCAGGCA-3′ 3′: 5′-CTATGCAGTTGATGAAGATGTCAAA-3′ |
94°C 30s, 58°C 30s, 72°C 40s |
| IL-6 | 5′: 5′-CGGAATTCATGTTCCCTACTTCACAAG-‘ 3′: 5′-GCCGTCGACCTAGGTTTGCCGAGTA-3′ |
94°C 30s, 60°C 30s, 72°C 40s |
Expression of CD44highCD62Llow on CD4+ T cells
Splenocytes were isolated on day 100 after the second immunization as described previously, and diluted to 1 × 106 cells/ml. The cells were suspended in 100μl phosphate-buffered saline (PBS) and blocked with 1 μl Fcγ monoclonal antibody (0.5 mg/ml). After incubation for 30 min at 4°C, cells were washed three times with PBS, and then incubated with anti-CD44-PE, anti-CD62L-FITC and anti- CD4-APC for 30 min at 4°C. The expression of CD44highCD62Llow was detected using a FACS Calibur.
Statistical analysis
The data were analyzed using a one-sided Student’s t-test. Differences were considered to be statistically significant with p < 0.05 indicated with an asterisk. (Author, please cite reference 24)
Acknowledgments
This work was supported in part by the National High-Tech R & D Program of China (2010AA022907). We would like to thank Dr. Jane Q.L. Yu and Zhonghuai He for their assistance in this study.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22105
Reference
- 1.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 2.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–62. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 5.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 6.Sprent J, Surh CD. Generation and maintenance of memory T cells. Curr Opin Immunol. 2001;13:248–54. doi: 10.1016/S0952-7915(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 7.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med. 2006;203:573–82. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–88. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 11.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–9. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K, Lee CK, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol. 1998;160:5735–41. [PubMed] [Google Scholar]
- 13.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 14.Khaled AR, Li WQ, Huang J, Fry TJ, Khaled AS, Mackall CL, et al. Bax deficiency partially corrects interleukin-7 receptor alpha deficiency. Immunity. 2002;17:561–73. doi: 10.1016/S1074-7613(02)00450-8. [DOI] [PubMed] [Google Scholar]
- 15.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–6. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–95. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller YM, Makar V, Bojczuk PM, Witek J, Katsikis PD. IL-15 enhances the function and inhibits CD95/Fas-induced apoptosis of human CD4+ and CD8+ effector-memory T cells. Int Immunol. 2003;15:49–58. doi: 10.1093/intimm/dxg013. [DOI] [PubMed] [Google Scholar]
- 18.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–26. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 19.Weigel BJ, Nath N, Taylor PA, Panoskaltsis-Mortari A, Chen W, Krieg AM, et al. Comparative analysis of murine marrow-derived dendritic cells generated by Flt3L or GM-CSF/IL-4 and matured with immune stimulatory agents on the in vivo induction of antileukemia responses. Blood. 2002;100:4169–76. doi: 10.1182/blood-2002-04-1063. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–81. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 21.Münz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–7. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su B, Wang J, Wang X, Jin H, Zhao G, Ding Z, et al. The effects of IL-6 and TNF-alpha as molecular adjuvants on immune responses to FMDV and maturation of dendritic cells by DNA vaccination. Vaccine. 2008;26:5111–22. doi: 10.1016/j.vaccine.2008.03.089. [DOI] [PubMed] [Google Scholar]
- 23.Jin H, Li Y, Ma Z, Zhang F, Xie Q, Gu D, et al. Effect of chemical adjuvants on DNA vaccination. Vaccine. 2004;22:2925–35. doi: 10.1016/j.vaccine.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook JFE, Maniatis T. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press, 1989. [Google Scholar]
- 25.Du X, Zheng G, Jin H, Kang Y, Wang J, Xiao C, et al. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J Gene Med. 2007;9:136–46. doi: 10.1002/jgm.1004. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Piriou L, Chilmonczyk S, Genetet N, Albina E. Design of a flow cytometric assay for the determination of natural killer and cytotoxic T-lymphocyte activity in human and in different animal species. Cytometry. 2000;41:289–97. doi: 10.1002/1097-0320(20001201)41:4<289::AID-CYTO7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGFbeta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–53. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang N, He YW. The antiapoptotic protein Bcl-xL is dispensable for the development of effector and memory T lymphocytes. J Immunol. 2005;174:6967–73. doi: 10.4049/jimmunol.174.11.6967. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–76. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 33.Di Santo JP, Aifantis I, Rosmaraki E, Garcia C, Feinberg J, Fehling HJ, et al. The common cytokine receptor gamma chain and the pre-T cell receptor provide independent but critically overlapping signals in early alpha/beta T cell development. J Exp Med. 1999;189:563–74. doi: 10.1084/jem.189.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]