Abstract
In a mastocytoma model expressing mutant c-KIT, a combination of the c-KIT inhibitor dasatinib and an OX40-specific monoclonal antibody synergized in triggering a strong antitumor T-cell response that promoted curative therapeutic effects. Along with a number of other recent studies, these data support the notion that combining targeted agents with immunotherapy may constitute a promising approach for cancer therapy.
Keywords: targeted therapy, immunotherapy, dasatinib, anti-OX40, C-KIT mutant tumors
The identification of cellular proto-oncogenes, one of the most important achievements in biomedical history, has quickly led to the development of a number of targeted drugs that can specifically eradicate neoplastic cells. c-KIT is a proto-oncogenic tyrosine kinase receptor that is overexpressed or mutated in several human malignancies, including gastrointestinal stromal tumors (GISTs), melanoma, mast cell leukemia, mastocytoma and germ cell tumors. The administration of c-KIT inhibitors has significantly improved the prognosis of GIST patients bearing c-KIT mutations, and has altered the natural history of this disease.1 However, although most targeted agents are capable of inducing dramatic responses in some patients, they rarely promote complete or durable antitumor effects. Indeed, due to the heterogeneous nature of cancer cells and the emergence of secondary resistance, most patients receiving targeted therapy eventually relapse. Still, accumulating evidence suggests that combining targeted therapy with immunotherapeutic regimens may improve disease outcome in cancer patients.
There are a number of rationales for combining immunotherapy with molecularly targeted drugs. First, although some targeted agents have been shown to induce very high response rates, these responses are most often transient. By contrast, immunotherapeutic approaches, which generally result in low response rates, can sometimes lead to complete responses that can last for years. Therefore, the combination of these two approaches may achieve both potent and long-lasting antitumor effects. In addition, targeted agents and immunotherapy exert antineoplastic effects by completely distinct mechanisms, raising the possibility that targeted drug-resistant tumor cells might be eradicated by cytotoxic lymphocytes, resulting in improved therapeutic effects. Second, recent studies have supported the notion that the constitutive activation of oncogene signaling upregulates a number of immunosuppressive mechanisms in vivo.2 Thus, the efficacy of immunotherapy may be increased by oncogene-targeting agents. In addition, some tyrosine kinase inhibitors exert beneficial off-target effects on immunosuppressive immune cells. For example, sunitinib treatment results in the inhibition of myeloid-derived suppressor cells (MDSCs) and FOXP3+ regulatory T cells (Tregs).3 Third, a number of cytotoxic regimens have been shown to induce antitumor immune responses by promoting massive tumor cell death and hence an intense release of tumor-associated antigens. In fact, there is very good evidence indicating that the development of an antitumor immune response might significantly contribute to the therapeutic benefits of chemotherapy, radiotherapy and targeted therapy.4 Thus, immunostimulatory strategies that augment such an immune response might result in improved antitumor effects. Our recent results demonstrate that such a combinatorial approach can indeed generate potent antitumor immunity, resulting in a superior therapeutic outcome.5
Using P815 mastocytomas as a model, we demonstrated that a 3-day dasatinib course triggers a strong antitumor T-cell response that significantly contributes to the therapeutic effects of this targeted agent. Tumor antigens from dying tumor cells likely promoted the development of this response, and decreased Treg levels following treatment may have also played a role in such an enhanced antitumor effect. We also found that dasatinib can significantly enhance T-cell responses upon vaccination of tumor-free mice.5 Similarly, other tyrosine kinase inhibitors including sunitinib, imatinib, sorafenib and nilotinib have been shown to significantly inhibit Tregs.6 Therefore, they are all potential candidates for reversing immunosuppression in the setting of targeted therapy-immunotherapy combinations.
A number of agonistic antibodies specific for T cell co-stimulatory molecules have generated promising results both in animal studies and in clinical trials. Among these molecules, OX40 is known to play an important role in the regulation of T-cell proliferation, activation, differentiation and migration. Notably, most mice in our study were cured when treated with dasatinib plus anti-OX40. We found that the major effect of anti-OX40 in this model is the enhanced recruitment of effector T cells into the tumor microenvironment. Significantly higher levels of tumor-specific T cells were present in the neoplastic lesions of mice receiving the combination regimen, and high levels of interferon γ (IFNγ)-induced TH1 cytokines such as CXCL9, CXCL10 and CXCL11, correlated with the local amounts of tumor-specific T cells. These data support a model in which a positive feedback loop composed of cytotoxic T lymphocytes (CTLs), IFNγ, and TH1 cytokines favors the migration of CTLs into malignant lesions (Fig. 1). Since dasatinib and anti-OX40 antibodies have both been shown to negatively interfere with the suppressive function of Tregs, this may have also contributed to the antitumor activity of our combination regimen.5 With the recent development of antibodies targeting CTLA-4 and PD-1/PD-L1, resulting in checkpoint blockade, a number of clinical trials combining these agents with oncogene-targeted therapies have been launched. It remains to be determined whether a similar positive feedback loop leading to antitumor immunity can be generated with these combination therapies.
