Abstract
Elevated levels of plasmacytoid dendritic cells (pDC) have been observed as breast cancer disseminates to the bone. The selective depletion of pDC in mice led to a total abrogation of bone metastasis as well as to an increase in TH1 antitumor response, suggesting that pDC may be considered as a potential therapeutic target for metastatic breast cancer.
Keywords: breast cancer, bone metastasis, osteoclasts, plasmacytoid dendritic cells
Osteolytic bone metastases are common in breast cancer (BCa). Approximately, 70% of patients dying from BCa show evidence of bone metastasis at postmortem examinations. The presence of such bone lesions usually signifies serious morbidity and a grave prognosis. Despite the complications deriving from bone metastasis, the therapies for metastatic BCa patients are limited and are not aimed at controlling the disease. Therefore, developing new strategies to control bone metastasis and to improve patient survival is an absolute necessity, which requires a deeper understanding of the molecular mechanisms involved in BCa metastatic dissemination.
As the primary tumor disseminates to the bone, it triggers the production of osteolytic cytokines and growth factors that—altogether—(1) result in osteoclast activation, (2) promote the growth of tumor cells and (3) facilitate the establishment of an immunosuppressive microenvironment. Moreover, the products of bone cells are critical for the normal development of the hematopoietic and immune systems. Thus, understanding the influence and interaction of metastasizing cancer cell with cells of the skeletal system and on cells of the immune system will provide clues for the design of preventive and therapeutic strategies for osteolytic bone metastasis.1
In a pre-clinical mouse model of metastatic BCa, we observed high numbers of plasmacytoid dendritic cells (pDC) in the bone, which continued to increase as the tumor growth progressed (Fig. 1).2 Increased pDC infiltration at both primary and the metastatic sites has been reported also in BCa patients, but the significance of these findings was unclear. Besides BCa, lung cancer and multiple myeloma, which primarily affects the skeleton, have been associated with an increased bone infiltration by pDC.3 This indicates that pDC may exert an important role in the establishment of bone metastases. But the question remains what role, if any, do these cells play?
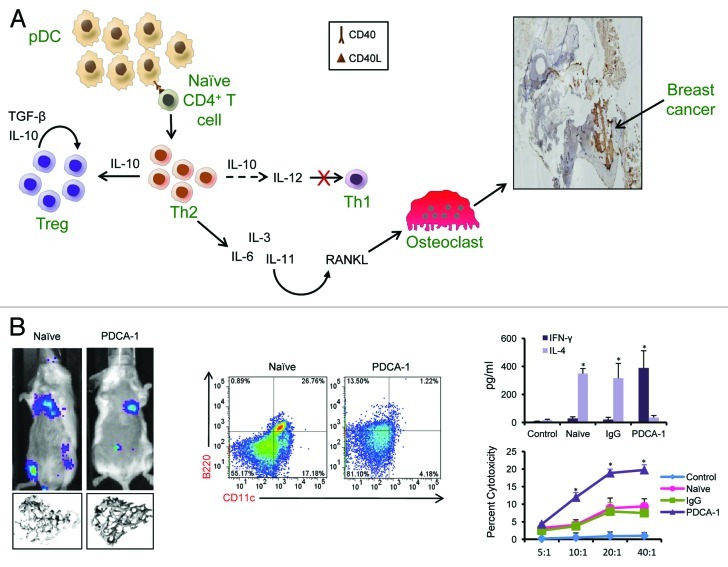
Figure 1. Relevance of plasmacytoid dendritic cells in bone metastasis. (A) As breast cancer (BCa) grows and disseminates to the bone, there is a rapid accumulation of plasmacytoid dendritic cells (pDC). By interacting with naïve CD4+ T cells, pDC promote the development of an immunosuppressive TH2 response that, in turn, blunts TH1 cell differentiatino and stimulates the accumulation of regulatory T cells (Tregs). Factors secreted by TH2 cells induce RANKL expression, leading to the activation of osteoclasts. These cells destruct the bone, hence allowing BCa cells to establish and grow within the bone microenvironment. (B) Data show that the depletion of pDC using an anti-PDCA-1 antibody leads to reduced tumor growth and prevents metastatic dissemination to the bone, as detected by the absence of bioluminescence from luciferase-expressing cancer cells in the bone and bone destruction study by micro-CT. Anti-PDCA-1 antibody administration was effective in depleting (B220+CD11c+) pDC in the bone and was accompanied by a skew of the immune response toward a TH1 phenotype, as seen by high interferon γ (IFNγ) levels and increased cytotoxicity of CD8+ T cells. These results are described in detail in Sawant et al.2
pDC can induce immunosuppression through a variety of mechanisms. In BCa, pDC promote tumor progression via the expression of ICOS-ligand and also as a result of CD40/CD40L interactions, which allow for the accumulation of immunosuppressive CD4+ T cells and hence limit the number and function of cytotoxic CD8+ T cells.2,4 In multiple myeloma, immune dysfunction is partially caused by pDC, which are incompetent relative to the Toll-like receptor 9 (TLR9) mediated interferon α (IFNα) production and hence exhibit a reduced ability to induce T cell proliferation. Increased infiltration by pDC is associated with high levels of interleukin (IL)-3, IL-6, IL-10, IL-15, IP-10, MCP-1 and RANTES in both breast carcinoma and myeloma.5 These chemokines and cytokines, besides being immunosuppressive, are known to induce osteoclastogenesis, either directly or indirectly. These soluble factors induce indeed the expression of receptor-activating nuclear factor-κB ligand (RANKL), which is critical for the osteoclast-mediate bone resorption, hence helping metastatic cells to grow. A recent publication has shown that pDC isolated from the bone marrow of rats express high levels of RANKL.6 This observation adds a further facet to the role of pDC in bone metastasis, whereby pDC-generated soluble RANKL may directly induce osteoclastogenesis by acting on bone marrow osteoclast progenitors.7 Using a murine BCa model, we have recently identified that, besides immunosuppressive T cell populations, myeloid-derived suppressor cells (MDSC) accumulated in high numbers together with pDC during BCa bone dissemination. Furthermore, MDSC in the cancer-bone microenvironment were found to function as novel osteoclast progenitors. Based on these findings, one could speculate that pDC-generated RANKL may directly act upon MDSC, inducing their differentiation into osteoclasts and thus promoting bone destruction and local BCa growth.
