Abstract
The polarization of TH1 or TH2 responses by dendritic cells (DCs) requires distinct maturation conditions. Our data indicate that quantitative differences in DC maturation dictate a TH1 or TH2-cell polarization outcome. We discuss how chromatin remodeling at DC loci coding for pro-inflammatory vs. polarizing cytokines may explain differential TH-cell polarization.
Keywords: chromatin, cytokines, dendritic cells, HDAC, histone, epigenetic, polarization, TH2
The requirements for TH2 polarization in terms of nature and activation state of antigen-presenting cells, and more specifically the dendritic cell (DC) subset and maturation stimuli, are not fully understood. In fact, TH2 cells may be generated under different conditions. Here, we discuss our recent findings about the common features of different murine TH2-polarizing DCs.1 Our data indicate that quantitative differences in DC maturation dictate TH1 vs. TH2 cell-polarization. While strong DC maturation signals activate up to 5,000 genes and lead to a TH1 shift, a weaker DC maturation stimulus induces 10- to 20-fold fewer genes and hence promotes the development of TH2 cells.
Reports on the requirements for TH2-cell polarization differ to considerable extents. Some authors report that TH2 responses can develop via a default pathway, i.e., that the absence of interleukin (IL)-12p70 production is sufficient for the maturation of TH2 cells.2 Other groups found that the differentiation of TH1 vs. TH2 effector cells depend on a peptide dose and/or binding affinity.3 Finally, differential expression of the Notch ligands Jagged-1 and -2 on antigen-presenting cells has been proposed as a decisive element for the development of TH2 responses.4 These observations suggest that the absence of an active polarizing signal, especially under weak T-cell stimulatory conditions, is sufficient to promote TH2 immunity, although specific ligands may exist that promote TH2-cell polarization by DCs.
It appears that helminth-derived products evoke only mild transcriptional alterations in DCs, resulting in a immature/partially mature DC phenotype,5 similar to that we observed when DCs were exposed to endogenous pro-inflammatory factors such as tumor necrosis factor α (TNFα).6 Partially mature DCs exert tolerizing but also TH2-cell polarizing functions. Partial DC maturation is characterized by the upregulation of MHC Class II and co-stimulatory molecules along with the absent production of cytokines.. Partially mature DCs as elicited by TNFα induce the differentiation of IL-4+ TH2 cells after a single round of T-cell stimulation in vitro and in vivo.1 Repetitive injections of TNFα-matured DCs prevented the induction of experimental autoimmune encephalomyelitis (EAE) by the shift from TH2 toward IL-10+ IL-13+ CD4+ T cells, compatible with a TR1-like regulatory T-cell phenotype.6 These observations support the concept that TNFα-induced partially mature DCs exhibit tolerogenic features. Although the scientific literature indicated that DC maturation profiles induced by helminths or parasites can be similar to those obtained with TNFα, a direct comparison had not yet been performed.
Therefore, we investigated how the genetic maturation signature and the corresponding TH2-cell differentiation potential may differ between DC exposed to TNFα and pathogens. To this aim, we selected two variant surface glycoproteins (VSGs) purified from Trypanosoma brucei that had previously been characterized for their immunomodulatory potential. Surprisingly, low concentrations of VSG elicited a weak Toll-like receptor (TLR)/myeloid differentiation primary response gene 88 (MYD88)-transduced signal, promoted a genetic program that is highly similar to that triggered by TNFα, and lead to a semi-mature DC phenotype.1 VSG-matured DCs were able to instruct TH2 priming in vitro and in vivo. A common signature including 24 pro-inflammatory genes was identified among three distinct types of TH2-cell polarizing DC populations analyzed in this study. Of note, DC maturation by lipopolysaccharide polarized TH1 responses while inducing almost 5,000 genes, including the 24 pro-inflammatory genes linked to the TH2 program as well as additional genes like those coding for the typical TH1-inducing cytokine IL-12p35 (constituting part of IL-12p70) and the Notch ligand Delta-4.4 Only a moderate shift of Jagged-2 could be observed in TH2 cell-polarizing DCs and no difference was observed in Jagged-1 expression in both TH1- or TH2-inducing DC.1 Taken together, these data indicate that relatively small pro-inflammatory gene signature characterizes TH2-inducing DCs, while many additional factors are required for the development of a TH1-inducing DC. Thus, our findings support the default theory of TH2 cell induction. In addition, our data support previous findings indicating that a low peptide dose favors TH2 polarization.3 In our experimental, the low peptide dose was mimicked by the partial maturation of DCs, leading to a comparatively less efficient antigen presentation that may allow for TH2 induction. In line with our observation, quantitative aspects about TH polarization have just been revisited.7
How can the strength of DC maturation signals mediate a TH2 to TH1 shift? Both TNFα and LPS are well-known inducers of the transcription factor NFκB. However, the accessibility of genes for NFκB binding may differ, resulting in completely distinct functional outcomes. In particular, the post-translational opening of chromatin following the activation of histone acetyltransferases or the inhibition of histone deacetylases (HDACs) can influence NFκB activity at different cytokine-encoding genetic loci. Indeed, the accessibility of the IL-12p35-coding locus in DCs requires nucleosome remodeling.8 In line with this notion, the release of TNFα, IL-1 and IL-6 by DCs was not influenced by HDAC inhibitors (or needed prolonged inhibition), while the secretion of IL-12p35, IL-12p40 and interferon β (IFNβ) was highly susceptible to HDAC inhibitors.9 In addition, the recruitment of the NFκB subunit RelA to the promoter region of the TNFα-coding gene was rapid, while it was delayed for the IL-12-coding locus.10
These data indicate that genes coding for prototypic pro-inflammatory cytokines can be activated easily and rapidly in DCs, while genes coding for polarizing cytokines may require stronger and/or prolonged stimuli that allow for chromatin modifications. In this setting, a mild maturation signal would give rise to TH2-inducing DCs, while stronger and extended stimuli would allow for the development of TH1-inducing DCs, most likely as a result of the differential accessibility of the IL-12-coding gene. Taken together, these findings support a model in which not only the quality of DC maturation signals, as determined by the activation of either pattern recognition receptors or cytokine receptors, but also quantitative differences in maturation signals that are conveyed by the same pattern recognition receptors can critically influence TH1/TH2 polarization (Fig. 1).
