Abstract
DNA vaccines have emerged as an attractive strategy to promote protective cellular and humoral immunity against the encoded antigen. DNA vaccines are easy to generate, inexpensive to produce and purify at large-scale, highly stable and safe. In addition, plasmids used for DNA vaccines act as powerful “danger signals” by stimulating several DNA-sensing innate immune receptors that promote the induction of protective adaptive immunity. The induction of tumor-specific immune responses represents a major challenge for DNA vaccines because most of tumor-associated antigens are normal non-mutated self-antigens. As a consequence, induction of potentially self-reactive T cell responses against such poorly immunogenic antigens is controlled by mechanisms of central and peripheral tolerance as well as tumor-induced immunosuppression. Although several DNA vaccines against cancer have reached clinical testing, disappointing results have been observed. Therefore, the development of new adjuvants that strongly stimulate the induction of antitumor T cell immunity and counteract immune-suppressive regulation is an attractive approach to enhance the potency of DNA vaccines and overcome tumor-associated tolerance. Understanding the DNA-sensing signaling pathways of innate immunity that mediate the induction of T cell responses elicited by DNA vaccines represents a unique opportunity to develop novel adjuvants that enhance vaccine potency. The advance of DNA adjuvants needs to be complemented with the development of potent delivery systems, in order to step toward successful clinical application. Here, we briefly discuss recent evidence showing how to harness DNA-induced immune response to improve the potency of cancer vaccines and counteract tumor-associated tolerance.
Keywords: DNA vaccine, DNA electroporation, innate immunity, adaptive immunity, cancer vaccines
Introduction
Adaptive immunity consists of a highly variable repertoire of antigen receptors expressed on the surface of CD4+ and CD8+ T (cellular response), and B cells (humoral response), which recognize infected host cells and microbial pathogens, respectively. The initiation of an adaptive immune response depends on the specific interaction between T cell receptor (TCR) expressed by naïve T cells and a cognate peptide–major histocompatibility protein complex (peptide–MHC) on the surface of antigen-presenting cells (APC).1 Among all APCs, dendritic cells (DCs) have the unique capacity to efficiently induce the activation of naïve CD8+ and CD4+ T cells during an adaptive immune response.2 Effector CD8+ cytotoxic T lymphocytes (CTLs) recognize and kill cells that present the respective epitope/MHC I complex, such as infected cells expressing viral or bacterial antigens and tumor cells expressing tumor antigens, whereas effector CD4+ T helper (Th) cells recognize epitopes in complex with MHC class II molecules and secrete diverse cytokines that modulate immune responses and thus promote the elimination of pathogens and tumors.
Traditional and Recombinant Vaccines
The immune system is highly efficient in recognizing and destroying invading pathogens and transformed cells, and stimulates strong immune responses specific for such agents conferring crucial protection against disease. For more than a century vaccines have been the most effective approach to prevent and guard against a wide range of diseases caused by viruses and bacteria and still continues to be one of the most cost-effective public health measures.3 The effectiveness of vaccines lies in their ability to induce protective adaptive immune responses that can last for decades. More than two centuries have passed since 1796, when Edward Jenner generated the first vaccine based on the transference of cowpox pus that conferred protection against the poxvirus to the patients. The name vaccine is derived from this procedure (in latin, vaccinus = cow).4 Almost a century later, Louis Pasteur developed the first bacterial vaccine utilizing attenuated bacterial cultures for anthrax treatment. In the early 1920s, toxoid or protein subunit vaccines appeared for the treatment of tetanus and diphtheria and almost two decades later, John Franklin Enders and Jonas Salk championed the development and production of virus vaccines for polio treatment. Current licensed vaccines commonly consist of killed (inactivated) or live-attenuated pathogens or pathogen subunits that elicit humoral and cellular immune responses. One of the most effective vaccines ever made is the live attenuated yellow fever-17D vaccine (YF-17D). The great efficacy of this vaccine lies in the coordinated activation of multiple pattern recognition receptors (PRRs) and downstream master transcription factors that integrate innate and adaptive immunity.5,6 PRRs are a group of evolutionary conserved receptors predominantly expressed on innate immune cells that detect pathogen-associated molecular patterns (PAMPs), and initiate the production of cytokines, type I IFNs and chemokines to promote activation and maturation of an immune response.7,8
Recombinant DNA technology has proved to be useful in the generation of entirely customizable vaccines, allowing for the selection and expression of only the desired antigen or antigens. This also reduces the risk of exposure to live pathogens during the manufacturing process as well as during administration.9 Thus, using this technology a new generation of vaccines are being produced that can be lauded for their safety, simplicity and versatility. An important advantage of these kinds of vaccines over traditional ones lie in the opportunity to generate vaccines against non-infectious diseases, like cancer, in which the specific target antigens have been identified. Recombinant antigens can be delivered either as purified proteins or encoded in both viral and non-viral genetic vectors. The first recombinant protein vaccine available was the Hepatitis B vaccine, which consists of virus-like particles (VLP) that have Hepatitis B antigen (HBsAg) expressed on the surface of the virus. These VLPs are purified from cultures of genetically engineered Saccharomyces cerevisiae, and are delivered as a vaccine in combination with aluminum hydroxide as adjuvant.10,11 This vaccine was licensed in 1986 but it took almost 20 years before the second recombinant protein vaccine, protecting against the human papilloma virus (based on the L1 structural protein), became approved using a very similar technical approach.12 One of the main reasons for having such a low number of recombinant protein vaccines in the market is that these vaccines lack multiple PAMPs. This lack of PAMPs leads directly to a decreased stimulation of innate immunity in contrast to traditional vaccines and, therefore requires the development of more immunogenic adjuvants. Unfortunately, despite two centuries of vaccine development, relatively little is known about how traditional vaccines stimulate the innate immune system and which PRR/PAMP downstream signaling pathways are required to induce protective immune responses. For this reason, only a few adjuvants have been approved for human use.
