Abstract
The advent of immunotherapies for cancer has resulted in robust clinical responses and confirmed that the immune system can significantly inhibit tumor progression. The recent success of adoptive cell therapy against melanoma suggests that endogenous T-cell responses have the potential to control cancer. However, the lack of responses in some patients receiving such therapy indicates a need for a better understanding of the host immune response to solid tumors. In this review, we summarize the current knowledge on the characteristics of adoptively transferred T cells associated with successful anti-melanoma immune responses in humans.
Keywords: adoptive cell therapy, CD8+ cells, memory T cells, TILs
Introduction
Melanoma is an aggressive skin cancer that leads to 48,000 deaths annually worldwide.1 It is the most common cancer in young adults aged 20 to 30 years old and the leading cause of cancer death in women aged 25 to 30 years old.2 Although the mortality rates of early stage melanoma are generally low, stage IV metastatic disease predicts poor disease outcome, with less than 10% survival rate at five years from diagnosis.3 Depending on the stage of the disease, treatments for melanoma include surgical resection of tumor and draining lymph nodes as well as the amputation of affected body parts. Other treatment modalities, including chemotherapy, are generally not as effective in melanoma as in other types of cancer. In contrast, treatments targeted at improving immunity such as high dose interleukin (IL)-2, anti-CTLA-4 antibodies, and interferon (IFN)α are efficacious, at least in selected subsets of melanoma patients, indicating a prominent role for the immune system in control of melanoma.4-6
The concept of immunotherapy for melanoma stemmed from early observations of intratumoral lymphocytic infiltrates, suggesting the existence of a local antitumor immune response.7 These tumor-infiltrating lymphocytes (TILs) included T cells, which could be expanded in culture with recombinant IL-2 and displayed cytolytic activity against autologous melanoma cells in vitro.8 The presence of CD4+ and CD8+ T cells among TILs positively correlates with favorable disease outcome, further demonstrating an important role for T cells in antitumor immunity.9-13 Since then, a broad spectrum of immunotherapies has been developed to boost protective T cell responses against melanoma (e.g., high-dose IL-2 and type I IFN) or to unleash endogenous responses to tumor (e.g., anti-CTLA-4 and anti-PD-1 monoclonal antibodies).
Adoptive cell therapy (ACT), a procedure whereby T cells are isolated from patients, expanded in culture, and eventually delivered back into the patient, have improved cure rates in melanoma patients by 20–40%.14 T cells used in ACT are derived from TILs of resected primary melanomas or, when TILs are not available, from peripheral blood T cells genetically modified to recognize tumor antigens.15,16 Retrospective analyses of ACT-based clinical trials have provided some insights into the characteristics of T cells that generate an effective antitumor response for melanoma. Most studies have focused on CD8+ T cell populations identified through the use of peptide-MHC tetramers or through the analysis of T-cell receptor (TCR) variable regions (TCRV) to examine antigen-specific T cells or T cell clonotypes, respectively.17 These methods are commonly used to monitor the frequency of specific T cells in the peripheral blood or among TILs, and are correlated with patient responses to therapy. Determining whether melanoma patients are good candidates for ACT is often challenging due to the variability of clinical presentation. An overriding question therefore must be whether certain features of T cells can be identified, such as cell surface phenotype, specificity or cytokine secretion profile, which might be used to predict patient survival. This review summarizes the characteristics of adoptively transferred T cells that promote tumor regression.
Phenotype
Cytotoxic CD8+ T cells have long been considered the principal immune cells involved in antitumor responses, based on their potent ability to directly kill tumor cells. The mechanisms for CD8+ T cell-mediated tumor cell killing are well established, and primarily involve the release of cytotoxic mediators such as granzymes and perforin upon the recognition of tumor antigens presented on MHC class I molecules.18 Identifying the T-cell populations that are most effective at promoting tumor regression is the first step in determining how to enhance the number and/or function of T cells to treat melanoma.