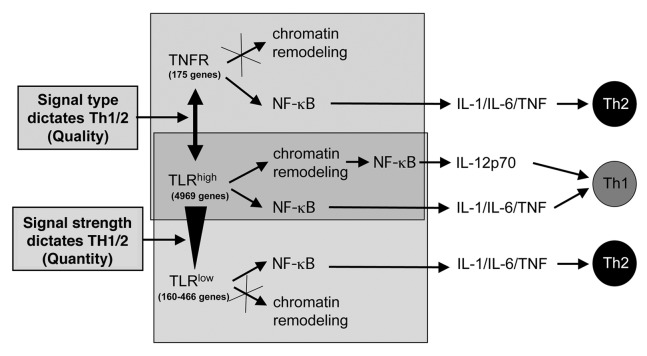
Figure 1. Qualitative and quantitative differences in dendritic cell maturation affect TH1/TH2 polarization. Dendritic cell (DC) maturation can be initiated by various types of pattern recognition receptors, such as Toll-like receptors (TLRs), or by the receptors for various pro-inflammatory cytokines, such as the tumor necrosis factor α (TNFα) receptor TNFR. The genetic signatures resulting from TNFR-conveyed and weak TLR-conveyed signals are remarkably small and highly similar to each other, sharing a common pro-inflammatory component. When DC maturation is triggered by TNFR or weak TLR signals (TLRlow), the transcription factor NFκB can rapidly bind to the promoter region of genes coding for interleukin (IL)-1, IL-6 and TNFα. This type of DC maturation promotes TH2-cell polarization. In response to these signals, no chromatin remodeling at the IL-12-coding gene promoter occurs to allow for the binding of NF-κB. In contrast, strong and prolonged TLR (TLRhigh) signals are required to allow for chromatin remodeling at promoter region of the IL-12-coding gene and hence for the (delayed) binding of NFκB, resulting in the maturation of TH1-polarizing DCs. This model integrates findings indicating that both DC maturation signal type (quality) and intensity (quantity) influence can TH1 vs. TH2-cell polarization.
Glossary
Abbreviations:
- EAE
experimental autoimmune encephalomyelitis
- DC
dendritic cell
- HDAC
histone deacetylase
- IL
interleukin
- IFN
interferon
- LPS
lipopolysaccharide
- MYD88
myeloid differentiation primary response gene 88
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- VSG
variant surface glycoproteins
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22796
References
- 1.Pletinckx K, Stijlemans B, Pavlovic V, Laube R, Brandl C, Kneitz S, et al. Similar inflammatory DC maturation signatures induced by TNF or Trypanosoma brucei antigens instruct default Th2-cell responses. Eur J Immunol. 2011;41:3479–94. doi: 10.1002/eji.201141631. [DOI] [PubMed] [Google Scholar]
- 2.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 3.Hosken NA, Shibuya K, Heath AW, Murphy KM, O’Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/S0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–7. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menges M, Rössner S, Voigtländer C, Schindler H, Kukutsch NA, Bogdan C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Garra A, Gabryšová L, Spits H. Quantitative events determine the differentiation and function of helper T cells. Nat Immunol. 2011;12:288–94. doi: 10.1038/ni.2003. [DOI] [PubMed] [Google Scholar]
- 8.Goriely S, Demonté D, Nizet S, De Wit D, Willems F, Goldman M, et al. Human IL-12(p35) gene activation involves selective remodeling of a single nucleosome within a region of the promoter containing critical Sp1-binding sites. Blood. 2003;101:4894–902. doi: 10.1182/blood-2002-09-2851. [DOI] [PubMed] [Google Scholar]
- 9.Bode KA, Schroder K, Hume DA, Ravasi T, Heeg K, Sweet MJ, et al. Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology. 2007;122:596–606. doi: 10.1111/j.1365-2567.2007.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode KA, Schmitz F, Vargas L, Heeg K, Dalpke AH. Kinetic of RelA activation controls magnitude of TLR-mediated IL-12p40 induction. J Immunol. 2009;182:2176–84. doi: 10.4049/jimmunol.0802560. [DOI] [PubMed] [Google Scholar]