Abstract
Transcription factors of the MYC family regulate several homeostatic cell functions, and their role as proto-oncogenes has been the focus of interest for decades. We have recently demonstrated that c-MYC is expressed by tumor-associated macrophages (TAMs) and regulates their phenotype and pro-tumor activities in vivo.
Keywords: angiogenesis, c-MYC, cancer, macrophage differentiation/maturation, tumor-associated macrophage
MYC transcription factors have long been known to affect many cellular processes, and recent experiments in Drosophila melanogaster have revealed yet another facet of these proto-oncogenes. Thus, in fly embryos, ‘winner’ cells with high dMyc levels actively eliminate neighboring ‘loser’ cells with lower dMyc activity to become the predominant cells.1 This so-called ‘cell competition’ phenomenon, which seems to be part of a developmental size and quality control program,1 has also been proposed to play an important role in mammalian cancer development, as an initial tumoral cell with higher expression/activity of proto-oncogenes, such as c-MYC, might have some advantages over non-transformed neighboring cells (e.g., increased metabolic rate, ability to resist apoptosis, high proliferative activity).2,3 The establishment of this competitive niche by tumor cells suggests the possibility that other cells in the tumor microenvironment might gain an advantage over neighbors upon MYC expression. Indeed, we have recently detected c-MYC expression in tumor-associated macrophages (TAMs) from human colon cancer.4
TAMs produce factors that promote angiogenesis, remodel tissues and dampen anti-tumor immune responses. Macrophages acquire specialized phenotypes in response to signals from the local microenvironment, hence polarizing toward a specific activation state.5 The tumor microenvironment has been shown to skew TAMs toward a wound healing/regulatory state that resembles several aspects of the alternatively-activated macrophage phenotype.6 We have recently shown that c-MYC is induced in human macrophages during alternative activation in vitro and controls the expression of several alternative activation-specific markers,4 suggesting that targeting c-MYC function in TAMs may limit tumor growth. To test this possibility in a pre-clinical setting, we crossed c-Mycfl/fl with LysMcre/+ mice to generate c-Mycfl/fl-LysMcre/+ mice exhibiting a myeloid cell-specific c-Myc inactivation (Mθ-c-Myc-KO).7 Compared with their counterparts with intact c-Myc (c-Mycfl/fl), bone-marrow derived macrophages and peritoneal macrophages from Mθ-c-Myc-KO mice showed lower c-Myc levels (70–100% reduction, across all analyzed mice), while the expression of c-Myc was unaffected in other tissues including the liver, kidney and testis. Under steady-state conditions, Mθ-c-Myc-KO mice exhibited normal immune system parameters, including numbers of total bone marrow cells and bone marrow hematopoietic precursors (e.g., multipotent, multiple erythroid, granulocyte-macrophage, common myeloid and macrophage progenitors, as well as long-term and short-term hematopoietic stem cells). Moreover, Mθ-c-Myc-KO and control c-Mycfl/fl mice showed similar circulating cell counts, including total white blood cells, lymphocytes, neutrophils, classical- and non-classical monocytes, granulocytes, erythrocytes and platelets, as well as comparable numbers of similarly distributed infiltrated CD68+ macrophages in the spleen, thymus and lymph nodes.
To investigate oncogenesis in mice with a macrophage-specific deletion of c-Myc, we injected B16-F10 melanoma cells carrying the firefly luciferase gene into the flanks of Mθ-c-Myc-KO and control c-Mycfl/fl mice and performed in vivo luciferase bioluminescent assays to assess tumor growth over time. These longitudinal studies together with post-mortem analysis (performed 15 d after tumor-cell inoculation) revealed a reduced tumor growth in Mθ-c-Myc-KO mice.7 The analysis of TAMs from neoplastic lesions developing in Mθ-c-Myc-KO mice showed a reduced expression of c-Myc but no effect on tumor cell proliferation or the apoptotic response of malignant cells, which, as in control animals, were very low. Interestingly, a detailed immunophenotyping (performed by flow cytometry) revealed a higher abundance of mature Ly6ClowMHCIIhigh TAMs in control animals, whereas TAMs isolated from Mθ-c-Myc-KO mice mostly dispayed an immature Ly6ChighMHCIIhigh phenotype. This observation is consistent with previous reports indicating that different tumor types or tumor grades are infiltrated by different TAM subtypes.8,9 Our results provide evidence for a previously unrecognized role of c-MYC in the control of TAM maturation in vivo. It would be interesting to study if, over time, tumors from Mθ-c-Myc-KO mice eventually reach the size of tumors observed in control animals and if, at that points, their TAMs have matured, somehow bypassing c-MYC inactivation. In this respect, it should be remembered that other transcription factors, such as NF-κB, are important drivers of cancer-related inflammation.10 It would therefore be interesting to analyze whether c-MYC and NF-κB act in parallel or sequentially, and if they are expressed in the same macrophages or in distinct macrophage populations exhibiting distinct intratumoral distributions.