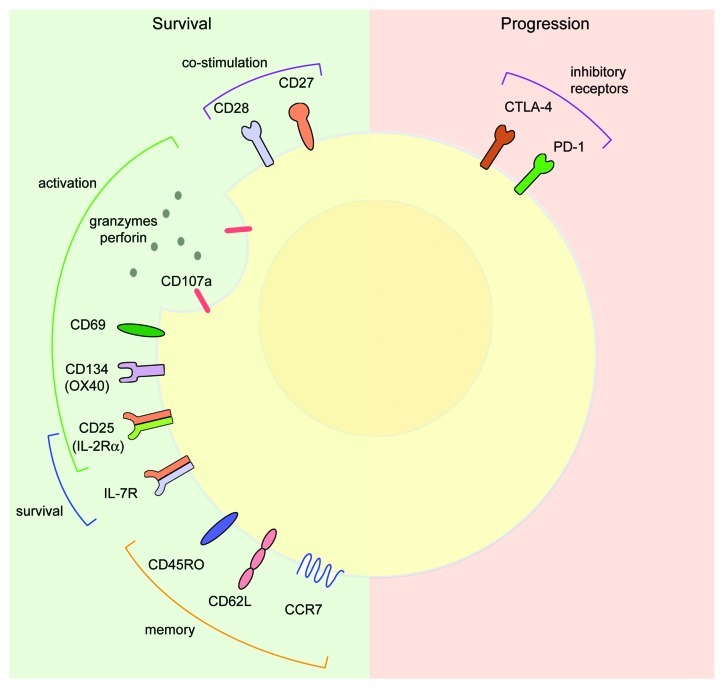
The activation phenotype of CD8+ T-cell populations has been used to predict clinical outcomes among melanoma patients. Independent studies that examined T-cell phenotypes in primary cutaneous melanoma showed that the expression of the T-cell activation markers CD69 within neoplastic lesions and CD25 (the α chain of the IL-2R) or CD134 (OX40) in peritumoral regions correlated with improved patient survival.19,20 Other T-cell activation markers associated with enhanced patient survival include CD107a (LAMP-1), a marker for T-cell degranulation, and CD45RO, a memory T cell marker.21,22 Thus, surface activation markers of CD8+ T cells may be used to screen for protective T-cell populations in melanoma (Fig. 1).
Figure 1. Cell surface phenotypes in melanoma. On the left (green): cell surface markers associated with melanoma survival. Co-stimulatory molecules: CD27 and CD28; activation markers: CD107a, CD69, CD134 (OX40) and CD25 (IL-2Rα); cytokine receptors that promote T-cell survival: CD25 (IL-2Rα) and IL-7R; memory T-cell markers: CD45RO, CD62L, and CCR7. On right (pink): cell surface markers associated with melanoma progression. Inhibitory receptors, CTLA-4 and PD-1.
Memory T cells constitute a specialized subset of antigen-experienced T cells that persists in vivo and confer long-lasting immunity against microbial infection. Memory T cell subsets include central memory T (TCM) and effector memory T (TEM) cells, which are characterized by distinct functional properties and migratory patterns.23 TCM cells constitutively express the lymph node homing receptors CCR7 and CD62L/L-selectin and are characterized by their ability to rapidly expand and to differentiate into potent effector cells in response to secondary antigen exposure.23,24 In contrast, TEM cells lack CCR7 and express low levels of CD62L, which allows for their migration to peripheral tissues for immediate effector functions. Adoptively transferred CD8+ TCM cells exhibited an enhanced recall response in vivo and significantly prolonged survival in mice with established B16 melanoma as compared with TEM cells, suggesting that TCM cells are more effective than TEM cells in mediating antitumor responses.25 Similar findings in melanoma patients showed that adoptively transferred CD8+ T cells that acquired a TCM phenotype correlate with complete tumor regression.26
Based on the ability of TCM cells to exert robust antitumor immunity in animal models, TCM cells were considered ideal candidates for ACT in clinical trials. Recently Wang and colleagues developed a method to screen for TCM and TEM cells in the peripheral blood upon antigen exposure, based on stoichiometric production of IL-2 and IFNγ.27 Melanoma-specific TCM cells produced high levels of IL-2, lower levels of IFNγ, and demonstrated superior proliferative capacity as compared with TEM cells, suggesting the potential of TCM cells to mediate durable cancer regression. Unfortunately, the adoptive transfer of TCM-derived effector cells failed to induce tumor regression in patients with melanoma. As the small patient sample may have been a limitation of this attempt, more studies are required to determine whether TCM cells can improve the therapeutic outcome of ACT in melanoma patients.
The failure of adoptively transferred TCM cells to mediate tumor regression suggests that selecting T cells based on memory phenotype alone may not be a reliable method of identifying the optimal antitumor cells for immunotherapy. Additionally, both high and low avidity CTL clones have a similar cell surface phenotype, indicating that cell phenotype does not necessarily correlate with immune function.28 Thus, cell surface phenotypes may be useful for characterizing activated T cells (Fig. 1), but functional studies may be more relevant to predict their efficacy in immunotherapies.
Another T-cell phenotype (whose relevance for melanoma has not yet been established) is constituted by the newly described skin resident memory T (TRM) cells. Unlike other memory T cells that recirculate between the blood and lymph nodes, skin TRM cells compose a unique population of memory T cells that remain in the skin and confer long-term immunity against secondary antigen exposure. Skin TRM cells express CD45RO and maintain high levels of skin homing receptors such as the cutaneous lymphocyte antigen (CLA), CCR4, and CCR6, which enable them to persist in skin for several months.29 TRM cells readily poised in skin respond to previously encountered pathogens faster than other memory T cells, which need to be recruited from circulation.30,31 Furthermore, skin TRM cells have been shown to populate the entire skin surface and to be superior to TCM cells at eliminating pathogens at distant skin sites as well as at the site of infection.30 The ability of these cells to mediate long-lasting cutaneous immunity indicates that TRM cells may be more effective than TCM cells in providing protection against melanoma. Interestingly, TRM cells appeared to be unaffected by drugs that targeted circulating T cells, suggesting that conventional immunotherapies designed to provide systemic immunity against melanoma may not affect TRM cell functions.31 Thus, it may be worthwhile to monitor TRM cells in melanoma patients receiving conventional immunotherapies or consider targeting these cells when designing future T cell-based treatments for melanoma.