DNA Vaccines
DNA-mediated immunization began in the early ‘90s with demonstrations that in vivo injection of plasmid DNA encoding the influenza A nucleoprotein led to protective necleoprotein-specific CTL response. This showed that the host’s cellular machinery itself could translate, process and present the DNA encoded antigens to elicit an adaptive immune response such as occurring during typical virus infection. The CTLs generated were able to protect from a subsequent challenge with different strains of influenza A virus, as measured by decreased viral lung titers, inhibition of mass loss, and increased survival.13-15 Since then, DNA vaccines have been developed to treat a broad spectrum of diseases including cancer, allergy, autoimmunity and infectious diseases.16 This type of vaccine has several advantages over other immunization strategies, such as, the desired antigen-encoding plasmid is easy to generate and fairly inexpensive to produce and purify in large quantities. The same production platform can be used for plasmids encoding any protein, obviating expensive purification procedures for each particular antigen. DNA vaccines are also very stable, thus facilitating long-term storage. Plasmids employed for DNA vaccines are non-infectious, non self-replicating (in mammals) and there is virtually no genomic integration after plasmid administration. It is no surprise then that DNA vaccines have been demonstrated to be safe in clinical trials.17 Importantly, plasmids employed for DNA vaccines are able to act as a powerful danger signal that trigger several innate immune receptors to initiate the production of immune-stimulatory molecules, the maturation of APCs and the induction of protective adaptive immunity.18
DNA-Mediated Innate Immune Activation
The interaction between DNA and DNA-sensing receptors is the initiating step to induce an adaptive immune response. Once the antigen-encoding plasmid is delivered to the organism, plasmid DNA or the expressed antigen is taken up by APCs, such as macrophages or DCs. The DNA-encoded antigen is then processed and presented on MHC to T cells.19,20 DNA itself acts as a powerful “built-in” adjuvant that provides the pro-inflammatory context necessary for maturating APCs that are able to induce effective immune responses.18 Accordingly, it was demonstrated that the immune-stimulatory effects of alum, one of the few adjuvants approved for human use, are mediated by immunogenic DNA released from dying cells.21
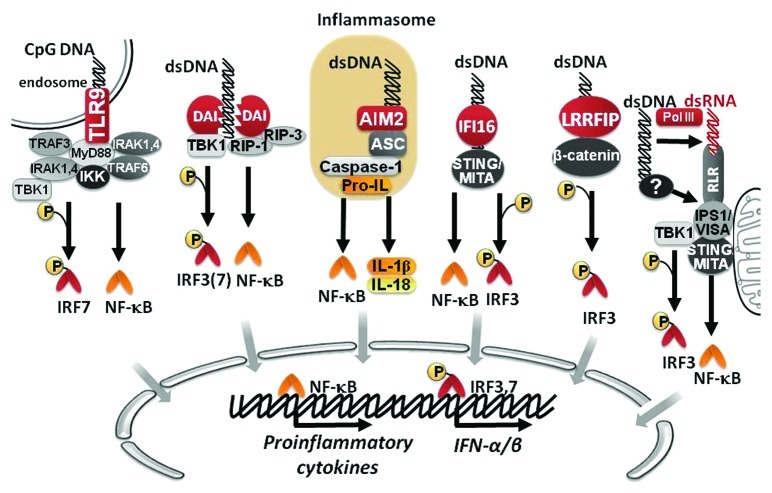
Toll-like receptor (TLR) 9 is the most studied DNA sensor, which preferentially recognizes unmethylated CpG motifs from bacterial and viral DNA within endocytic vesicles, a common entry route for pathogens, and triggers the activation of nuclear factor-kappa B (NF-κB) and activating protein-1 (AP-1) transcription factors that lead to the production of type I IFNs and proinflammatory cytokines.22,23 Although initially TLR9 was described as a receptor unique in its ability to sense foreign DNA, studies showed that TLR9 knockout mice and cells were still able to respond to DNA, suggesting that other DNA-sensing receptors could be involved in DNA recognition.24 Indeed, new DNA sensors have been described, including: DNA-dependent activator of interferon-regulatory factors (DAI, also known as ZBP1 or DLM-1),25 absent in melanoma 2 (AIM2),26,27 IFI-16,28 leucine-rich repeat flightless-interacting protein1 (LRRFIP1)29 and DNA-dependent RNA polymerase III (Pol-III).30,31 DAI recognizes double-stranded DNA (dsDNA) present in the cytosol in a sequence-independent but length-dependent fashion, and triggers the expression of type I IFNs, chemokines and proinflammatory cytokines via two distinct signaling pathways.32 One pathway involves DAI mediated phosphorylation of the TANK-binding kinase-1 (TBK-1) and subsequent activation of the transcription factor IFN regulatory factor (IRF) 3.25,32 The second pathway requires phosphorylation of the receptor interacting protein-1 kinase (RIP-1), leading to phosphorylation of IκB-α, and the subsequent activation of the transcription factor NF-κB.33 Similarly, interferon-inducible protein IFI16 also mediates the induction of IFN-I through the activation of IRF3 and NF-κB by recruiting the stimulator of interferon genes (STING) protein.28 AIM2 recognizes DNA and activates the inflammasome, a multiprotein complex that activate caspase-1 to induce the proteolysis and secretion of interleukin (IL)-1β and IL-18.34 The recently described DNA sensor LRRFIP1 recruits and phosphorylates β-catenin, promoting IRF3 phosphorylation and the induction of type I IFNs.29 Pol-III is different from the aforementioned DNA-sensing receptors; since Pol-III transcribes AT-rich cytosolic dsDNA into AU-rich dsRNA that in turns acts as a ligand for the cytosolic RNA sensor RIG-I, leading to type I IFN production.30,31 A summary of DNA signaling pathways is schematized in Figure 1.
Figure 1. Signaling pathways of innate immunity involved in DNA-sensing. DNA can be recognized by different sensors, including TLR9, DAI, AIM2, IFI16 and LRRFIP1. As result of downstream signaling, NF-κB and IRF transcription factors become activated to promote the expression of different proinflammatory cytokines, and IFN-α/β.
The importance of DNA-induced innate immune activation for the immunogenicity of DNA vaccines is confirmed by studies showing that adaptive immune response elicited by DNA vaccination was almost completely abrogated in mice lacking adaptor proteins involved in some of these DNA recognition pathways, such as TBK-1 or STING.31 These studies have shown that the IRF3-type I IFN signaling axis is the main mechanism that dictates the immunogenicity of DNA vaccines.35,36 However, we have evidence demonstrating that neither IRF337 nor type I IFN-receptor but rather NF-κB is essential for the induction of antitumor T cell responses by intradermal DNA vaccination, through a cytosolic DNA sensing-mediated mechanism (Ligtenberg et al., unpublished data).
Although it is known that DNA promotes the generation of immune-stimulatory signals, DNA can also induce immunosuppressive pathways involved in the regulation of the immune response, which could potentially dampen the immune response triggered by DNA vaccines. For example, it has been shown that TLR9 promotes Signal Transducer and Activator of Transcription (STAT) 3 activation in DCs, decreasing the production of pro-inflammatory cytokines and chemokines thus constraining the induction of both innate and adaptive immune responses.38 As expected, STAT3 ablation in the hematopoietic compartment increases proinflamatory cytokine production and greatly improves the therapeutic effect of intratumoral CpG oligonucleotide administration.38 Recently, Miles et al. has assigned a direct tolerogenic role to TLR9 by driving IL-10 production in B cells stimulated with apoptotic cells.39 This effect was abrogated in B cells lacking TLR9 or when treating apoptotic cells with DNase, showing a direct effect of the DNA recognition over the TLR9-mediated immunosuppressive effect.39 In another study, IFI16 has been shown to mediate anti-inflammatory effects observed by type I IFNs by directly associating with AIM2 and blocking AIM2 functionality by inhibiting the AIM2-ASC inflammasome-mediated activation of caspase-1.40 Thus, it seems that DNA-induced innate immune activation is a self-limiting process that is important for initiating adaptive immune responses but needs to be tightly regulated to avoid self-injury.
