Abstract
Myeloid-derived suppressor cells (MDSCs) promote immune evasion, a key feature of oncogenesis. The restoration of immunosurveillance by immunomodulatory antibodies improves the survival of a subset of cancer patients. Preclinical studies suggest that the ablation of monocytic MDSCs may be a useful adjunct to available immunotherapeutic strategies against cancer.
Keywords: CCR2, myeloid derived suppressor cells, GM-CSF, immunotherapy, adoptive T-cell transfer
A multitude of overlapping immunomodulatory pathways has evolved to maintain the normal immune homeostasis. Prominent immunosuppressive mechanisms involve transmembrane receptors expressed by effector T cells, such as CTLA-4, and immunosuppressive cell populations such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Tumor growth occurs in a complex microenvironment, along with the formation of a stroma admixed with both immune effector and immune suppressor cells. Results from preclinical studies suggest that favorable disease outcomes are associated with increases in effector to suppressor T-cell ratios at the tumor site.1 In humans, an increased density of CD8+ effector T cells, memory cells and TH1 cytokines at the tumor site have been shown to correlate with a favorable clinical outcome (reviewed in ref. 2). Thus, enabling effector tumor infiltration by TH1 cells and overcoming immunosuppressive factors of the tumor stroma appears to be critical for the success of immunotherapy. In this commentary, we highlight our recent work dissecting MDSC heterogeneity and highlighting how a monocytic MDSC subset limits intratumoral T-cell accumulation and hence the efficacy of T-cell immunotherapy (Fig. 1).3
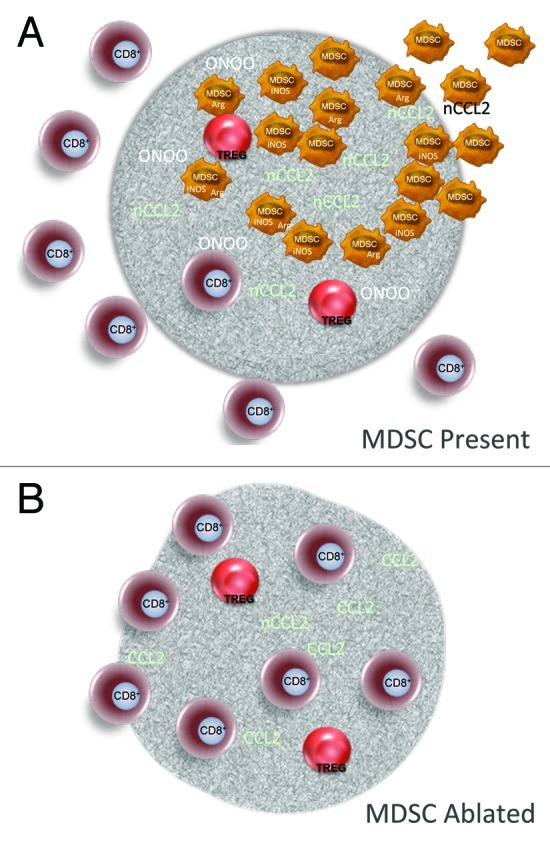
Figure 1. Targeting MDSCs to improve the therapeutic outcome of anticancer immunotherapy. (A) The accumulation of myeloid derived suppressor cells (MDSCs) at the tumor site leads to the formation of peroxynitrite via the concerted action of inducible nitric oxide synthetase (iNOS) and arginase. The nitrosylation of substrates such as CCL2 constitutes one mechanism limiting the intratumoral accumulation of CD8+ T cells. (B) The ablation of MDSCs limits CCL2 nitrosylation, enhances antigen specific CD8+ T-cell activation, and allows for the intratumoral accumulation of T cells, leading to tumor shrinkage.
MDSCs constitute an important fraction of the tumor stroma and can also be detected in the peripheral blood and lymphoid organs. MDSCs are a heterogeneous population of myeloid cells that interfere with T-cell function.4 More than 20 secreted factors have been reported to expand functional MDSCs, which in mice express CD11b and (usually) GR1 (Ly6G and Ly6C). Interestingly, immunosuppressive functions have been ascribed to both monocytic and granulocytic cells based on variations in GR1 expression levels as well as on the expression of other surface markers (e.g., CD115, F4/80 and IL-4Rα). These variations may perhaps reflect to type of tumor under investigation.4 Nevertheless, as a result of this heterogeneity, both surface markers and the ability to suppress T-cell proliferation in vitro are required to correctly identify MDSCs.