T Cell Persistence In Vivo
Two ACT-based Phase I clinical trials that failed to achieve objective clinical responses reported a rapid decline in adoptively transferred T-cell populations, which were virtually undetectable in the peripheral blood of patients two to three weeks following transfer.32,33 These trials provided evidence for a relationship between T-cell persistence in vivo and tumor regression. The pre-conditioning of patients with non-myeloablative, but lymphodepleting chemotherapy to eliminate regulatory T cells and other tolerogenic mechanisms before the transfer of polyclonal TILs plus high-dose IL-2 significantly improved T-cell persistence in the peripheral blood to up to four months and led to objective clinical responses in 6 out of 13 patients.15 A second study using a larger cohort of patients revealed that the levels of T-cell persistence in ACT responders were significantly higher than those of non-responders.34 Together, these findings suggest that long-term T-cell persistence in vivo correlates with a favorable outcome in metastatic melanoma patients. Identifying long-term persistent T cell receptor (TCR) clones, or clonotypes, should hence make immunotherapies more effective.
Long-term persistent T cell clonotypes possess significantly longer telomeres, differentiate more slowly than less persistent clonotypes, and express higher levels of CD27 and CD28 (Fig. 1).35 The amount of time that TILs passed in culture also influences in vivo T-cell persistence, so that minimally cultured TILs appear to persist for comparatively longer periods in patients, correlating with objective clinical responses.36 Unlike TILs cultured for long time periods, minimally cultured TILs also retain CD27 and CD28 expression.37 Recently, Li and colleagues have demonstrated that CD28, but not CD27, is required for maintaining T-cell survival in vivo. Indeed, the loss of CD28 after the expansion of TILs in vitro with IL-2 resulted in impaired T-cell proliferation in response to antigenic stimulation.38 In contrast, CD28-expressing TILs retained expansion capability and exhibited improved survival in vivo.
T-cell persistence in vivo after ACT can be dramatically influenced by the cytokines that are used to expand T-cell populations from TILs in vitro. The traditional approach to expand TILs for ACT involves culturing tumor cell suspensions with recombinant IL-2. However, recent reports indicate that IL-15 and IL-21 may be more effective than IL-2 in generating long-term persistent T-cell clones.38 CD8+ T cells cultured with IL-15 and IL-21 express higher levels of CD28 and divided more proficiently after tumor peptide stimulation than cells expanded with IL-2. Persistent T-cell clones commonly expressed IL-7Rα, implying that IL-7 may also be important for long-term T-cell survival.39 Interestingly, both IL-7 and IL-15 are implicated in the homeostatic proliferation of memory T cells and both cytokines signal through a common receptor γ chain, which is also shared by IL-2 and IL-21.40,41 Whether ACT can be improved by identifying a cytokine cocktail that would enhance the persistence of T cells in vivo is currently unknown, but undoubtedly warrants further investigation.
Specificity for Tumor Antigens
Identifying the antigen specificity of endogenous T cells and hence defining those proteins that might be used to select for tumor-specific T cells in ACT might dramatically enhance the therapeutic efficacy of this approach. The first successful ACT for the treatment of metastatic melanoma used highly reactive T-cell populations directed against MART-1/Melan A and gp100, highlighting the importance of antigen-specific T cells in effective cancer immunotherapy.15 Several melanoma-associated antigens and their corresponding CD8+ T cell epitopes have been identified through biochemical and molecular approaches, including mass spectrometry after peptide elution from HLA molecules, or by screening cDNA expression libraries with melanoma-reactive T cells.42,43 Based on the expression patterns of parental proteins, T cell-relevant tumor antigens were categorized into four major classes: overexpressed antigens, melanocyte differentiation antigens (e.g., MART-1/Melan A and gp100), cancer/testis antigens (e.g., NY-ESO-1 and MAGE) and mutated antigens.42 Many tumor-associated antigens are also expressed in normal tissues. Thus, selecting the appropriate antigen that preferentially targets tumor cells while avoiding toxicity to normal tissues is a major challenge in designing immunotherapies.
Antigen-specific T-cell populations from a single biological sample can be identified by a high throughput approach using dual color peptide MHC multimers.44 A total of 90 T-cell responses were identified against melanoma antigens in TILs (63 TILs from 19 patients) using this approach.45 T-cell reactivity against overexpressed antigens, which represent the largest class of antigens contributing to known T-cell epitopes relevant for melanoma, were rare and appeared to have no impact on clinical responses. Although TILs most frequently responded to melanoma differentiation and cancer/testis antigens, only reactivity against the latter correlated with patient sensitivity to TIL-based therapy.46 These data suggest that the recognition of cancer/testis antigens by infused T-cell populations may be associated with favorable therapeutic outcomes.
