Abstract
Myleodysplastic syndromes (MDS) are pre-malignant hematopoietic diseases that can progress to acute myeloid leukemia (AML) progression in conjunction with changes in immune function. In this model of leukemia evolution, the expansion of immunosuppressive regulatory T cells (Tregs) contributes to immune escape. Here, we discuss the importance of Treg-memory phenotype switching as a poor prognostic indicator in MDS.
Keywords: regulatory T cells, myelodysplastic syndrome, immunoediting, immune escape, leukemia
In immunocompetent hosts, developing neoplasms are shaped by a dynamic evolutionary process coined “immunoediting” that involves changes in the tumor, local microenvironment and the immune system.1 The “immunoediting” model is heavily based on elegant mouse studies of chemically induced neoplasms and consists of three stages: elimination, equilibrium and escape. Newly transformed cells are susceptible to immune-mediated clearance, (i.e., elimination) owing to the presentation of altered or overexpressed self antigens, or through mechanisms mediated by innate immunity. Under this control, neoplastic cells undergo prominent antigenic and biological changes (i.e., in the equilibrium phase), which eventually allow them to growth unrestrained by the immune system (i.e., escape).
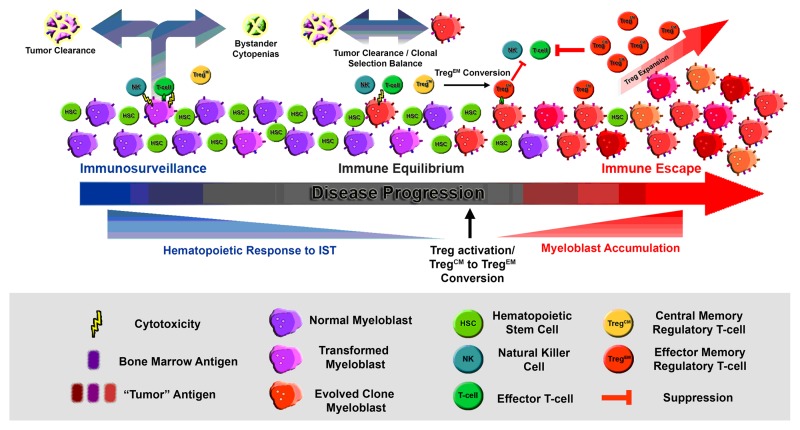
The processes leading to permissive growth of naturally arising human tumors is poorly understood, but is linked to elevated levels of regulatory T cells (Tregs), which suppress productive antigen-specific antitumor effector responses. Tregs are expanded in non-small cell lung carcinoma, ovarian cancer, breast, pancreatic and colon cancer in response to tumor-associated antigens.2 Myelodysplastic syndromes (MDS) are genetically and morphologically diverse hematopoietic neoplasms that may be subjected to a process of immunoediting during development. Evidence is mounting that the heterogeneity of MDS and leukemia progression is driven though inflammation and immune suppression.3 Consistent with an effector phase, it is clear that key immunologic molecules function as extrinsic tumor suppressors in some patients affected by this disease. These molecules are not only toxic to developing leukemic blasts, but also destroy normal hematopoietic progenitors through antigen cross-reactivity or through indirect mechanisms of cytokine-mediated suppression (Fig. 1). The production of interferon γ—which has the ability to kill bone marrow progenitors, tumor necrosis factor, FAS ligand and TRAIL—is elevated within the bone marrow of MDS patient subses.3-6 Features of an “effector” disease state in MDS are pancytopenia, low blast counts, dysplasia and T-cell responses to leukemia-associated antigens.6 Years of clinical evidence have shown that immunosuppressive therapy (IST) based on agents such as cyclosporine or anti-thymocyte globulin (ATG) can effectively improve hematopoiesis in this highly selected subgroup of patients.5 Mechanistic evidence of an equilibrium phase is less clear in MDS, but variable periods of dormancy prior to disease progression are well documented.7
Figure 1. A schematic representation of regulatory T-cell memory phenotype switching in the context of MDS immunoediting as it relates to immunosuppression and response to immunosuppressive therapy.
