Abstract
Immunosuppressive tumor microenvironments limit the efficacy of T cell-based immunotherapy. We have recently demonstrated that the inhibition of BRAFV600E with vemurafenib relieves interleukin-1 (IL-1)-induced T-cell suppression as mediated by melanoma tumor associated fibroblasts (TAFs). These results suggest that inhibitors of the MAPK pathway in combination with T cell-based immunotherapies may induce long-lasting and durable responses.
Keywords: BRAF, interleukin-1, tumor-associated fibroblasts, immune suppression, cytotoxic T cells, PD-1 ligands, COX-2
T cell-based immunotherapies have the potential to induce long-lasting complete remissions in cancer patients. However, their efficacy is often limited by immunosuppressive cells and factors found within the tumor microenvironment, which protect the tumor from immune recognition and killing by cytotoxic T lymphocytes (CTLs).1 While several tumor-specific mechanisms of immunosuppression have been described, how immunosuppression is initiated and sustained within neoplastic lesions to promote tumor growth remains to be elucidated. We have recently shown that immunosuppression can be initiated by oncogenic BRAF, which is mutated to a constitutively active form, BRAFV600E, in > 50% of melanoma patients.2 This pathway of immunosuppression involves a molecular cross-talk between BRAFV600E-expressing tumor cells and tumor-associated fibroblasts (TAF) and features the upregulation of a transcriptional program involving multiple immunomodulatory genes known to inhibit immune responses (Fig. 1). Collectively, our results suggest that cancer-associated chronic immunosuppression may be relieved through the pharmacological inhibition of the MAPK signaling pathway in tumor cells, and predict that such an intervention would strongly synergize with immunotherapy.
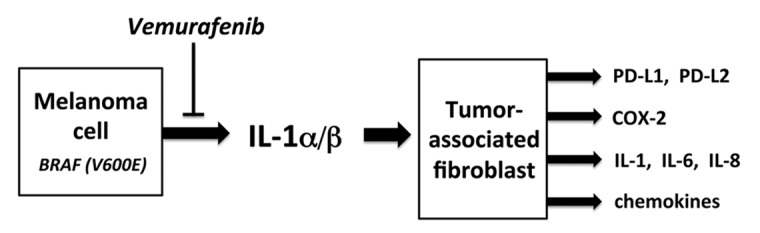
Figure 1. Mechanisms of BRAFV600E-driven immunosuppression. The activation of the MAPK signaling pathway in melanoma cells by oncogenic BRAFV600E leads to the production of interleukin 1 (IL-1)α/β. Tumor-associated fibroblasts (TAFs) respond to IL-1 by upregulating an immunomodulatory transcriptional program resulting in the production of COX-2, PD-1 ligands and chemokines, as well as in the amplification of IL-1 signaling. Collectively, these factors and signaling circuitries suppress cytotoxic T lymphocyte (CTL) functions.
Although the BRAFV600E-mediated activation of the MAPK signaling pathway in tumor cells has been linked to tumor cell proliferation, survival and metastasis, attempts to gain a simplified understanding of its transcriptional impact have presented multiple challenges, including the lack of reproducibility of transcriptional array-based signatures obtained with panels of melanoma cell lines.3 In our study, we initiated a screen of immunologically relevant transcriptional targets through the ectopic expression of oncogenic BRAF in primary human melanocytes, thus measuring differential expression in the absence of other mutations. Although we observed the BRAFV600E-induced transactivation of genes coding for both interleukin-1α (IL1A) and interleukin-1β (IL1B) in melanocytes and wild-type BRAF-expressing melanoma cells, the analysis of clinical specimens failed to demonstrate a clear correlation between the V600E mutation and the production of these cytokines. For example, we found that IL-1α was expressed in ~85% of melanoma metastases examined, far exceeding the rate of V600E mutations. In addition, IL-1β was detected in only ~15% of tumors analyzed, suggesting that the expression of these cytokines is under complex regulation in vivo. Nevertheless, when we examined tumor biopsies from patients prior to therapy and on-treatment with the BRAFV600E inhibitor vemurafenib, we observed a clear reduction of IL-1α expression in 11 of 12 patients evaluated, confirming our findings in V600E-positive melanoma cell lines treated with verurafinib in vitro.