Despite the prediction that T-cell responses to cancer/testis antigens would lead to favorable outcomes in melanoma, vaccination with the cancer/testis antigen MAGE-3.A1 peptide resulted in complete tumor regression only in 10–20% melanoma patients.47 Unexpectedly, patients responsive to peptide vaccination had a very low frequency of MAGE-3.A1-specific CD8+ cells in the peripheral blood, but a high frequency of CD8+ T-cell precursors specific for other tumor antigens.48 In some cases, antitumor T-cell clonotypes were observed in the blood before vaccination, while in other cases, antitumor T cells only emerged after vaccination.49 The presence of antitumor T-cell precursors in the blood of non-vaccinated individuals suggested that spontaneous antitumor T cells that were quiescent or ineffective became active upon vaccination. In contrast, the appearance of new antitumor clonotypes after vaccination demonstrated clonal spreading, or the emergence of new CTL clones against previously targeted antigens. Whether new antitumor T-cell clonotypes were a product of vaccination or naturally appeared during the course of disease has not been elucidated. New antitumor clonotypes specific for tumor antigens not targeted by vaccination may also arise from antigen spreading, or the development of CTL clones against previously ignored antigens.50 Evidence for antigen spreading has previously been reported in one patient undergoing a complete regression that developed antitumor responses against a mutated antigen, for which no T cells could be detected prior to vaccination.50 In a similar report, a patient who experienced tumor regression after receiving NY-ESO-1-specific TILs also demonstrated reactivity to MART-1 and MAGE-3 antigens.51 It has been postulated that antigen spreading may result from new tumor antigens being released from killed tumor cells that are processed by antigen-presenting cells and used to activate new T cells. Collectively, these findings suggest that clonal or antigen spreading may increase the repertoire of tumor-reactive T cells to improve the effectiveness of immunotherapies. With this in mind, the use of several tumor-reactive T-cell clones (to target multiple melanoma antigens) rather than a single one may increase the therapeutic efficacy of ACT.
Although several immunotherapies target T cells that are specific for shared melanoma antigens, neo-antigens may also emerge from genetic mutations. For instance, approximately 50% of melanomas harbor a mutation in BRAF, a gene coding for a protein kinase involved in the RAS-RAF signaling pathway.52 Although other mutated antigens in melanoma have also been identified, the magnitude of T-cell responses directed against mutated antigens for melanoma is very low.46 Nevertheless, highly reactive T cells directed against mutated antigens have been reported to mediate complete regression in patients with metastatic melanoma.53,54 In an attempt to identify genetic mutations in melanoma, Wei and colleagues performed whole exome sequencing of 14 matched normal and metastatic tumor DNA from untreated melanoma patients. This analysis allowed them to confirm previously identified mutations in BRAF and to identify new mutations in genes that had not previously been associated with melanoma, including TRRAP (coding for transformation/transcription domain-associated protein) and GRIN2A (coding for a glutamate receptor subunit).55 Interestingly, most genetic mutations associated with melanoma encoded members of the glutamate signaling pathway. While this study helped to identify novel melanoma-specific mutations, the use of exome-sequencing to analyze potential target genes in all melanoma patients may be time consuming and costly. Thus, the development of more efficient methods to screen for patients with relevant mutations and neo-antigens is still required.
Regardless of the approach used to identify novel tumor antigens and the development of corresponding T-cell reactive clones, some patients that initially respond to ACT also experience relapse.56 Recent evidence suggests that tumor resistance to therapy occurs when cancer cells lose melanoma antigens or undergo a process of “dedifferentiation,” enabling them to escape the recognition by antigen-specific T cells. Landsberg and colleagues identified tumor necrosis factor α (TNFα), a pro-inflammatory cytokine produced by T cells, as a critical factor responsible for dedifferentiation in melanoma cells.56 Thus, future ACT approaches should consider targeting several tumor antigens to minimize or prevent the development of tumor resistance.