Although the above mentioned observations pointed to a possible role for pDC in promoting bone metastasis, a more direct and substantiated evidence was necessary. This led us to deplete pDC in vivo using an anti-PDCA-1 antibody, which causes a selective and effective pDC depletion. Our data clearly show that pDC-depleted mice fail to develop BCa bone metastasis and also that the overall tumor growth is dramatically reduced in pDC-depleted mice as compared with their normal counterparts.2 Further evidence in support of this observation was established in IFNα receptor-deficient (Ifnar−/−) mice, which lack functional pDC and also fail to develop BCa-derived bone metastasis. pDC-depleted mice exhibited low levels of osteoclastogenesis-promoting cytokines and growth factors. Reduced tumor burdens in these animals were the result of a skew in the immune response toward a TH1 profile, resulting in increased levels of cytotoxic CD8+ T cells as well as in an overall decrease of immunosuppressive cells.
Taken together, our data suggest that pDC may play a key role in the establishment of BCa bone metastasis. This novel function of pDC makes them a viable target for the development of novel therapeutic strategies. Both in humans and mice, pDC express the DC immunoreceptor (DCIR), which is a putative C-type lectin receptor (CLR). DCIR-mediated antigen uptake by human pDC leads to efficient antigen presentation and results into the induction of a memory T cell response.8 Besides DCIR, human pDC also express sialic acid binding Ig-like lectin H (Siglec-H). Antigen presentation to pDC via Siglec-H induces a TH1/TH17 polarization of CD4+ T cells without skew toward a TH2 or regulatory T (Treg) profile.9 Therefore, targeting DCIR and Siglec-H may be useful in the treatment of BCa. BCa-associated pDC are irresponsive, meaning that they fail to produce Type I IFNs, to TLR9 agonists such as CpG-A. Nevertheless, the therapeutic activation of pDC with imiquimod (a TLR7 agonist) has been shown to result in pDC-dependent Type I IFN production.10 Hence, several approaches may be used for the development of better therapeutic strategies against metastatic BCa and could possibly be extended to the treatment of other carcinomas associated with osteolytic bone pathology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22983
References
- 1.Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone. 2011;48:6–15. doi: 10.1016/j.bone.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A, et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol. 2012;189:4258–65. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–23. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 2012;72:6130–41. doi: 10.1158/0008-5472.CAN-12-2409. [DOI] [PubMed] [Google Scholar]
- 5.Pinto A, Rega A, Crother TR, Sorrentino R. Plasmacytoid dendritic cells and their therapeutic activity in cancer. Oncoimmunology. 2012;1:726–34. doi: 10.4161/onci.20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anjubault T, Martin J, Hubert FX, Chauvin C, Heymann D, Josien R. Constitutive expression of TNF-related activation-induced cytokine (TRANCE)/receptor activating NF-κB ligand (RANK)-L by rat plasmacytoid dendritic cells. PLoS One. 2012;7:e33713. doi: 10.1371/journal.pone.0033713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, et al. Myeloid derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Can Res. 2012;73:672–82. doi: 10.1158/0008-5472.CAN12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–53. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 9.Loschko J, Heink S, Hackl D, Dudziak D, Reindl W, Korn T, et al. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J Immunol. 2011;187:6346–56. doi: 10.4049/jimmunol.1102307. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch I, Caux C, Hasan U, Bendriss-Vermare N, Olive D. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol. 2010;31:391–7. doi: 10.1016/j.it.2010.07.004. [DOI] [PubMed] [Google Scholar]