Tregs are known to play a prominent immunosuppressive role in patients with de novo AML,8 and recent studies have confirmed the presence of increased numbers of Tregs in patients with high-risk MDS.9 Blast expansion in MDS occurs through the progressive accumulation of genetic mutations accompanied by immune suppression, but the mechanism(s) contributing to this pivotal switch in pathophysiology is unknown. In a study by Mailloux, et al.,10 the authors hypothesized that phenotypic features associated with conventional effector cell activation may also be linked to Treg activation and/or expansion during the progression of MDS to leukemia. In a retrospective study, these authors investigated the phenotypic features of Treg subsets including naïve, central memory and effector memory cells, in association with disease progression. They found that the majority of peripheral Tregs in healthy individuals display a central memory phenotype (TregCM: CD3+CD4+FOXP3+CD25+CD127dimCD27+CD45RA-), while a subset of MDS patients display a significant shift toward an effector memory phenotype (TregEM: CD3+CD4+FOXP3+CD25+CD127dimCD27-CD45RA-). Patients with increased percentage and absolute number of TregEM cells had a higher percentage of abnormal bone marrow myeloblasts compared with patients with normal Treg profiles, or to patients with high numbers of other Treg subtypes. If they were analogous to conventional T cells, TregCM cells may represent an inactive, long-term memory population, while TregEM cells are likely to constitute a currently or more recently activated population. In support of this notion, isolated TregEM cells were shown to be significantly more suppressive than TregCM cells in vitro. Importantly, TregEM cells were seen in many patients without an increased number of total Tregs, suggesting that a transition to the TregEM phenotype, possibly through antigen exposure, may precede expansion of the entire Treg compartment.
It was then hypothesized that the presence of TregEM cells may serve as a prognostic indicator. There are several well-defined prognostic models to estimate survival and risk for leukemia progression of MDS patients. The most widely used models include the International Prognostic Scoring System (IPSS), which is based on newly-diagnosed cases, and the MD Anderson Risk Assessment Model (MDAS) with improved prognostic potential in patients with established disease.7 Overall survival was examined using univariate and multivariate analyses based on patients grouped by Treg status (normal, high TregCM, high TregEM and high overall Treg numbers). The presence of high TregEM cells was associated with significantly reduced overall survival in this patient population. Moreover, the presence of such highly suppressive TregEM cells was shown to be independent from that of other Treg subtypes as well as from established MDS risk factors in multivariate models.10
While future studies of isolated Treg subpopulations are needed to determine if TregEM cells are recently activated in response to tumor-associated antigens, monitoring their induction may serve as a surrogate marker denoting immune escape and disease progression (Fig. 1). If fully validated, the analysis of the TregEM phenotype using flow cytometry may be a simple and useful tool to predict an early immune escape in MDS patients. Beyond MDS, this could have utility in other premalignant diseases such as cervical dysplasia, pre-malignant head and neck cancer, colorectal polyps or inflammatory bowel disease, in which the transition to tumor-induced immunosuppression may have prognostic importance for covert malignant transformation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22654
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–36. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epling-Burnette PK, List AF. Advancements in the molecular pathogenesis of myelodysplastic syndrome. Curr Opin Hematol. 2009;16:70–6. doi: 10.1097/MOH.0b013e3283257ac7. [DOI] [PubMed] [Google Scholar]
- 4.Calado RT. Immunologic aspects of hypoplastic myelodysplastic syndrome. Semin Oncol. 2011;38:667–72. doi: 10.1053/j.seminoncol.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sloand EM, Barrett AJ. Immunosuppression for myelodysplastic syndrome: how bench to bedside to bench research led to success. Hematol Oncol Clin North Am. 2010;24:331–41. doi: 10.1016/j.hoc.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sloand EM, Melenhorst JJ, Tucker ZC, Pfannes L, Brenchley JM, Yong A, et al. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood. 2011;117:2691–9. doi: 10.1182/blood-2010-04-277921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–61. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118:5084–95. doi: 10.1182/blood-2011-07-365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110:847–50. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 10.Mailloux AW, Sugimori C, Komrokji RS, Yang L, Maciejewski JP, Sekeres MA, et al. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J Immunol. 2012;189:3198–208. doi: 10.4049/jimmunol.1200602. [DOI] [PMC free article] [PubMed] [Google Scholar]