T-cell Cytokine Secretion Profile
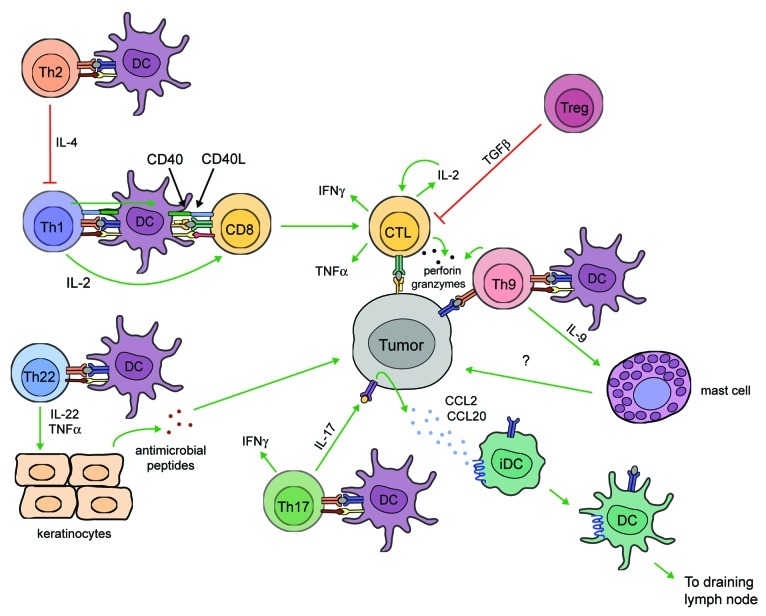
T cells used for ACT are often selected from bulk TIL populations based on their ability to exert antitumor effector functions. Various in vitro assays have been used to assess the reactivity of effector CD8+ T cells, including chromium release assays, which measure the killing capacity of T cells against chromium-labeled tumor cell targets, as well as assays that measure T-cell degranulation based on granzyme B release or CD107a expression.21,33 T cell reactivity for tumor antigens may also be evaluated through the production of IFNγ or TNFα.15,57 These parameters for measuring T-cell functions define the quality of the T-cell response. However, simply measuring a single functional parameter may not accurately predict the magnitude of an antitumor T-cell response in vivo. For example, assays that measure T-cell degranulation lack the sensitivity for distinguishing high- and low-avidity CTL populations, which may account for differences in patient responses to ACT.28 T-cells with a better quality, or those exhibiting a broader repertoire of effector functions, are able to elicit enhanced protection against microbial infection as compared with cells with a single functional profile.58 In the context of tumor immunity, T cells exhibiting high avidity for peptide-MHC multimers, an elevated degranulation potential (based on CD107a expression), and producing IFNγ, IL-2 and TNFα, demonstrated superior tumor recognition (Fig. 2).28 Thus, selecting T cells based on multifunctional capability rather than a single functional parameter may dramatically improve the therapeutic outcome of ACT.
Figure 2. Functional T-cell populations in tumor immunity. Antitumor T-cell functions. Cytotoxic CD8+ T lymphocytes (CTLs) directly kill tumor cells by releasing cytotoxic granules containing perforin and granzymes and secrete interleukin (IL)-2, interferon (IFN)γ, and tumor necrosis factor (TNF)α, which contribute to antitumor functions in several ways. IL-2 promotes CD8+ cell survival and proliferation. IFNγ enhances CD4+ TH1 cell differentiation, inhibits angiogenesis, and activates antitumor biochemical pathways in macrophages and dendritic cells (DCs) (not shown). TNFα directly induces tumor cell death and promotes CD8+ cell proliferation and recruitment. CD4+ TH1 cells promote antitumor responses by secreting IL-2 and IFNγ and prime DCs to activate CD8+ T cells through CD40-CD40 ligand (CD40L) interactions. TH17 cells secrete IFNγ and IL-17, the latter of which induces tumor cells to release CCL2 and CCL20, which promote DC recruitment to tumor tissues. DCs bearing tumor antigens migrate to draining lymph nodes and activate tumor-specific CD8+ T cells. In mice, TH9 cells directly kill tumor cells through the release of granzymes and secrete IL-9, which promotes antitumor responses through an unknown mechanism that involves mast cells. TH22 cells secrete IL-22 and TNFα, which activates keratinocytes to produce antimicrobial peptides that exhibit antitumor activity. Pro-tumor T-cell functions. TH2 cells facilitate tumor growth by inhibiting the functions of TH1 cells. Naturally occurring regulatory T cells expressing CD25 and FOXP3 also promote tumor cell growth by suppressing the proliferation and activation of effector T cells.
CD8+ T-cell functions in cancer immunotherapies often overshadow functional responses by CD4+ T cells, which make up to 19% of the total TIL population from primary melanomas.59 Protective immune responses in melanoma may involve specific subsets of CD4+ helper T (TH) cells that recognize tumor antigens presented on MHC Class II molecules. However, the characterization of protective CD4+ populations in humans is poor. Although CD4+ T cells are generally thought to provide help to enhance the cytolytic functions of CD8+ T cells, CD4+ T cells have also been shown to exert direct cytolytic activity against tumor cells.60-62 Moreover, the adoptive transfer of both tumor-specific CD4+ and CD8+ T cell populations induced better clinical outcomes in melanoma patients than that of CD8+ T cells alone, illustrating the importance of CD4+ T cells in antitumor immunity.15
Although studies correlating CD4+ T cells and cancer outcome have been performed in animal models, the characterization of CD4+ cells in human melanoma is understudied and controversial. TH1 cells play a protective role in antitumor immunity in animal models by enhancing the cytolytic activity of CD8+ cells, whereas—according to most reports—TH2 cells contribute to tumor progression.63,64 Similar findings have been reported in the human setting, in which CTL responses were enhanced by TH1 and TH0 cells, but not by TH2 cells.65 Additionally, elevated serum levels of IL-4, IL-6 and IL-10 and lower levels of IFNγ and IL-2 have been found in patients with malignant melanoma, suggesting that an imbalance between TH1 and TH2 cytokines contributes to melanoma development.64 Interestingly, the prognostic value of IFNγ levels failed to reach statistical significance in regressing melanomas, suggesting that IFNγ may not be a reliable marker for favorable outcome.66 Nevertheless, IFNγ production is often used as a marker to select for tumor-reactive T-cell populations from TILs. At least two clinical cases of tumor regression in patients treated with melanoma-reactive CD4+ TILs have been reported, with one case resulting in long-term complete remission.51,67 In both studies CD4+ TILs were expanded in vitro and selected for reactivity to melanoma antigens based on IFNγ production, indicating that tumor-specific CD4+ cells were predominantly TH1 cells.