IL-1 is a pleiotropic cytokine that is expressed by many tumor types. Although in different experimental models it exerts either protumor or antitumor functions4, rheumatoid arthritis patients receiving a recombinant IL-1 receptor antagonist, Anakinra, do not develop tumors at an increased frequency.5 Since IL-1 has been reported to influence stromal cell migration and survival, we became interested in how IL-1 may affect the expression of immunomodulatory genes in TAFs. We found that fibroblasts isolated from melanoma tumors, when pre-treated with IL-1α, suppressed interferon γ (IFNγ) secretion by CTLs and their degranulation. Gene expression studies revealed significant changes in the transcription of roughly 200 genes in TAFs exposed to IL-1. These included at least 10 genes linked to T-cell immunosuppression, including PDL1, PDL2 and COX2, a signature that was consistent across TAFs obtained from lung, soft tissue and lymph node metastases. Antibody-mediated blockade and COX-2 inhibition experiments showed that IL-1, COX-2, and PD-1 ligands all contribute—to partial extents—to TAF-mediated T-cell suppression. This said, our microarray data suggest that other mechanisms may contribute to this process. Indeed, the administration of vemurafenib to three distinct BRAFV600E-expressing melanoma cell lines largely compromised their capacity to generate secreted factors that stimulate TAF-mediated CTL immunosuppression.
Our results imply that the oncogene-driven activation of the MAPK pathway can promote the production of a number of immunomodulatory factors by tumor cells. Among these factors, IL-1 alone can induce a transcriptional program that stimulates the immunosuppressive activity of TAFs. Thus, the effects of oncogene activation on the tumor microenvironment are significant and multifactorial and in general seem to favor immunosuppression (Fig. 1). Overall, our data predict that the use of immune-sparing BRAFV600E inhibitors may increase the efficacy of T cell-based immunotherapy, even in synergistic fashion.6 Recent studies from other groups support this notion. BRAF inhibition has been shown to increase T-cell tumor infiltration in patients, stimulate the expression of melanocyte differentiation antigens in tumor cells, and ameliorate antitumor responses in murine models.7,8 A number of planned or ongoing clinical trials that combine oncogene-targeted agents with immunotherapies will test this hypothesis in a clinical setting.
Since IL-1α was expressed in ~85% of melanoma tumor specimens examined, it is possible that mechanisms other than the activation of the MAPK pathway drive IL-1 production in this setting. These may include signaling pathways such as those driven by NRAS, GNAQ, GNA11, or C-KIT, all of which are mutated in a fraction of melanoma patients. At present, no specific clinical inhibitors are available for these proteins. However, our results suggest that the blockade of IL-1 may provide clinical benefit to wild-type BRAF-expressing patients, especially when combined with immunotherapy. Anakinra and other clinical reagents that block IL-1 are already available, and clinical trials in cancer patients are currently ongoing to test the efficacy of this compound as a standalone intervention. In murine models, IL-1 has been shown to be necessary for the recruitment of myeloid derived suppressor cells (MDSCs) in spontaneous and implanted tumors.9 Additional studies have shown that IL-1 can directly promote the growth and survival of melanoma cells.10 Hence, it appears that there is a strong rationale for clinically targeting IL-1 within the melanoma tumor microenvironment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22745
References
- 1.Lizée G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 2006;12:4794–803. doi: 10.1158/1078-0432.CCR-06-0944. [DOI] [PubMed] [Google Scholar]
- 2.Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, et al. Oncogenic BRAF(V600E) Promotes Stromal Cell-Mediated Immunosuppression Via Induction of Interleukin-1 in Melanoma. Clin Cancer Res. 2012;18:5329–40. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoek KS. DNA microarray analyses of melanoma gene expression: a decade in the mines. Pigment Cell Res. 2007;20:466–84. doi: 10.1111/j.1600-0749.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 4.Apte RN, Voronov E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–41. doi: 10.1111/j.1600-065X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, Pollono EN, Cueto JP, Gonzales-Crespo MR, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA. 2012;308:898–908. doi: 10.1001/2012.jama.10857. [DOI] [PubMed] [Google Scholar]
- 6.Hong DS, Vence L, Falchook G, Radvanyi LG, Liu C, Goodman V, et al. BRAF(V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clin Cancer Res. 2012;18:2326–35. doi: 10.1158/1078-0432.CCR-11-2515. [DOI] [PubMed] [Google Scholar]
- 7.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 8.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–94. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 9.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 10.Qin Y, Ekmekcioglu S, Liu P, Duncan LM, Lizée G, Poindexter N, et al. Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol Cancer Res. 2011;9:1537–50. doi: 10.1158/1541-7786.MCR-11-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]