DNA Vaccination Against Cancer
DNA vaccines have shown to be particularly efficient in inducing specific MHC class I–restricted CTLs and MHC class II–restricted CD4+ Th1 cells, which are important in controlling cells infected with intracellular pathogens and malignantly transformed cells. Induction of protective T cell responses has been demonstrated in mice immunized against a variety of antigens, including hepatitis B surface and core antigens,41-43 HIV Env and Gag antigens,44,45 listeriolysin O from Listeria monocytogenes,46 as well as tumor antigens47 providing CTL-mediated tumor protection in different cancer models. CTLs can specifically eliminate malignantly transformed cells expressing tumor antigens without affecting other tissues. Accordingly, intratumoral CTL infiltration is often associated with favorable clinical outcomes such as decreased disease recurrence and prolonged survival in diverse malignancies.48-53 Furthermore, trials using adoptive transfer of tumor-specific CTLs that control disease progression in metastatic melanoma patients have provided direct evidence about the therapeutic efficacy of CTLs.54 Several clinical trials have shown that generating tumor-reactive CTLs in patients with different types of cancer is possible.,55,56 However, these responses seem to be insufficient to prevent or cause regression of tumor growth and metastasis. Therefore, DNA vaccines represent a cost-effective alternative to initiate or boost anti-tumor T cell responses that promise to significantly reduce the side effects associated with current cancer therapies.
Since the characterization of the first tumor antigen termed melanoma antigen (MAGE) - 1 in 1991,57 the identification of a growing amount of tumor antigens and the availability of genomic and bioinformatics tools has prompted the development of gene-based vaccines encoding tumor antigens. Tumor-specific antigens, resulting from random somatic mutations, carcinogenic substances or splicing aberrations that are exclusively expressed by tumors and absent in normal cells are very rare and heterogeneous among different individuals. In effect, most tumor antigens that are overexpressed in malignant cells can also be present in normal tissues, though at lower levels or at specific stages during lifetime and development. Examples of such kind of antigens, also called tumor-associated antigens (TAAs), are carcinoembryonic antigen (CEA),58 human epidermal growth factor receptor 2 (HER2/neu) protein,59 cancer-testis (CT) antigens,59,60 as well as tissue-specific differentiation antigens, such as tyrosinase61 and prostate-specific antigen (PSA).62 In order to help and guide the proper antigen selection, the National Cancer Institute (NCI) has elaborated a priority-ranked list of cancer antigens using a structured technique and mathematical model known as the Analytic Hierarchy Process that includes criteria such as therapeutic potential, immunogenicity, and role in oncogenicity, specificity, among others.63 Although none of the 75 antigens evaluated have met all of the characteristics of the ideal tumor antigen, the top ten ranking, in descending order, are as follows: WT1, MUC1, LMP2, HPV E6 E7, EGFRvIII, HER-2/neu, Idiotype, MAGE A3, p53 non-mutant and NY-ESO-1.64
Despite the encouraging results obtained in preclinical models, very few studies using cancer DNA vaccines have shown objective clinical responses. Although T cells have the potential to eliminate cells expressing tumor antigens, they need to be present in sufficient amounts and display an activated phenotype. One major obstacle for successful cancer vaccination is that most tumor-associated antigens are non-mutated self-antigens expressed in normal cells. Therefore, induction of potentially self-reactive T cell responses against such antigens is controlled by mechanisms of self-tolerance, which involves central and peripheral tolerance.65
Tumor-Associated Tolerance and Immunosuppression
One of the key characteristics of the immune system is the ability to discriminate between self and non-self-antigens. During the establishment of central tolerance in the thymus, self-reactive T cell clones are deleted by a process known as negative selection.66 Alternatively, self-reactive T cells can be transformed into natural Foxp3+ regulatory T cells (nTregs), a T cell subset with immunosuppressive properties that limit the magnitude of effector T cell responses.67 As consequence of central tolerance, tumor-specific T cell precursors are present at low frequencies, display sub-optimal TCR affinity or present an immunosuppressive phenotype. Potentially self-reactive T cells that escape from central tolerance are controlled in the periphery by steady-state APCs, which present tumor antigens to T cells in the absence of costimulatory signals (e.g., CD40 and CD86) or in the presence of coinhibitory receptors (e.g., Programmed cell death ligands (PD-L1 and PD-L2)), rendering T cells hypo-responsive (anergic) or transforming them into inducible Tregs (iTregs). Both nTregs and iTregs further suppress self-reactive T cells by antigen-specific and non-specific mechanisms that involve the secretion of anti-inflammatory cytokines, such as TGF-β and IL-10, and the expression of inhibitory molecules, such as Cytotoxic T-Lymphocyte Antigen (CTLA)4, that suppress DC-mediated induction and function of effector T cells.37,68,69
Moreover, tumors can actively promote an immunosuppressive microenvironment that strongly limit infiltration and effector function of T cells.70 This tumor-induced immunosuppression is achieved by secretion of different cytokines, recruitment and activation of immunosuppressive cells, and by modulating the phenotype of myeloid populations and DCs.71 This microenvironment promotes tumor progression through immune and non-immune mechanisms that include the production of pro-angiogenic factors, matrix metalloproteases (MMPs)72 as well as by hematopoiesis deregulation that render cell-mediated anti-tumor immunity dysfunctional.73 This dysfunction extends to tumor antigen-specific T cells as well as components of the innate immune system such as natural killer (NK) cells, macrophages and monocytes. Tumors induce the accumulation of a heterogeneous population of immature myeloid cells with a suppressive phenotype, called myeloid-derived suppressor cells (MDSCs), which can be found in high frequencies within the tumor microenvironment and also in distant lymphatic organs and peripheral blood of tumor-bearing individuals.74 MDSCs have been found to accumulate in mice with transplanted or spontaneous tumors and in patients with different types of cancer,57 where MDSC levels correlate with clinical cancer stage and prognosis.75 MDSCs directly suppress antigen-specific T cell responses76 through the production of reactive oxygen and nitrogen species,77,78 cysteine77,78 and tryptophan79 depletion and mechanisms involving the production of IL-10 and TGF-β.49,72 Tregs also accumulate within the tumor microenvironment inhibiting antitumor immune responses.80,81 There are several examples of cancer associated with increased frequencies of Tregs in circulation or in the tumor tissues.82,83 Tumor-infiltrating DCs and macrophages are to be likely involved in the local activation and expansion of Tregs inside tumor lesions.84 Accordingly, deletion of Tregs results in efficient anti-tumor immune responses leading complete tumor regression.80,85 Tregs counteract anti-tumor functions of immune effector cells through both contact-dependent and independent mechanisms. Contact-dependent mechanisms include upregulation of CTLA-4 which interacts with CD80 and CD86 on DC surface with higher affinity than CD28 and therefore out-compete CD28-CD80/86 stimulatory signaling.86 Furthermore, Tregs induce indoleamine 2,3-dioxygenase (IDO) expression in DCs via interaction with CD80 and CD86, which generates immunosuppressive metabolites, attenuating T cell function.37,69,87 Contact independent mechanism include the production of cytokines, such as IL-10, TGF-β and IL-35, which promote the switch of effector T cells to regulatory-like T cells.88
Tumor-associated macrophages (TAMs) are another group of cells present in tumors capable of suppressing immune responses.89 These cells constitute an important part of the tumor-infiltrating immune cells,90 derived from monocytic precursors circulating in blood and recruited to tumor site by chemokines like Chemokine (C-C motif) Ligand (CCL) -5 and CCL2, VEGF and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF).91 TAMs have an immune-regulatory function associated with the production of epidermal growth factor (EGF), TGF-β, IL-10 and VEGF, which contribute to the growth of blood vessels that are essential for tumor proliferation and neo-angiogenesis.92 TAMs also secrete metalloproteinases and cathepsins that favor tumor expansion, motility and invasion.89 Studies in different types of cancer, including intestinal gastric cancer,93 thyroid cancer94 and pancreatic cancer95 indicate that the presence of TAMs in the tumor environment is associated with a poor prognosis.