TAMs isolated from Mθ-c-Myc-KO mice also showed an attenuation of pro-tumor functions (such as a reduced expression of vascular endothelial growth factor, VEGF, matrix metalloproteinase 9, MMP9 and hypoxia-inducible factor 1α, HIF1α), and this was associated with impaired tissue remodeling and angiogenesis in vivo (as assessed by molecular fluorescence tomography) as well as with a reduced development of new blood vessels (as revealed by the post-mortem confocal microscopy analysis of tumors).7 Consistent with these findings, Mθ-c-Myc-KO bone marrow-derived macrophages exposed to tumor-conditioned medium in vitro exhibited lower expression levels of VEGF, MMP9 and HIF1α than c-Myc-proficient macrophages, and this was accompanied by a decreased pro-tumor activity, as revealed by reduced MMP activity in zymogram assays, by inhibition of endothelial cell proliferation and migration in wound healing assays, as well as by inhibition of CD8+ T lymphocyte proliferation.
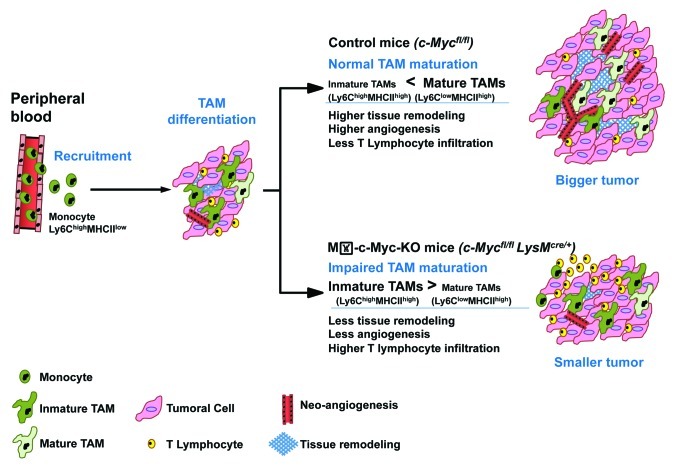
Our work identified c-MYC as an important regulator of TAM biology and maturation in vivo (Fig. 1). Compared with control animals, Mθ-c-Myc-KO mice, which specifically lack c-Myc in myeloid cells, exhibit defective tumor angiogenesis and reduced melanoma and fibrosarcoma development in xenographt models. These findings point to c-MYC inactivation as an attractive strategy for cancer treatment. We believe that the new Mθ-c-Myc-KO mouse model will also be useful for analyzing the role of c-MYC in other inflammatory diseases associated with myeloid cell infiltration.
Figure 1. Role of c-MYC in tumor-associated macrophages and cancer progression. Upon recruitment from the peripheral blood, monocytes differentiate within the tumor into immature tumor-associated macrophages (TAMs), which do not exert full-blown pro-tumor functions. In control c-Mycfl/fl mice (with intact c-Myc expression), TAMs mature and express high level of vascular endothelial growth factor (VEGF), matrix metalloproteinase 9 (MMP9) and hypoxia-inducible factor 1α (HIF1α), hence contributing to tissue remodeling, angiogenesis and tumor growth. The myeloid-specific deletion of c-Myc in Mθ-c-Myc-KO mice impairs TAM maturation and the expression of pro-tumor factors, thus inhibiting oncogenesis.
Glossary
Abbreviations:
- TAM
tumor-associated macrophage
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22984
References
- 1.Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–29. doi: 10.1016/S0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 2.Baker NE, Li W. Cell competition and its possible relation to cancer. Cancer Res. 2008;68:5505–7. doi: 10.1158/0008-5472.CAN-07-6348. [DOI] [PubMed] [Google Scholar]
- 3.Moreno E. Is cell competition relevant to cancer? Nat Rev Cancer. 2008;8:141–7. doi: 10.1038/nrc2252. [DOI] [PubMed] [Google Scholar]
- 4.Pello OM, De Pizzol M, Mirolo M, Soucek L, Zammataro L, Amabile A, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–21. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 5.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmieder A, Michel J, Schönhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol. 2012;22:289–97. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Pello OM, Chèvre R, Laoui D, De Juan A, Lolo F, Andrés-Manzano MJ, et al. In vivo inhibition of c-MYC in myeloid cells impairs tumor-associated macrophage maturation and pro-tumoral activities. PLoS One. 2012;7:e45399. doi: 10.1371/journal.pone.0045399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 9.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 10.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–46. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]