Unlike TH1 cells, which are traditionally regarded as the CD4+ helper/effector cells mainly involved in antitumor responses, the role of TH17 cells in tumor immunity is unclear. Tumor-specific TH17 cells eradicated transplanted B16 tumors in mice more efficiently than tumor-reactive TH1 cells, suggesting a superior protective role for TH17 cells in tumor immunity.68 Surprisingly, the antitumor effects of TH17 cells were dependent on IFNγ production, as the depletion of IFNγ but not that of IL-17A or IL-23, inhibited tumor rejection. Furthermore, TH17 cells induced the production of CCL2 and CCL20 by tumor cells, leading to the recruitment of dendritic cells (DCs), which were able take up tumor antigens and migrate to draining lymph nodes to activate tumor-specific CD8+ T cells (Fig. 2).69 In humans, TH17 cells have been shown to play a protective role in ovarian and prostate cancers but a pathogenic role in hormone-resistant prostate cancer.70-72 Patients with hormone-resistant prostate cancer had high levels of circulating TH17 cells prior to immunotherapy, and this correlated with faster disease progression.72 In other types of cancer, the prevalence of peripheral blood TH17 cells appears to be comparable to that of healthy individuals, suggesting that elevated levels of circulating TH17 cells may indicate an underlying infection.70 Thus, the criteria for patient selection may account for conflicting results across different studies. This said, additional efforts to investigate the impact of TH17 cells on the survival of melanoma patients are warranted.
Naturally occurring regulatory T cells (Tregs), which express CD25 and the transcription factor FOXP3, are also present in the tumor microenvironment. Tregs suppress the proliferation and activation of effector T cells and their abundance correlate with poor prognosis in melanoma patients. Accordingly, treatments that overcome the suppressive effects of Tregs or reduce their frequency in the peripheral blood improve patient survival (Fig. 2).73-75 Combination therapies that boost the responses of tumor-reactive T cells while removing the suppressive effects of Tregs have shown encouraging results in animal models, but these still need to be confirmed in humans.76
T-cell subsets implicated in skin immunity may further complicate the identification of protective T cells in melanoma (Fig. 2). In animal models, IL-9-producing T (TH9) cells demonstrated cytolytic activity against B16F10 melanomas and recombinant IL-9 inhibited tumor growth in a mast cell-dependent manner, suggesting a role for TH9 cells and IL-9 in antitumor immunity.77 IL-22 producing T (TH22) cells may also promote antitumor immune responses by activating keratinocytes to produce antimicrobial peptides, which can contribute to antitumor immunity.78,79 Lastly, CD1-restricted T cells, in particular CD1a-restricted T cells, recognize lipid antigens presented by Langerhans cells to produce IL-22 and mediate epidermal immunity.80 A role for CD1-restricted T cells has not been described in the context of melanoma, but given the role of these cells in maintaining epidermal homeostasis, further studies are warranted. The relative contribution of understudied T cells to melanoma should be evaluated before considering the use of these cells for immunotherapy.
Concluding Remarks
Metastatic melanoma continues to be associated with a high mortality rate despite the development of novel therapeutic strategies, and current therapies can result in significant morbidity. An overriding goal of these therapies is to liberate endogenous immunity with minimal collateral damage. Achieving this goal will result from identifying the most effective T-cell populations with regard to phenotype, persistence in vivo, antigen specificity and function, as all these parameters can influence tumor regression. This said, the selection of T cells solely based on cell surface phenotype is an unreliable approach for predicting clinical outcomes and functional studies should always be performed to confirm the efficacy of T cells for immunotherapy. Multifunctional T cells, as determined by cytokine production and granule release, may constitute promising mediators of antitumor responses, as this has been previously shown for microbial infection. An approach that is optimal with regards to all these parameters and/or that simultaneously targets several melanoma antigens may generate the host response that mediates tumor regression in the greatest number of patients.
Acknowledgments
The authors are supported in part by National Institutes of Health ( AR59126–3) and the Joseph B. Gould Foundation.