There is an association between the presence of Tregs in tumors and TAMs. It has been shown that Tregs begin to infiltrate the tumor at an early stage of tumor development. The homing to the tumor is due to increased CCL20 production by TAMs in the tumor environment and the concomitant expression of high levels of Chemokine Receptor 6 (CCR6) by Tregs.96 When TAMs were conditionally eliminated, a decreased expression of CCL20 was observed as well as a marked decrease in FoxP3 positive Tregs infiltration of the mouse colorectal cancer, resulting in a significant inhibition of colorectal cancer.97
Clinical Trials Using DNA Vaccines Against Cancer
The first clinical trials of cancer DNA vaccines involved the immunization against the idiotypic portion of the B cell receptor expressed by human B-cell lymphomas.98 The intramuscular injection of this DNA vaccine to patients with follicular lymphoma in clinical remission following chemotherapy, promoted anti-idiotypic antibody production in 38% of the patients.98,99 A second approach involved the fusion of the single-chain variable fragment (scFv) with IgG2a and κ mouse heavy- and light-chain constant regions, promoting cellular and humoral immune response in 50% of the patients.100 However, the preparation of specific idiotypic DNA vaccines for every patient represents a time-consuming and expensive strategy, which promoted the development of DNA vaccines encoding tumor antigens that were more broadly expressed. There are several examples of clinical studies using DNA vaccines encoding tumor antigens such as HER-2/neu for breast cancer,101 gp100 for melanoma,102 or E7 for cervical cancer,103 among others. As an example, a plasmid containing the prostate-specific membrane antigen (PSMA) fused to a domain of Fragment C of tetanus toxin has shown to elicit both CD4+ (directed to tetanus toxin) and CD8+ (directed to PSMA) T cell responses in 3 of 3 patients with recurrent prostate cancer.104 Another phase I/IIa clinical trial using a DNA vaccine encoding prostatic acid phosphatase (PAP) together with 200 μg of GM-CSF were co-administrated to 22 prostate cancer patients. Interestingly, 41% of these patients developed PAP-specific CD4+ and/or CD8+T-cell response, and it was also accompanied by increased prostate-specific antigen doubling time, which is associated with a good prognosis and lower risk of death from prostate cancer.105 These studies, as summarized in Table 1, showed that this approach is well tolerated by the patients, safe and without evident adverse events. However the low immunogenicity observed has prompted the development of new delivery approaches, optimization of antigen design strategies, novel formulations and the inclusion of immune adjuvants in the same plasmid to induce a strong immune response against tumors.106,107 Clinical results from DNA vaccine trials have been able to show immunological responses to antigens targeted for treatment.108-110 These responses have not been able to result in significant improvements to patient clinical outcomes as shown in Table 1. The hurdles that DNA vaccines must overcome to be successful are starting to be addressed. The first obstacle that needs to be surmounted is the issue of scale, and translating success from rodent models to human patients. Where preclinical rodent models use DNA doses ranging around 10–100 µg, human clinical studies have wildly ranging doses from 8 µg (NCT00988559) to 8 mg (NCT00680589), though typically dose escalation studies end around 1 mg (Table 1). This 10–100-fold increase is still low compared with the 2,000-fold escalation than could be expected, considering average rodent (35 g) compared with human weight (70 kg).111 Though it is generally accepted that there must be a considerable increase in DNA dose between preclinical to clinical, what is often neglected is an appreciable increase in the volume of vaccination. Considering an injection volume as low as 10 ul for administrating DNA vaccines in rodents, a volume of 20 ml would be expected to be used in humans resulting from the 2000-fold rodent-to-human escalation. Volume issue is not generally being addressed by clinical trials but can certainly limit an adequate rodent-to-human escalation and impact the efficacy of DNA vaccines.
Table 1. DNA vaccination clinical trials targeting cancer.
| Tumor | Target | Site of administration | Device | DNA dose | Treatment dose | Immunological Response | NCT Listing |
|---|---|---|---|---|---|---|---|
|
Melanoma |
Tyrosinase |
IM + EP |
TriGrid Delivery System |
N.D. |
5 doses |
N.D. |
NCT00471133 |
|
Tyrosinase |
IM |
N.D |
0.5, 2.0, 4.0, 8.0 mg |
6 doses |
N.D. |
NCT00680589 |
|
| gp100 |
IM or PMED |
Biojector2000 or PowderMed |
1 mg (IM) or 0.002 mg (PMED) |
16 doses |
5/27 CD8+ tetramer+ response, PMED shows trend to increased CD8+ IFN-γ generation (Ginsberg et al., 2010) |
NCT00398073 |
|
| gp100 |
IM |
Biojector2000 |
0.1, 0.5, 1.5 mg |
6 doses |
5/19 CD8+ tetramer+ response |
NCT00104845 |
|
| (Yuan et al., 2009) | |||||||
|
Tyrosinase |
IM |
Biojector2000 |
0.1, 0.5, 1.5 mg |
6 doses |
7/18 CD8+ tetramer+ response |
NCT00698100 |
|
| (Wolchok et al., 2007) | |||||||
|
Cervical Intraepithelial Neoplasia |
HPV16/18 E6/E7 |
IM + EP |
Cellectra |
0.6, 2.0, 6.0 mg |
3 doses |
N.D. |
NCT00685412 |
| HPV16 E7 |
ID or IM or IL |
GeneGun |
0.008 or 0.016 mg (ID), 1 or 3 mg (IM), 1 or 3 mg (IL) |
4 doses |
N.D. |
NCT00988559 |
|
| HPV16 E7 |
IM |
N.D. |
0.5, 1.0, 3.0 mg |
3 doses |
No Vaccine induced antibodies, |
|
|
| 5/9 IFN-γ Elispot (Trimble et al., 2009) | |||||||
|
Prostate Cancer |
PSA |
ID + EP |
DermaVAX |
0.05, 0.15, 0.4, 1.0 mg |
5 doses |
N.D. |
NCT00859729 |
| PSMA |
IM + EP |
Elgen Twinjector |
0.8, 1.6, 3.2 mg |
3 doses |
16/32 PSMA specific CD8+ response, 21/32 vaccine induced antibodies, trend to improved clinical outcome (Chudley et al., 2012) |
|
|
| PAP |
ID |
N.D. |
0.1, 0.5, 1.5 mg |
6 doses |
3/22 CD8 antigen specific IFNg |
NCT00582140 |
|
| (McNeel et al., 2009) | |||||||
| PSA |
ID + IM |
N.D. |
0.1, 0.3, 0.9 mg |
5 doses |
PSA specific antibodies, 2/9 IFN gamma response to PSA (Pavlenko et al., 2004) |
|
|
|
Ovarian and Breast Cancer |
Her-2/Neu |
ID |
N.D. |
0.01, 0.1 mg |
3 doses |
13/21 Her2 specific Tcell response |
NCT00436254 |
| J Clin Oncol 27:15s, 2009 (suppl; abstr 3054) | |||||||
| Her-2/Neu |
IM + IC |
N.D. |
0.03 (IC) and 0.27 (IM) |
3 doses |
Measurable T cell response, increased Her-2 specific binding antibodies |
|
|
| (Norell et al., 2010) | |||||||
| Hematological Malignancy | WT1 |
IM + EP |
N.D. |
1 mg |
6 x 4 doses |
N.D. |
NCT01334060 |
| Idiotype | IM + ID | Biojector | 0.2, 0.6, 1.8 mg | 3 doses | 8/12 T-cell or Antibody specific response to MsIG(Timmerman et al., 2002) |
IM, intramuscular; ID, intradermal; IL, intralesional; IC, intracutaneous; EP, electroporation; N.D., not described; PMED, particle-mediated epidermal delivery; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostatic acid phosphatase.