The complexity of correctly identifying MDSCs in turn creates challenges in dissecting the biology of these cells in vivo. In an effort to address this issue with a simple experimental model, we used a B16 murine melanoma system engineered to overexpress granulocyte macrophage colony-stimulating factor (GM-CSF), a growth factor that is critically involved in the expansion and activation of MDSCs. The absence of functional MDSC expansion by wild-type, parental B16 cells allowed us to assess the effects of one single growth factor on the biology of MDSCs. In line with previous reports, we found that GM-CSF stimulates the proliferation of MDSCs.5 GM-CSF-expanded cell populations were a mixture of granulocyte- and monocyte-derived myeloid cells. CCR2, a chemokine receptor expressed at the highest density by inflammatory monocytes and required for monocyte to exit the bone marrow, allowed for the discrimination of these 2 cell subsets and further functional studies. Hence, we demonstrated that only monocytic (CCR2+CD11b+) MDSCs harbors T-cell suppressive function in this model. Importantly, CCR2+ MDSCs can be identified in other tumor models as well as in melanoma patients, suggesting that CCR2 is a useful marker for the identification of monocytic MDSCs in general.
The accumulation of MDSCs at the tumor site can contribute to the paucity of T cells and to immune escape via a number of mechanisms, including arginine depletion and the release of reactive nitrogen species.6 The latter reduce the numbers of CD8+ T cells by a proximity-dependent inhibition of priming or via the nitrosylation of chemokines such as CCL2, the main ligand of CCR2.6,7 CCL2 nitrosylation promotes the intratumoral recruitment of monocytic MDSCs over that of CD8+ T cells, due to the fact that MDSCs express a high density of CCR2 on their plasma membrane. Our findings suggest that these mechanisms can be reversed and that a clinically relevant accumulation of CD8+ T cells at the tumor site can be restored through the therapeutic ablation of monocytic MDSCs.
The role of MDSCs in the immune escape of cancer in patients receiving active immunotherapy is not as well characterized as their immunosuppressive activity in preclinical models. We have evaluated the phenotype and function of CD14+HLA-DRlow/- monocytic cells, which have been described as MDSCs in melanoma patients.8 Our group has recently observed that a decrease in monocytic MDSCs is associated with a striking clinical response in a melanoma patient treated with radiation and the CTLA-4-blocking antibody ipilimumab.9 Although generalized conclusions cannot be drawn from a single case, these findings suggest that a low frequency of MDSCs may be a convenient clinical marker of a switch from immune evasion to the immune-mediated elimination of melanoma. Interestingly, a preliminary study assessing MDSC frequency and function in melanoma patients prior to treatment with ipilimumab found a correlation between MDSC frequency and overall survival, suggesting that MDSC may serve both as a predictive and pharmacodynamic marker of treatment outcome.10
Although strategies that alter MDSC differentiation, function or abundance are currently being evaluated in various phases of clinical development, a therapeutic reagent that specifically ablates monocytic MDSCs in patients will have to overcome the phenotypic diversity of these cells.8 In addition, for the development of meaningful MDSC-targeting strategies, such a heterogeneity will have to be characterized detail in patients with distinct clinical outcomes. We believe that our preliminary observations provide strong rationale for the exploration of therapeutic strategies that specifically target monocytic MDSCs in humans in combination with active immunotherapeutic approaches such as adoptive T-cell therapy and CTLA-4 blockade.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22764
References
- 1.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 3.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–86. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35:107–15. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 9.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitano S, Postow MA, Cortez C, et al. Myeloid-derived suppressor cell quantity prior to treatment with ipilimumab at 10mg/kg to predict for overall survival in patients with metastatic melanoma. ASCO Annual Meeting. Vol. 30. Chicago, IL: J Clin Oncol; 2012. [Google Scholar]