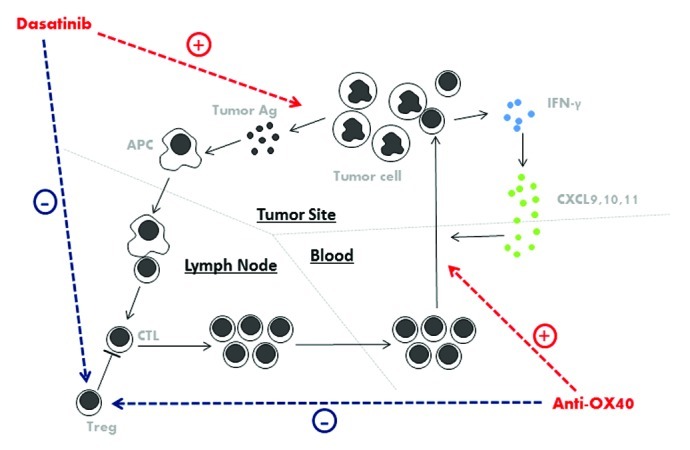
Figure 1. Mechanisms underlying the antineoplastic effects of dasatinib combined with anti-OX40 antibody. Dasatinib leads to the demise of tumor cells expressing mutant c-KIT, hence promoting the release of tumor antigens. Antigen-presenting cells take up the tumor-cell debris, process them and present tumor-associated antigens to T cells, thus promoting tumor-specific T-cell priming. Co-stimulatory signals provided by anti-OX40 helps circulating cytotoxic T lymphocytes (CTLs) to migrate into the tumor microenvironment. In response to interferon γ (IFNγ) released by T cells, CXCL9, CXCL10 and CXCL11 are produced, recruiting further CTLs to the tumor site. Besides, both dasatinib and anti-OX40 exert inhibitory effect on FOXP3+ regulatory T cells (Tregs).
Potential deleterious off-target effects of tyrosine kinase inhibitors on lymphocytes are of concern for immunotherapy-based approaches. Dasatinib has been reported to negatively regulate T-cell proliferation and function, by interfering with signaling of Src family members.7 However, our study demonstrates that a short-term (3 days) dasatinib course elicits potent tumor-specific T-cell responses, while prolonged (8 days) administration does not.5 These data suggest that short-term pulses or the intermittent administration of tyrosine kinase inhibitors might be optimal. Hence, preserving the immune response elicited by molecularly targeted agents may be achieved with careful dosing and scheduling.
Recently, several publications have reported synergistic effects between oncogene-targeted agents and immunotherapy. For example, the oncogenic B-RAF inhibitor vemurafenib plus adoptive T-cell transfer resulted in superior antitumor effects in a mouse melanoma model.8 Imatinib, another c-KIT inhibitor, was shown to synergize with anti-CTLA-4 antibodies in a GIST mouse model.9 Finally, the combination of imatinib with IFNα has also generated promising results in a recent clinical trial enrolling GIST patients.10 These studies support our experimental results and suggest that combinations of targeted therapy and immunotherapy may result in improved antitumor responses. Future potential clinical trials may combine oncogene-targeted agents with adoptive T-cell transfer, cancer vaccines, or co-stimulatory/checkpoint blockade antibodies. Based on the accumulated evidence, we believe that the general strategy of combining targeted therapies with immunotherapies will provide benefits to many cancer patients in the years to come.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22730
References
- 1.Lennartsson J, Rönnstrand L. The stem cell factor receptor/c-Kit as a drug target in cancer. Curr Cancer Drug Targets. 2006;6:65–75. doi: 10.2174/156800906775471725. [DOI] [PubMed] [Google Scholar]
- 2.Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, et al. Oncogenic BRAF(V600E) Promotes Stromal Cell-Mediated Immunosuppression Via Induction of Interleukin-1 in Melanoma. Clin Cancer Res. 2012;18:5329–40. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Liu C, Peng W, Lizée G, Overwijk WW, Liu Y, et al. Anti-tumor T cell responses contribute to the effects of dasatinib on c-KIT mutant murine mastocytoma and are potentiated by anti-OX40. Blood. 2012:in press. doi: 10.1182/blood-2012-02-407163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine A, Held SA, Bringmann A, Holderried TA, Brossart P. Immunomodulatory effects of anti-angiogenic drugs. Leukemia. 2011;25:899–905. doi: 10.1038/leu.2011.24. [DOI] [PubMed] [Google Scholar]
- 7.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111:1366–77. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012;72:3928–37. doi: 10.1158/0008-5472.CAN-11-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LL, Chen X, Choi H, Sang H, Chen LC, Zhang H, et al. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol Immunother. 2012;61:1113–24. doi: 10.1007/s00262-011-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