Another daunting hurdle is patient selection and treatment. In many preclinical models rodents are used and treated with DNA vaccination either therapeutically or prophylactically. In the prior this is typically a transplanted tumor model in healthy young mice followed by DNA vaccine treatment commencing once the tumor is palpable; in the later, spry rodents are treated with DNA vaccines and then challenged with matching tumor models. Either case does not come close to mimicking the true patient situation. For patients enrolled in most clinical trials the tumor has had ample time to establish itself83,112 leading to the above-described immune-suppressive environment. Concurrent with this immune-suppressive environment the patient typically has undergone “classical” therapies including chemotherapy or radiation therapy; both can lead to severe immune-dysfunction. Clinicians are often trying to elicit a strong anti-tumor immune response from a severely hampered immune system. DNA vaccines may be better suited to early stage and less aggressive cancers or patients with minimal residual disease after classical therapies.
Electroporation, an Efficient Method for Delivering DNA Vaccines
DNA vaccines have shown a limited response in clinical trials, in part because of the low in vivo transfection efficiency and therefore the development of potent delivery systems is a necessary step toward clinical application.106,107,113 Delivery methods that use needles and syringes can be classified into transcutaneous, epidermal, intradermal, subcutaneous and intramuscular. The World Health Organization highlights the use of intradermal delivery as it is easier to administer and being more immunogenic than intramuscular or subcutaneous delivery because intradermally delivered vaccines would be more likely to be taken up by APCs such as Langerhans cells and other DC subsets.114 Immunogenicity of vaccines delivered by intramuscular and intradermal injections was compared by Arnou et al. using the same quantity of antigen but less volume during intradermal injection, which showed a higher immune response in the latter.115
In vivo electroporation has emerged as a simple, efficient and clinically applicable method for delivering DNA vaccines that greatly enhances plasmid uptake, antigen expression and immune responses.113 Electroporation was initially described in the ‘70s116 as the application of controlled brief electric pulses of high intensity to induce transient membrane permeabilization without compromising cell integrity.117 This permeabilization allows for the entrance of small molecules like hydrophilic chemotherapeutic drugs that otherwise would not penetrate into the cell.118 Permeabilization also facilitates the entrance of large molecules, such as plasmid DNA, which was first described in the early ‘80s.119 Since then electroporation has emerged as a promising technique due to its simplicity and efficiency. The first clinical trials involving electroporation began in the ‘90s in which this technique was applied for the introduction of chemotherapeutic drugs into tumors in cancer patients.120,121 Electroporation has been widely used to deliver pDNA to skin, testis, cartilage, liver,122,123 brain,124,125 cornea,126,127 prostate and skeletal muscle128 in biological models from rodents to non-human primates. Electroporation of DNA encoding immune-stimulatory molecules, such as IL-12, have been tested in preclinical and clinical studies.129,130 Interestingly, DNA electroporation induces the infiltration of immune cells and the production of pro-inflammatory cytokines and chemokines that contribute to the induction of adaptive immune responses against the encoded antigen.131,132 A recent clinical trial using DNA electroporation in patients with prostate cancer showed enhanced humoral and CD8+ T cell responses against prostate cancer compared with DNA vaccine alone.104,133 Another study showed increased immunogenicity of an HIV-1 DNA vaccine injected intramuscularly followed by electroporation in healthy volunteers.48 Employing electroporation directly following may have an impact on DNA vaccine trials, Chudley et al. have shown that intramuscular injection followed by electroporation resulted in increased specific CTL response,133 and outcomes from other clinical trials utilizing electroporation are eagerly awaited. Although most of the studies have been conducted using intramuscular delivery, intradermal electroporation with plasmids encoding tumor antigens has shown to be highly efficient in inducing specific CD8+ T cell responses and conferring protection against tumors in mouse models.134,135 Several clinical studies testing the efficacy of intradermal DNA electroporation are currently ongoing.
According to the Journal of Gene Medicine, naked DNA vectors are currently the third most used gene transfer approach for clinical trials (18.5% of clinical trials) only exceeded by adenovirus (23.3%) and retrovirus (20%) mediated gene-transfer. (www.wiley.com//legacy/wileychi/genmed/clinical/). Approximately 337 clinical trials worldwide have used naked DNA, with 146 of them are focused on cancer diseases, to date there have been no reports of problems related with vector immunity. Moreover, DNA vaccines have been commercially approved by the FDA for veterinary use in dogs, fish and horses29 whereas, DNA vaccines for humans are being tested in clinical trials.