Glossary
Abbreviations:
- ACT
adoptive cell transfer
- CCR
C-C chemokine receptor
- CLA
cutaneous lymphocyte antigen
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- DC
dendritic cell
- IFN
interferon
- IL
interleukin
- MHC
major histocompatibility complex
- PD-1
programmed cell death 1
- TCM
central memory T
- TCR
T cell receptor
- TEM
effector memory T
- TH
helper T
- TIL
tumor infiltrating lymphocyte
- TNFα
tumor necrosis factor α
- Treg
regulatory T cell
- TRM
resident memory T
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22889
References
- 1.Lucas R, McMichael T, Smith W, Armstrong B. Solar Ultraviolet Radiation: Global burden of disease from solar ultraviolet radiation. In: Pruss-Ustun A, Zeeb H, Mathers C, Repacholi M, eds. Environmental Burden of Disease. Geneva, Switzerland: WHO, 2006:1-250. [Google Scholar]
- 2.Center M, Siegel R, Jemal A. Global Cancer Facts & Figures 2nd Edition. Atlanta, Georgia: American Cancer Society, 2011. [Google Scholar]
- 3.Bastiaannet E, Beukema JC, Hoekstra HJ. Radiation therapy following lymph node dissection in melanoma patients: treatment, outcome and complications. Cancer Treat Rev. 2005;31:18–26. doi: 10.1016/j.ctrv.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–8. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maccarty WC. Longevity in Cancer: A Study of 293 Cases. Ann Surg. 1922;76:9–12. [PMC free article] [PubMed] [Google Scholar]
- 8.Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987;138:989–95. [PubMed] [Google Scholar]
- 9.Clark WH, Jr., Elder DE, Guerry D, 4th, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 10.Tefany FJ, Barnetson RS, Halliday GM, McCarthy SW, McCarthy WH. Immunocytochemical analysis of the cellular infiltrate in primary regressing and non-regressing malignant melanoma. J Invest Dermatol. 1991;97:197–202. doi: 10.1111/1523-1747.ep12479662. [DOI] [PubMed] [Google Scholar]
- 11.Clemente CG, Mihm MC, Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.van Houdt IS, Sluijter BJR, Moesbergen LM, Vos WM, de Gruijl TD, Molenkamp BG, et al. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. Int J Cancer. 2008;123:609–15. doi: 10.1002/ijc.23543. [DOI] [PubMed] [Google Scholar]
- 13.Al-Batran S-E, Rafiyan M-R, Atmaca A, Neumann A, Karbach J, Bender A, et al. Intratumoral T-cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res. 2005;65:3937–41. doi: 10.1158/0008-5472.CAN-04-4621. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4:ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman JD, Davis MM. MHC-Peptide Tetramers to Visualize Antigen-Specific T Cells. Curr Prot Immunol: John Wiley & Sons, Inc., 2001. [DOI] [PubMed] [Google Scholar]
- 18.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–87. doi: 10.1016/0092-8674(94)90376-X. [DOI] [PubMed] [Google Scholar]
- 19.Hillen F, Baeten CI, van de Winkel A, Creytens D, van der Schaft DW, Winnepenninckx V, et al. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother. 2008;57:97–106. doi: 10.1007/s00262-007-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladányi A, Somlai B, Gilde K, Fejös Z, Gaudi I, Tímár J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10:521–30. doi: 10.1158/1078-0432.CCR-1161-03. [DOI] [PubMed] [Google Scholar]
- 21.Baumgaertner P, Jandus C, Rivals J-P, Derré L, Lövgren T, Baitsch L, et al. Vaccination-induced functional competence of circulating human tumor-specific CD8 T-cells. Int J Cancer. 2012;130:2607–17. doi: 10.1002/ijc.26297. [DOI] [PubMed] [Google Scholar]
- 22.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 24.Hamann D, Baars PA, Rep MHG, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapuis AG, Thompson JA, Margolin KA, Rodmyre R, Lai IP, Dowdy K, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci U S A. 2012;109:4592–7. doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang A, Chandran S, Shah SA, Chiu Y, Paria BC, Aghamolla T, et al. The stoichiometric production of IL-2 and IFN-γ mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci Transl Med. 2012;4:ra120. doi: 10.1126/scitranslmed.3004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilde S, Sommermeyer D, Leisegang M, Frankenberger B, Mosetter B, Uckert W, et al. Human antitumor CD8+ T cells producing Th1 polycytokines show superior antigen sensitivity and tumor recognition. J Immunol. 2012;189:598–605. doi: 10.4049/jimmunol.1102165. [DOI] [PubMed] [Google Scholar]
- 29.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-i, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–31. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra7. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–67. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–66. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 37.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–51. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 2010;184:452–65. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 39.Powell DJ, Jr., Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–50. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 41.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 42.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–93. doi: 10.1016/S0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 43.Kawakami Y. New cancer therapy by immunomanipulation: development of immunotherapy for human melanoma as a model system. Cornea. 2000;19(Suppl):S2–6. doi: 10.1097/00003226-200005001-00002. [DOI] [PubMed] [Google Scholar]
- 44.Hadrup SR, Bakker AH, Shu CJ, Andersen RS, van Veluw J, Hombrink P, et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat Methods. 2009;6:520–6. doi: 10.1038/nmeth.1345. [DOI] [PubMed] [Google Scholar]