Genetic Adjuvants to Boost the Potency of DNA Vaccines
The versatility of gene-based vaccines facilitates the co-delivery of genetic adjuvants encoding immune-stimulatory molecules. Historically, genes encoding single cytokines and chemokines, typically IL-2 and GM-CSF have been extensively used as genetic adjuvants.136-138 However, strategies conceiving the concerted action of several molecules achieve a more potent and broader activation of the immune responses.138 The production of several immune-stimulatory molecules can be achieved by stimulation of innate immune PRRs and promote long-lasting protective adaptive immunity.5,6,139 Understanding how DNA vaccines trigger the innate-immune system to drive an adaptive anti-tumor immune response will be essential in designing potent DNA vaccines to treat later stage patients. Despite the fact that DNA vaccines act as adjuvants by themselves, strategies that boost innate immune signaling by co-expressing DNA-sensing PRRs, intracellular adaptor molecules and downstream transcription factors have been shown to further enhance DNA vaccine potency.140
Among the DNA-sensing PRRs, coexpression of high-mobility group box 1 protein (HMGB1), a nuclear protein that cooperates in sensing nucleic acids,52 has shown strong adjuvant activity by acting as a potent maturation signal for DCs and inducing immunity against influenza.141 We have shown that overexpression of DAI can be used as an adjuvant strategy to stimulate innate immunity and promote the generation of effector and memory CTLs as well as CD4+ Th1 responses against tumor-associated antigens, conferring long-term antitumor protection.37 Strategies using dual-promoter plasmids co-expressing adaptor molecules such as the Toll-interleukin-1 receptor domain-containing adaptor-inducing β interferon (TRIF) or myeloid differentiation factor 88 (MyD88) along with influenza HA antigen or the tumor-associated antigen E7 have shown to enhance antigen-specific cellular and humoral immune responses and confer superior protection. Examples of co-expression of transcriptional factors downstream to PRRs, include the IRF-1, IRF-3 and IRF-7, which increase IFN-γ-producing CD8+ and CD4+ T cells.142,143
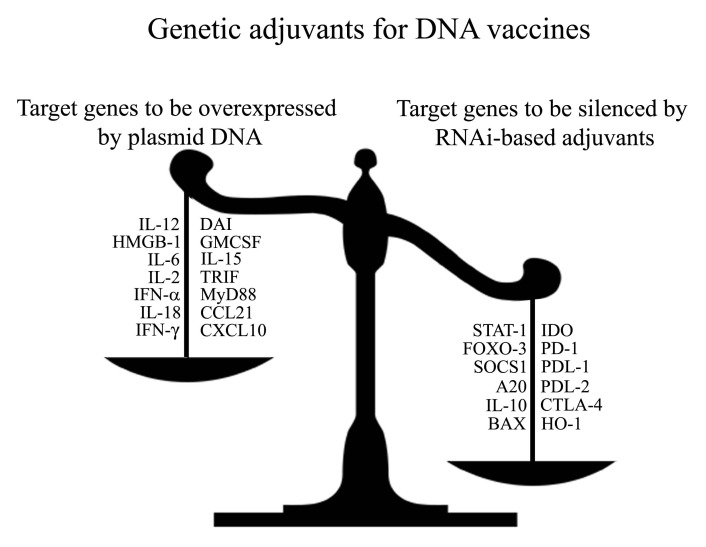
RNA interference (RNAi) technology anti-tumor can also be used to modulate the type and magnitude of the immune responses. Studies targeting either proapototic80,144 or immunosuppressive145 molecules in DCs, have shown to enhance Th1 and CTL antitumor immune responses. RNAi-based genetic adjuvants silencing molecules that inhibit DC function and/or T cell activation represent a different strategy to enhance DNA vaccine potency. A study showed that targeting the key immunosuppressive molecule IDO enhances antitumor protective immunity with or without co-administrating a DNA vaccine encoding a tumor-associated antigen.146,147 Multiple immunosuppressive molecules could be targeted for RNAi, including receptors involved in blocking T cell activation, such as CTLA-4 or PD-1, or their ligands, like PD-L1 or PD-L2.148,149 Additionally, transcriptional factor such as FOXO3, a negative regulator of DC function, could be targeted for suppression in order to promote strong adaptive immune responses.150 Further research is required to evaluate the potential of using RNAi against key immune-suppressive target, as an approach to generate more potent and specific DNA vaccine adjuvants that promote strong immune responses against cancer (Fig. 2). Already preclinical models are showing how plasmid encoded adjuvants, such as DAI,37 or combinations with RNAi technologies targeting immune-suppressive factors can potentiate the immune system.146 Taking these considerations into account and moving new techniques from the preclinical setting to the patients should effectively protect and eliminate cancer.
Figure 2. Genetic adjuvants for enhancing the potency of DNA vaccines against cancer: Overexpression vs. silencing. Genetic (DNA-encoded) adjuvants used for DNA vaccines can be designed to either overexpress immune-stimulatory molecules (left side of the scale), or downregulate the gene expression of inhibitory molecules (right side of the scale).
Acknowledgments
CONICYT Program PFB-16, CONICYT 791100038, FONDECYT 11110525, CORFO-Innova 12IDL2–13348
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22345
References
- 1.Germain RN, Stefanová I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/S0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 3.Lambert PHLM, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med. 2005;11(Suppl):S54–62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 4.Jenner E. Two cases of Small-Pox Infection communicated to the Fœtus in Utero under peculiar circumstances, with additional remarks. Med Chir Trans. 1809;1:271–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–31. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–24. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 9.Fox JL, Klass M. Antigens produced by recombinant DNA technology. Clin Chem. 1989;35:1838–42. [PubMed] [Google Scholar]
- 10.Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347–50. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 11.Stephenne J. Recombinant versus plasma-derived hepatitis B vaccines: issues of safety, immunogenicity and cost-effectiveness. Vaccine. 1988;6:299–303. doi: 10.1016/0264-410X(88)90173-9. [DOI] [PubMed] [Google Scholar]
- 12.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 13.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–4. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 14.Ulmer JBDJ, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 15.Fynan EFWR, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993;90:11478–82. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Wang S, Grimes-Serrano JM. Current progress of DNA vaccine studies in humans. Expert Rev Vaccines. 2008;7:175–91. doi: 10.1586/14760584.7.2.175. [DOI] [PubMed] [Google Scholar]
- 18.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–9. [PubMed] [Google Scholar]
- 19.Doe B, Selby M, Barnett S, Baenziger J, Walker CM. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc Natl Acad Sci U S A. 1996;93:8578–83. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–60. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 23.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 24.Spies B, Hochrein H, Vabulas M, Huster K, Busch DH, Schmitz F, et al. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J Immunol. 2003;171:5908–12. doi: 10.4049/jimmunol.171.11.5908. [DOI] [PubMed] [Google Scholar]
- 25.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 26.Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–72. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 27.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–8. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–94. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 30.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–72. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–91. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Choi MK, Ban T, Yanai H, Negishi H, Lu Y, et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci U S A. 2008;105:5477–82. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181:6427–34. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–13. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeshita F, Tanaka T, Matsuda T, Tozuka M, Kobiyama K, Saha S, et al. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J Virol. 2006;80:6218–24. doi: 10.1128/JVI.00121-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirota H, Ishii KJ, Takakuwa H, Klinman DM. Contribution of interferon-beta to the immune activation induced by double-stranded DNA. Immunology. 2006;118:302–10. doi: 10.1111/j.1365-2567.2006.02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lladser A, Mougiakakos D, Tufvesson H, Ligtenberg MA, Quest AF, Kiessling R, et al. DAI (DLM-1/ZBP1) as a genetic adjuvant for DNA vaccines that promotes effective antitumor CTL immunity. Mol Ther. 2011;19:594–601. doi: 10.1038/mt.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kortylewski M, Kujawski M, Herrmann A, Yang C, Wang L, Liu Y, et al. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res. 2009;69:2497–505. doi: 10.1158/0008-5472.CAN-08-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc Natl Acad Sci U S A. 2012;109:887–92. doi: 10.1073/pnas.1109173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS One. 2011;6:e27040. doi: 10.1371/journal.pone.0027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schirmbeck R, Böhm W, Ando K, Chisari FV, Reimann J. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice. J Virol. 1995;69:5929–34. doi: 10.1128/jvi.69.10.5929-5934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhröber A, Wild J, Pudollek HP, Chisari FV, Reimann J. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int Immunol. 1997;9:1203–12. doi: 10.1093/intimm/9.8.1203. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Dong SF, Sun SH, Wang Y, Li GD, Qu D. Coimmunization with IL-15 plasmid enhances the longevity of CD8 T cells induced by DNA encoding hepatitis B virus core antigen. World J Gastroenterol. 2006;12:4727–35. doi: 10.3748/wjg.v12.i29.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiver JW, Perry HC, Davies ME, Freed DC, Liu MA. Cytotoxic T lymphocyte and helper T cell responses following HIV polynucleotide vaccination. Ann N Y Acad Sci. 1995;772:198–208. doi: 10.1111/j.1749-6632.1995.tb44745.x. [DOI] [PubMed] [Google Scholar]
- 45.Boyer JD, Chattergoon M, Shah A, Ginsberg R, MacGregor RR, Weiner DB. HIV-1 DNA based vaccine induces a CD8 mediated cross-clade CTL response. Dev Biol Stand. 1998;95:147–53. [PubMed] [Google Scholar]
- 46.Uchijima M, Yoshida A, Nagata T, Koide Y. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J Immunol. 1998;161:5594–9. [PubMed] [Google Scholar]
- 47.Schirmbeck R, Riedl P, Kupferschmitt M, Wegenka U, Hauser H, Rice J, et al. Priming protective CD8 T cell immunity by DNA vaccines encoding chimeric, stress protein-capturing tumor-associated antigen. J Immunol. 2006;177:1534–42. doi: 10.4049/jimmunol.177.3.1534. [DOI] [PubMed] [Google Scholar]
- 48.Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011;6:e19252. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–95. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 50.Kang Y, Zheng G, Chen A, Wang J, Hu Y, Li J, et al. Tolerogenic DNA vaccine for prevention of autoimmune ovarian disease. Immunol Invest. 2012;41:249–60. doi: 10.3109/08820139.2011.622828. [DOI] [PubMed] [Google Scholar]
- 51.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–6. [PubMed] [Google Scholar]
- 52.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boon T, van Baren N. Immunosurveillance against cancer and immunotherapy--synergy or antagonism? N Engl J Med. 2003;348:252–4. doi: 10.1056/NEJMe020165. [DOI] [PubMed] [Google Scholar]
- 56.Germeau C, Ma W, Schiavetti F, Lurquin C, Henry E, Vigneron N, et al. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J Exp Med. 2005;201:241–8. doi: 10.1084/jem.20041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marshall J. Carcinoembryonic antigen-based vaccines. Semin Oncol. 2003;30(Suppl 8):30–6. doi: 10.1016/S0093-7754(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 59.Renard V, Leach DR. Perspectives on the development of a therapeutic HER-2 cancer vaccine. Vaccine. 2007;25(Suppl 2):B17–23. doi: 10.1016/j.vaccine.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 60.Zendman AJ, Ruiter DJ, Van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194:272–88. doi: 10.1002/jcp.10215. [DOI] [PubMed] [Google Scholar]
- 61.Robbins PF, el-Gamil M, Kawakami Y, Stevens E, Yannelli JR, Rosenberg SA. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124–6. [PubMed] [Google Scholar]
- 62.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 63.Saaty TL. The analytic hierarchy process: planning, priority setting, resource allocation. McGraw-Hill International Book Co., 1980. [Google Scholar]
- 64.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 69.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 70.Mittendorf EA, Sharma P. Mechanisms of T-cell inhibition: implications for cancer immunotherapy. Expert Rev Vaccines. 2010;9:89–105. doi: 10.1586/erv.09.144. [DOI] [PubMed] [Google Scholar]
- 71.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–77. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–31. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 77.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng S, Kim TW, Lee JH, Yang M, He L, Hung CF, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005;16:584–93. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22:327–34. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng HH, Tseng GY, Yang HB, Wang HJ, Lin HJ, Wang WC. Increased numbers of Foxp3-positive regulatory T cells in gastritis, peptic ulcer and gastric adenocarcinoma. World J Gastroenterol. 2012;18:34–43. doi: 10.3748/wjg.v18.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolchok JD, Yuan J, Houghton AN, Gallardo HF, Rasalan TS, Wang J, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15:2044–50. doi: 10.1038/sj.mt.6300290. [DOI] [PubMed] [Google Scholar]
- 84.Qin FX. Dynamic behavior and function of Foxp3+ regulatory T cells in tumor bearing host. Cell Mol Immunol. 2009;6:3–13. doi: 10.1038/cmi.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heinze E, Baldwin S, Chan G, Hansen J, Song J, Clements D, et al. Antibody-mediated FOXP3 protein therapy induces apoptosis in cancer cells in vitro and inhibits metastasis in vivo. Int J Oncol. 2009;35:167–73. doi: 10.3892/ijo_00000325. [DOI] [PubMed] [Google Scholar]
- 86.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 88.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 90.Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol. 2011;2011:565187. doi: 10.1155/2011/565187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 92.Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4:141–54. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawahara A, Hattori S, Akiba J, Nakashima K, Taira T, Watari K, et al. Infiltration of thymidine phosphorylase-positive macrophages is closely associated with tumor angiogenesis and survival in intestinal type gastric cancer. Oncol Rep. 2010;24:405–15. doi: 10.3892/or_00000873. [DOI] [PubMed] [Google Scholar]
- 94.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–74. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–9. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 96.Guan Y, Zhang M, Li Y, Cao W, Ji M, Liu Y. Vaccination with IA-2 autoantigen can prevent late prediabetic nonobese diabetic mice from developing diabetes mellitus. Diabetes Res Clin Pract. 2012;95:93–7. doi: 10.1016/j.diabres.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 97.Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, et al. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6:e19495. doi: 10.1371/journal.pone.0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hawkins RE, Zhu D, Ovecka M, Winter G, Hamblin TJ, Long A, et al. Idiotypic vaccination against human B-cell lymphoma. Rescue of variable region gene sequences from biopsy material for assembly as single-chain Fv personal vaccines. Blood. 1994;83:3279–88. [PubMed] [Google Scholar]
- 99.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–20. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 100.Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, et al. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 2002;62:5845–52. [PubMed] [Google Scholar]
- 101.Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, et al. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein--based vaccine. J Clin Oncol. 2004;22:1916–25. doi: 10.1200/JCO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 102.Perales MA, Yuan J, Powel S, Gallardo HF, Rasalan TS, Gonzalez C, et al. Phase I/II study of GM-CSF DNA as an adjuvant for a multipeptide cancer vaccine in patients with advanced melanoma. Mol Ther. 2008;16:2022–9. doi: 10.1038/mt.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15:361–7. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20:1269–78. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- 105.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–54. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jechlinger W. Optimization and delivery of plasmid DNA for vaccination. Expert Rev Vaccines. 2006;5:803–25. doi: 10.1586/14760584.5.6.803. [DOI] [PubMed] [Google Scholar]