- 45.Andersen RS, Thrue CA, Junker N, Lyngaa R, Donia M, Ellebæk E, et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012;72:1642–50. doi: 10.1158/0008-5472.CAN-11-2614. [DOI] [PubMed] [Google Scholar]
- 46.Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. 2012;1:409–18. doi: 10.4161/onci.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchand M, Weynants P, Rankin E, Arienti F, Belli F, Parmiani G, et al. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer. 1995;63:883–5. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 48.Germeau C, Ma W, Schiavetti F, Lurquin C, Henry E, Vigneron N, et al. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J Exp Med. 2005;201:241–8. doi: 10.1084/jem.20041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lurquin C, Lethé B, De Plaen E, Corbière V, Théate I, van Baren N, et al. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201:249–57. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corbière V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethé B, et al. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71:1253–62. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 51.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, El-Gamil M, Dudley ME, Li YF, Rosenberg SA, Robbins PF. T cells associated with tumor regression recognize frameshifted products of the CDKN2A tumor suppressor gene locus and a mutated HLA class I gene product. J Immunol. 2004;172:6057–64. doi: 10.4049/jimmunol.172.10.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei X, Walia V, Lin JC, Teer JK, Prickett TD, Gartner J, et al. NISC Comparative Sequencing Program Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet. 2011;43:442–6. doi: 10.1038/ng.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–6. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 57.Walker EB, Haley D, Miller W, Floyd K, Wisner KP, Sanjuan N, et al. gp100(209-2M) peptide immunization of human lymphocyte antigen-A2+ stage I-III melanoma patients induces significant increase in antigen-specific effector and long-term memory CD8+ T cells. Clin Cancer Res. 2004;10:668–80. doi: 10.1158/1078-0432.CCR-0095-03. [DOI] [PubMed] [Google Scholar]
- 58.Darrah PA, Patel DT, De Luca PM, Lindsay RWB, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 59.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goedegebuure PS, Lee K-Y, Matory YL, Peoples GE, Yoshino I, Eberlein TJ. Classification of CD4+ T helper cell clones in human melanoma. Cell Immunol. 1994;156:170–9. doi: 10.1006/cimm.1994.1162. [DOI] [PubMed] [Google Scholar]
- 62.Wong R, Lau R, Chang J, Kuus-Reichel T, Brichard V, Bruck C, et al. Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma. Clin Cancer Res. 2004;10:5004–13. doi: 10.1158/1078-0432.CCR-04-0241. [DOI] [PubMed] [Google Scholar]
- 63.Lasek W, Basak G, Switaj T, Jakubowska AB, Wysocki PJ, Mackiewicz A, et al. Complete tumour regressions induced by vaccination with IL-12 gene-transduced tumour cells in combination with IL-15 in a melanoma model in mice. Cancer Immunol Immunother. 2004;53:363–72. doi: 10.1007/s00262-003-0449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lauerova L, Dusek L, Simickova M, Kocák I, Vagundová M, Zaloudík J, et al. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma. 2002;49:159–66. [PubMed] [Google Scholar]
- 65.Lee K-Y, Goedegebuure PS, Linehan DC, Eberlein TJ. Immunoregulatory effects of CD4+ T helper subsets in human melanoma. Surgery. 1995;117:365–72. doi: 10.1016/S0039-6060(05)80054-6. [DOI] [PubMed] [Google Scholar]
- 66.Lowes MA, Bishop GA, Crotty K, Barnetson RS, Halliday GM. T helper 1 cytokine mRNA is increased in spontaneously regressing primary melanomas. J Invest Dermatol. 1997;108:914–9. doi: 10.1111/1523-1747.ep12292705. [DOI] [PubMed] [Google Scholar]
- 67.Friedman KM, Prieto PA, Devillier LE, Gross CA, Yang JC, Wunderlich JR, et al. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J Immunother. 2012;35:400–8. doi: 10.1097/CJI.0b013e31825898c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derhovanessian E, Adams V, Hähnel K, Groeger A, Pandha H, Ward S, et al. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–9. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 73.Viguier M, Lemaître F, Verola O, Cho M-S, Gorochov G, Dubertret L, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–53. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 74.Ménard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, et al. Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14:5242–9. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 75.De Panfilis G, Campanini N, Santini M, Mori G, Tognetti E, Maestri R, et al. Phase- and stage-related proportions of T cells bearing the transcription factor FOXP3 infiltrate primary melanoma. J Invest Dermatol. 2008;128:676–84. doi: 10.1038/sj.jid.5701046. [DOI] [PubMed] [Google Scholar]
- 76.Sorensen MR, Holst PJ, Steffensen MA, Christensen JP, Thomsen AR. Adenoviral vaccination combined with CD40 stimulation and CTLA-4 blockage can lead to complete tumor regression in a murine melanoma model. Vaccine. 2010;28:6757–64. doi: 10.1016/j.vaccine.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 77.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–53. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl 2):ii17–21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Jong A, Peña-Cruz V, Cheng T-Y, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat Immunol. 2010;11:1102–9. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]