- 108.Ginsberg BA, Gallardo HF, Rasalan TS, Adamow M, Mu Z, Tandon S, et al. Immunologic response to xenogeneic gp100 DNA in melanoma patients: comparison of particle-mediated epidermal delivery with intramuscular injection. Clin Cancer Res. 2010;16:4057–65. doi: 10.1158/1078-0432.CCR-10-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91:688–94. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuan J, Ku GY, Gallardo HF, Orlandi F, Manukian G, Rasalan TS, et al. Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun. 2009;9:5. [PMC free article] [PubMed] [Google Scholar]
- 111.Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:439. doi: 10.1186/1471-2458-12-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Norell H, Poschke I, Charo J, Wei WZ, Erskine C, Piechocki MP, et al. Vaccination with a plasmid DNA encoding HER-2/neu together with low doses of GM-CSF and IL-2 in patients with metastatic breast carcinoma: a pilot clinical trial. J Transl Med. 2010;8:53. doi: 10.1186/1479-5876-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luxembourg A, Evans CF, Hannaman D. Electroporation-based DNA immunisation: translation to the clinic. Expert Opin Biol Ther. 2007;7:1647–64. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- 114.Stoitzner PSF, Sparber F, Tripp CH. Langerhans cells as targets for immunotherapy against skin cancer. Immunol Cell Biol. 2010;88:431–7. doi: 10.1038/icb.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arnou RIG, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 116.Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol. 1972;10:279–90. doi: 10.1007/BF01867861. [DOI] [PubMed] [Google Scholar]
- 117.Gabriel BJT, Teissié J. Control by electrical parameters of short- and long-term cell death resulting from electropermeabilization of Chinese hamster ovary cells. Biochim Biophys Acta. 1995;1266:171–8. doi: 10.1016/0167-4889(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 118.Mir LMGL, Glass LF, Sersa G, Teissié J, Domenge C, Miklavcic D, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer. 1998;77:2336–42. doi: 10.1038/bjc.1998.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.E. Neumann MS-R Y. Wang, Ph. Hofschneider. Gene transfer into mouse lyoma by electroporation in high electric field. EMBO J. 1982;1:841–5. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heller RJM, Jaroszeski MJ, Glass LF, Messina JL, Rapaport DP, DeConti RC, et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer. 1996;77:964–71. doi: 10.1002/(SICI)1097-0142(19960301)77:5<964::AID-CNCR24>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 121.Mir LMBM, Belehradek M, Domenge C, Orlowski S, Poddevin B, Belehradek J, Jr., et al. [Electrochemotherapy, a new antitumor treatment: first clinical trial] C R Acad Sci III. 1991;313:613–8. [PubMed] [Google Scholar]
- 122.Heller RJM, Jaroszeski M, Atkin A, Moradpour D, Gilbert R, Wands J, et al. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996;389:225–8. doi: 10.1016/0014-5793(96)00590-X. [DOI] [PubMed] [Google Scholar]
- 123.Suzuki TSB, Shin BC, Fujikura K, Matsuzaki T, Takata K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett. 1998;425:436–40. doi: 10.1016/S0014-5793(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 124.Camille Boutin SD, et al. Efficient In Vivo Electroporation of the Postnatal Rodent Forebrain. PLoS ONE. 2008 doi: 10.1371/journal.pone.0001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xue-Feng Ding Y-QZ, et al. Efficient Gene Transfer into Neonatal Mouse Brain Using Electroporation. Neurochem Res. 2012:0–6. doi: 10.1007/s11064-012-0742-0. [DOI] [PubMed] [Google Scholar]
- 126.Blair-Parks KWB, Weston BC, Dean DA. High-level gene transfer to the cornea using electroporation. J Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang Y, Zhao J, Liu Y, Chen A, Zheng G, Yu Y, et al. FK506 as an adjuvant of tolerogenic DNA vaccination for the prevention of experimental autoimmune encephalomyelitis. J Gene Med. 2009;11:1064–70. doi: 10.1002/jgm.1387. [DOI] [PubMed] [Google Scholar]
- 128.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–70. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 129.Lucas MLHL, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther. 2002;5:668–75. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 130.Lucas MLHR, Heller R. IL-12 gene therapy using an electrically mediated nonviral approach reduces metastatic growth of melanoma. DNA Cell Biol. 2003;22:755–63. doi: 10.1089/104454903322624966. [DOI] [PubMed] [Google Scholar]
- 131.Ahlén G, Söderholm J, Tjelle T, Kjeken R, Frelin L, Höglund U, et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007;179:4741–53. doi: 10.4049/jimmunol.179.7.4741. [DOI] [PubMed] [Google Scholar]
- 132.Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, et al. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol. 2004;110:1–10. doi: 10.1016/j.jbiotec.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 133.Chudley L, McCann K, Mander A, Tjelle T, Campos-Perez J, Godeseth R, et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1270-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lladser A, Ljungberg K, Tufvesson H, Tazzari M, Roos AK, Quest AF, et al. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer Immunol Immunother. 2010;59:81–92. doi: 10.1007/s00262-009-0725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;13:320–7. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Agadjanyan MG, Chattergoon MA, Holterman MJ, Monzavi-Karbassi B, Kim JJ, Dentchev T, et al. Costimulatory molecule immune enhancement in a plasmid vaccine model is regulated in part through the Ig constant-like domain of CD80/86. J Immunol. 2003;171:4311–9. doi: 10.4049/jimmunol.171.8.4311. [DOI] [PubMed] [Google Scholar]
- 137.Kim JJ, Yang JS, Dentchev T, Dang K, Weiner DB. Chemokine gene adjuvants can modulate immune responses induced by DNA vaccines. J Interferon Cytokine Res. 2000;20:487–98. doi: 10.1089/10799900050023906. [DOI] [PubMed] [Google Scholar]
- 138.Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–601. [PubMed] [Google Scholar]
- 139.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–25. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther. 2006;17:1051–61. doi: 10.1089/hum.2006.17.1051. [DOI] [PubMed] [Google Scholar]
- 141.Fagone P, Shedlock DJ, Bao H, Kawalekar OU, Yan J, Gupta D, et al. Molecular adjuvant HMGB1 enhances anti-influenza immunity during DNA vaccination. Gene Ther. 2011;18:1070–7. doi: 10.1038/gt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sasaki S, Amara RR, Yeow WS, Pitha PM, Robinson HL. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J Virol. 2002;76:6652–9. doi: 10.1128/JVI.76.13.6652-6659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Castaldello A, Sgarbanti M, Marsili G, Brocca-Cofano E, Remoli AL, Caputo A, et al. Interferon regulatory factor-1 acts as a powerful adjuvant in tat DNA based vaccination. J Cell Physiol. 2010;224:702–9. doi: 10.1002/jcp.22169. [DOI] [PubMed] [Google Scholar]
- 144.Dharmapuri S, Aurisicchio L, Biondo A, Welsh N, Ciliberto G, La Monica N. Antiapoptotic small interfering RNA as potent adjuvant of DNA vaccination in a mouse mammary tumor model. Hum Gene Ther. 2009;20:589–97. doi: 10.1089/hum.2008.210. [DOI] [PubMed] [Google Scholar]
- 145.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–53. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 146.Yen MC, Lin CC, Chen YL, Huang SS, Yang HJ, Chang CP, et al. A novel cancer therapy by skin delivery of indoleamine 2,3-dioxygenase siRNA. Clin Cancer Res. 2009;15:641–9. doi: 10.1158/1078-0432.CCR-08-1988. [DOI] [PubMed] [Google Scholar]
- 147.Huang TT, Yen MC, Lin CC, Weng TY, Chen YL, Lin CM, et al. Skin delivery of short hairpin RNA of indoleamine 2,3 dioxygenase induces antitumor immunity against orthotopic and metastatic liver cancer. Cancer Sci. 2011;102:2214–20. doi: 10.1111/j.1349-7006.2011.02094.x. [DOI] [PubMed] [Google Scholar]
- 148.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 149.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 150.Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–13. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]